Penicillium verruculosum Strain BS3 Produces Aurantioclavine and Rugulosuvine B Alkaloids
Ravunni Maya, Sailas Benjamin
Ravunni Maya and Sailas Benjamin*
Enzyme Technology Laboratory, Biotechnology Division, Department of Botany, University of Calicut, Kerala, India
Received date: August 17, 2016; Accepted date: September 31, 2016; Published date: October 07, 2016
Citation: Maya R, Benjamin S. Penicillium verruculosum Strain BS3 Produces Aurantioclavine and Rugulosuvine B Alkaloids. Electronic J Biol, 12:4
Abstract
The present study describes rapid screening of Penicillium verruculosum strain BS3 (novel strain previously reported from this laboratory) for the production of secondary metabolites in potatodextrose agar and broth media. Metabolites were extracted from both mycelia (intracellular) and supernatant (extracellular). For intracellular secondary metabolites, the 5 days old mycelia were extracted in the solvent system containing methanol, dichloromethane and ethyl acetate (1:2:3 ratio, respectively) with 1% volume of formic acid; while the extracellular metabolites in the supernatant were extracted in ethyl acetate. The concentrated intraand extra-cellular extracts were pooled in methanol for analysis using liquid chromatograph/quardapole -time of flight/mass spectrometry (LC/Q-TOF/MS). Alkaloids such as aurantioclavine and rugulosuvine B were identified from the extract of P. verruculosum BS3, upon the database search of 465 secondary metabolites produced by various fungi. It indicates that P. verruculosum BS3 is a potent fungus capable of producing valuable leads for drugs such as aurantioclavine, an intermediate for the synthesis of fungal communesin and rugulosuvine B, an antitumor and antimicrobial agent.
https://marsbahislinki.com https://marsbetgiris.com https://mobilebahiss.com https://1xbetsgirisi.com https://onwinegiris.com https://betistegiris.com https://piacasinogiris.com https://holiganbahiss.com https://holiganbetting.com https://girisholigan.com https://mostbetegiris.com https://mariobetoyna.com https://meritgirisi.com https://meritkinge.com https://kingmerite.com https://nanogiris.com https://casinoplusa.com https://casinoplusgiris.com https://betriyal.net https://pluscasinogiris.com
Keywords
Penicillium verruculosum BS3; Aurantioclavine; Rugulosuvine B; LC/Q-TOF/MS.
1. Introduction
Microbial metabolites are biologically active compounds produced to fulfill the metabolism and growth activities of microorganisms. They are of two types, i.e., primary and secondary metabolites. Among microorganisms, fungi are the rich source of thousands of secondary metabolites, comprising many groups of low molecular weight compounds that are usually regarded as not essential for their life. Most of the secondary metabolites are produced towards the end of the growth phase of the organism. The secondary metabolites isolated from fungi exhibits some antimicrobial (antibacterial, antifungal and antiprotozoal), antitumor or antiviral activities [1]. Fungal secondary metabolites can be divided in to four main chemical classes; polyketides, terpenoids, shikimic acid derived compounds and non-ribosomal peptides [2]. Of diverse bioactive metabolites synthesized by the fungus Penicillium verruculosum; ergot and quinoline alkaloids and diketopiperzines got special consideration. Recently, profile of secondary metabolites offers an important component of fungal identification and classification. Selective extraction procedures required for obtaining a full-fledged profile of secondary metabolites. Secondary metabolites have been extracted and separated on the basis of chemical polarity using diverse array of chromatographic techniques such as Thin Layer Chromatography (TLC), High Pressure Liquid Chromatography (HPLC), Liquid Chromatography - Mass Spectrometry (LC-MS) and Liquid Chromatograph/Quadrapole-Time of Flight/ Mass Spectrometry (LC-Q-TOF/MS), etc. [3,4].
Many members of the genus, Penicillium are known to synthesize a variety of secondary metabolites of alkaloid in nature, i.e., ergots, diketopiperazines, quinolines, quinazolines, benzodiazepines and polyketide [5]. Out of them, aurantioclavine and rugulosuvine received much attention [6]. Aurantioclavine, the ergot alkaloidis believed to be a biological precursor to the communes in (a class of heptacyclic indole alkaloids with cytotoxic and insecticidal properties) family [7,8]. The bioactive rugulosuvines are diketopiperazine alkaloids [9]. Thus, the focus of this study is to screen Penicillium verruculosum strain BS3 for the production of bioactive secondary metabolites, with the following objectives: (a) cultivation and screening of P. verruculosum for the secondary metabolites; (b) extraction of potent secondary metabolites in suitable solvents; and (c) biophysical characterization of the active compounds extracted.
2. Materials and Methods
2.1 Materials
Analytical grade chemicals from HiMedia (India) and Nice (India) were used for the preparation of culture media and reagents for the extraction of metabolites.
2.2 Source of organism
Pure fungal culture, Penicillium verruculosum strain BS3 (GenBank accession No. HQ876770), reported previously from this Laboratory, was used in the present study [10].
2.3 Culture medium
Stock cultures were maintained on basal salt medium (BSM)-agar slants supplemented with carboxy methyl cellulose (CMC) at 28°C. The fungus was cultured on/in potato dextrose-agar (PDA) or potato dextrose (PD) broth. The PDA contained the following ingredients (g/l): 200 potato infusion; 20 dextrose; 15 agar in 1 L distilled water, and PD broth contained: 200 potato infusion; 20 dextrose in 1 L distilled water.
2.4. Extraction of secondary metabolites
2.4.1.Extraction of intracellular metabolites
Secondary metabolites were extracted from the mycelia of 5 days old P. verruculosum strain BS3 culture in solvent mixture (methanol, dichloromethane and ethyl acetate in ratio 1:2:3; containing 1% by volume formic acid), by crushing the mycelia using a mortar and pestle, and subsequent centrifugation at 12800 × g for15 min. The fungal extracts were stored at 4°C for further analysis.
2.4.2. Extraction of extracellular metabolites
Extracellular secondary metabolites were extracted from the mycelia of 5 days old culture of P. verruculosum from potato-dextrose broth in ethyl acetate. Using 100 ml separating funnel, the extract was concentrated in rotary evaporator at 77°C. Finally, intra- and extra-cellular extracts in methanol were pooled for further analysis.
2.5 Preliminary screening by TLC
The crude sample was loaded on silica gel coated plate. Solvent mixtures were toluene, ethyl acetate and formic acid in ratio 3:2:1 and 4:3:2, respectively. The chromatograms on the TLC plates were visualized by iodine reagent.
2.6. LC/Q-TOF/MS analysis
The crude extract was used for LC/Q-TOF/MS analysis using the facility available at the Inter- University Instrumentation Center, MG University in Kottayam. The Instrument used was Acquity H class (Waters) Ultra Performance Liquid Chromatography and Xevo G2 (Waters) Quadrapole-Time-of-Flight (Q-TOF). BEH C18 column (50 mm × 2.1 mm × 1.7 μm) was used at a flow rate of 0.3 ml/min. The total run time was 8 min. The source type was Electro- Spray Ionization (ESI) with the capillary temperature of 135°C. Capillary voltage of positive mode of ESI was 3.50 KV and for negative mode, it was 2.50 KV. The mobilization gas flow was nitrogen at 0.3 ml/min, approximately.
3. Results and Discussion
3.1. Effects of culture media
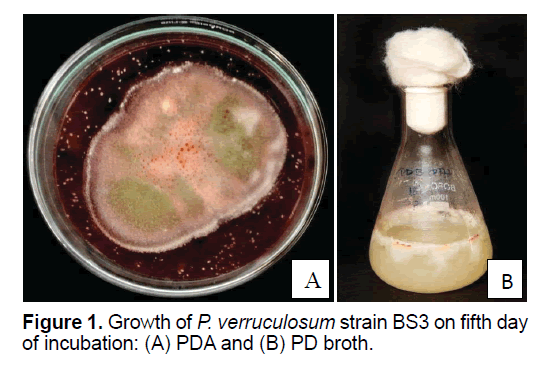
P. verruculosum strain BS3 grown in BSM supplemented with CMC in PDA and PD broth media showed significant growth within 5 days of incubation at 25ºC in both solid (on PDA) and liquid culture states. The fungus at its mycelial stage was whitish, and then upon sporulation (after 4 days of incubation), its color notably changed to light green. Well-developed fruiting bodies with spores appeared after 4 days of incubation at 25ºC (Figures 1 A and 1B). Brick-red colored pigment was released in 5 days of growth, which contained the secondary metabolites; thus 5 days old culture was used for the extraction of secondary metabolites. The brick-red colored pigment production was prominent on PDA medium. In PD broth, the mycelia were appeared as a viscous mat.
The components of culture media would affect production of secondary metabolite in fungi. The carbon or nitrogen ratio can affect the type and yield of the synthesis of secondary metabolites. Glucose, phosphate or ammonium at higher concentrations is generally regarded as the repressors of secondary metabolism. Several reports convincingly demonstrate that the profiles of fungal secondary metabolites varied under different culture conditions [11]. Growth of P. verruculosum strain BS3 onBSMCMC, PDA media and PD broth showed significant growth in 4 days. The carbon and nitrogen sources in PDA and PD broth media influenced the growth of P. verruculosum strain BS3.
3.2. Extraction of secondary metabolite
Fungal secondary metabolites may be cell bound (intracellular) or secretary (extracellular); therefore, both mycelia and supernatant were subjected to extraction.
3.2.1. Extraction of intracellular metabolites
The intracellular metabolites were extracted using 6 ml solvent mixture containing methanol, dichloromethane and ethyl acetate (1:2:3) in 1% of formic acid (v/v). The mycelia were disrupted using mortar and pestle or by sonication, with an output voltage of 15 dbs in 15 min. The extract was soluble in methanol, giving a pale yellowish color; which was stored at 4ºC for further analysis.
3.2.2. Extraction of extracellular metabolites
The extracellular metabolites were extracted in ethyl acetate. The upper layer containing the dissolved metabolites was collected and the lower layer was discarded (Figure 2). The extract was concentrated by evaporating the ethyl acetate using a rotary evaporator (77ºC at 120 rpm).
3.3. Screening by TLC
Preliminary screening of fungal secondary metabolites was carried out by TLC method. The solvent mixture contained toluene, ethyl acetate and formic acid in 3:2:1 and 4:3:2 ratios, respectively. A clear light orange-red spot was observed on both the TLC plate, run in two different solvent systems (Figure 3). The Rf of 3:2:1 solvent system was 0.557 cm, while that of 4:3:2 solvent system was 0.371 cm.
3.4. LC/Q-TOF/MS analysis
The analysis provided data based on mass to charge (m/z) ratio, chemical formula and molecular mass of the analytes from the crude sample. Using the information provided by LC/Q-TOF/MS analysis, the set of known secondary metabolites were identified from the database of fungal secondary metabolites [12]. The data from the LC/Q-TOF/MS obtained in both positive and negative modes. Many peaks with corresponding mass, m/z ratio and chemical structure are utilized for the identification of present study.
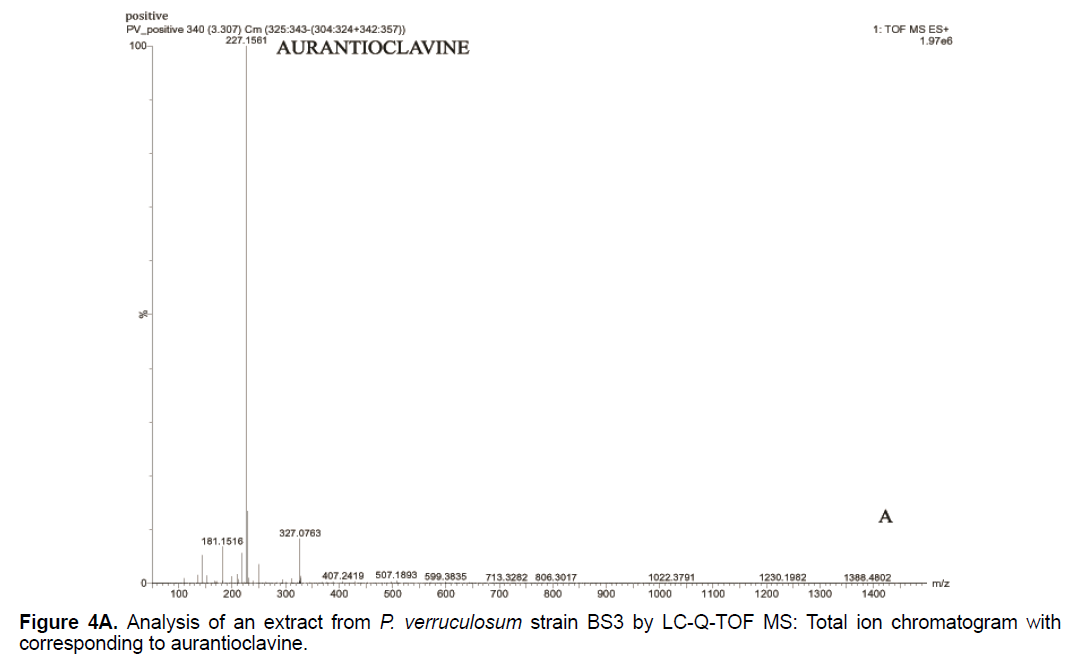
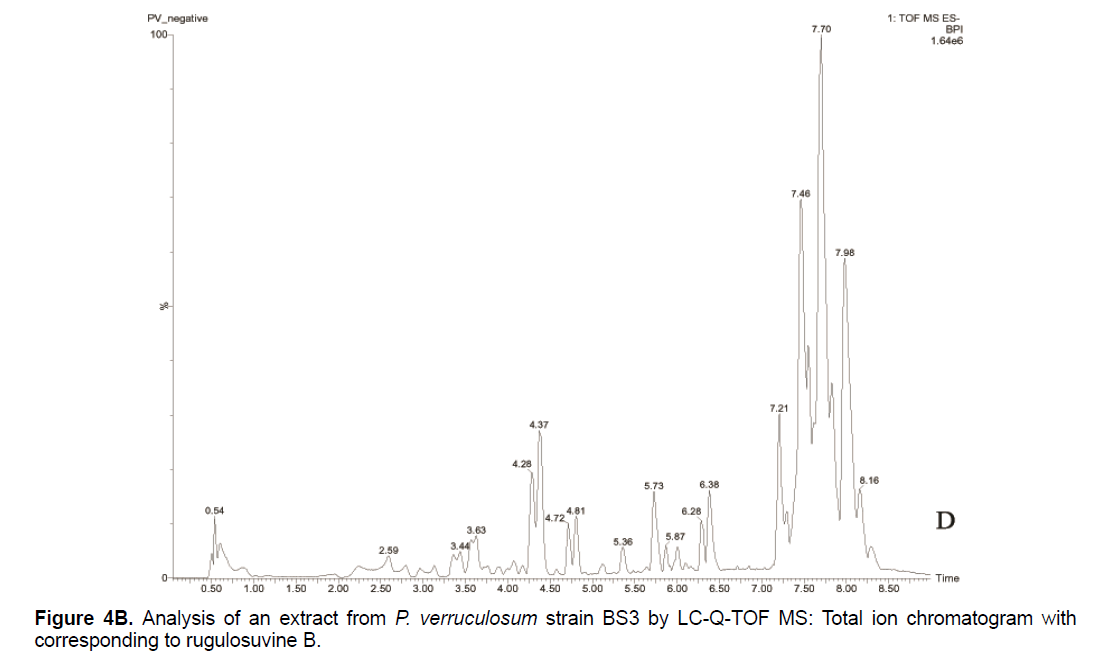
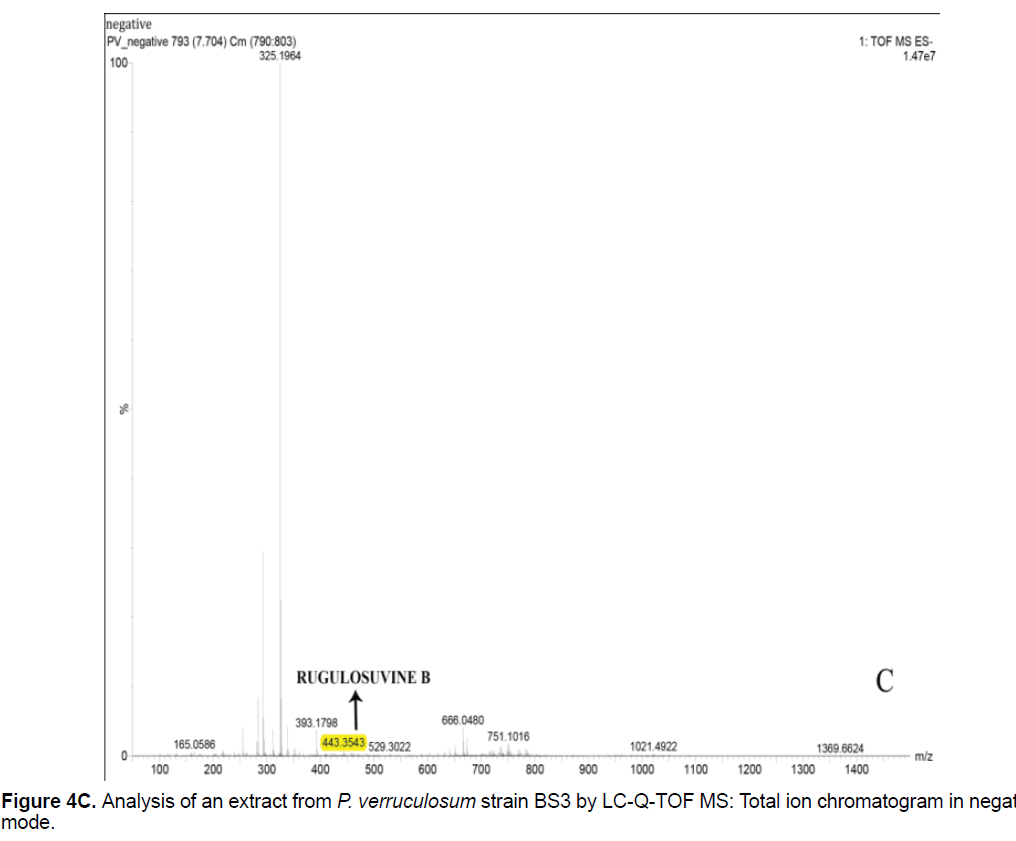
Based on the LC/Q-TOF/MS profile and of the database, aurantioclavine and rugulosuvine B alkaloids were identified as the significant compounds (Figures 4A-4C). As seen in Figure 4A, about 19 components were detected from the total ion chromatogram obtained from the extract of P. verruculosum strain BS3. The peak with m/z 227.1548 at 3.3 of RT was identified as aurantioclavine. Aurantioclavine is an ergot alkaloid, whose molecular structure is characterized by a tetracyclic ergoline system with a seven carbon ring and a ring D [7,8]. A resourceful producer of this alkaloid is P. nalgiovense Lax (1932) VKM F-229, formerly known as P. aurantiovirens VKM F-229. Optimal media for the synthesis of aurantioclavine and other ergot alkaloids by Penicillium spp. fungi contain succinate and manitol as carbon source and ammonium salt as nitrogen source [13]. It serves as an intermediate in the biosynthesis of fungal communesin. The dynamics of aurantioclavine in the liquid culture of the fungus also displayed a biphasic pattern. The extracellular concentration of the alkaloids increased concurrently with the biomass during the lag phase of active growth in succinate, and it will decrease in log phase. With manitol, increased active growth was seen, whereas at the beginning of the stationary growth phase, the concentration of alkaloid reached the maximum [14]. Within the retention time 7.7 of mass 443.3543 is identified as rugulosuvine B. Kozlovsky isolated the diketopiperazine of tryptophan and phenylalanine, an alkaloid metabolite from the culture of P. rugulosum VKM F-182 and named it after the producer (rugulosuvine) [15]. Absolute structure of rugulosuvine B was demonstrated as identical to that of rugulosuvine A. Rugulosuvine B showed antibiotic and antitumor activities [6,15]. Penicillium verruculosum (IMI 3521 19) was found to produce three closely related macro cyclic polylactones: BK223-A, BK223-B and BK223-C. BK223-A was identified as NG-012, a nerve growth factor potentiator isolated from P. verruculosum F-45421; BK223-Band BK223-C, were found as novel. All these compounds exhibit growth inhibiting activity against plant pathogenic fungi including Botrytis cinerea, Phoma lingam, Phoma betae, Pyrenophora teres, Sclerotinia sclerotiorum, Monilinia fructigena, Ascochyta pisi and Alternaria alternate [16].
Literature shows that aurantioclavine is produced both as intracellular and extracellular metabolite [13]; while, rugulosuvine B is produced chiefly as an extracellular metabolite [6,17]. Thus, both extracellular and intracellular metabolites are pooled for the rapid screening of useful secondary metabolites by LC/Q-TOF/MS technique.
5. Conclusion
P. verruculosum strain BS3 sub-cultured in media such as basal salt medium supplemented with carboxy methyl cellulose, PDA and PD broth. Two weeks old culture was extracted in ethyl acetate, which was subjected to LC-QTOF-MS analysis. Aurantioclavine and rugulosuvine B were the major secondary metabolites identified from the analysis of data. Rugulosuvine B is known to have antibiotic and antitumor properties.
6. Acknowledgement
The financial assistance (Grant No. 51/SPS54/2015/ CSTE) from Kerala State Council for Science, Technology and Environment (KSCSTE), Government of Kerala, is gratefully acknowledged.
References
- Berdy J. (2005). Bioactive microbial metabolites. The Journal of Antibiotics. 58: 1-26.
- Collemare J, Billard A, Böhnert HU, et al. (2008). Biosynthesis of secondary metabolites in the rice blast fungus Magnaporthe grisea: The role of hybrid PKS-NRPS in pathogenicity. Mycological Research. 112: 207-215.
- Smedsgaard J. (1997). Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. Journal of Chromatography. 760: 264-270.
- Klitgaard A, Iversen A, Andersen MR, et al. (2014). Aggressive dereplication using UHPLC–DAD–QTOF: screening extracts for up to 3000 fungal secondary metabolites. Analytical and Bioanalytical Chemistry. 406: 1933-1943.
- Kozlovsky AG, Zhelifonova VP, Antipova TV. (2013). Fungi of the genus Penicillium as producers of physiologically active compounds (Review). Applied Biochemistry and Microbiology. 49: 1-10.
- Kozlovsky AG, Zhelifonova VP, Adanin VM et al. (2001). The biosynthesis of low-molecular-weight nitrogen-containing secondary metabolite-alkaloids by the resident strains of Penicillium chrysogenum and Penicillium expansum isolated on the board of the Mir space station. Mikrobiologiia. 71: 773-777.
- Brak K, Ellman JA. (2010). Synthesis of (-)-Aurantioclavine. Synfacts. 9: 0978-0978.
- Suetsugu S, Tsukano C, Takemoto Y. (2016). Synthetic Studies towards communesins: Diastereoselective oxidative rearrangement of aurantioclavine derivatives. European Journal of Organic Chemistry. 1: 108-115.
- Bra¨se S, Encinas A, Keck J, et al. (2009). Chemistry and biology of mycotoxins and related fungal metabolites. Chemical Reviews. 109: 3903-3990.
- Sajith S, Sreedevi S, Priji P, et al. (2015). Production and partial purification of cellulase from a new isolate, Penicillium verruculosum BS3. British Microbiology Research Journal. 9.
- Miao LI, Kwong TF, Qian PY. (2006). Effect of culture conditions on mycelial growth, antibacterial activity, and metabolite profiles of the marine-derived fungus Arthrinium of saccharicola. Applied Microbiology and Biotechnology. 72: 1063-1073.
- Nielsen KF, Ma°nsso M, Rank C, et al. (2011). Dereplication of microbial natural products by LC-DAD-TOFMS. Journal of Natural Products. 74: 2338-2348.
- Zhelifonova VP, Kulakovskaya TV, Kozlovskii AG. (2003). On the mechanisms of the excretion and uptake of the alkaloid aurantioclavine during the growth of the fungus Penicillium nalgiovense VKM F-229. Microbiology. 72: 152-156.
- Zhelifonova VP, Antipova TV, Kozlovsky AG. (2010). Secondary metabolites in taxonomy of the Penicilliumfungi. Microbiology. 79: 277-286.
- Kozlovsky AG, Adanin VM, Dahse HM, et al. (2001). Rugulosuvines A and B, diketopiperazine alkaloids of Penicillium rugulosum and Penicillium piscarium fungi. Applied Biochemistry and Microbiology. 37: 253-256.
- Breinholt J, Jensen GW, Nielsen RI, et al. (1993). Antifungal macrocyclic polylactones from Penicillium verruculosum. The Journal of Antibiotics. 46: 1101-1108.
- Kozlovsky AG, Zhelifonova VP, Antipova TV. (2009). Secondary metabolites in the taxonomy of fungi of the subgenus Penicillium. Microbiology. 78: 618-623.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences