Wharton Jelly Mesenchymal Stem Cells Derived from Human Umbilical Cord Capable to Differentiate into Neural-Like Cells and their Potential Use in Neurological Disorders
Panagiotis Mallis, Gregory Papadopoulos, Efstathios Michalopoulos, Catherine Stavropoulos-Giokas
Hellenic Cord Blood Bank, Biomedical Research Foundation Academy of Athens, 4 Soranou Ephessiou Street, Athens, Greece.
- Corresponding Author:
- Efstathios Michalopoulos
Hellenic Cord Blood Bank
Biomedical Research Foundation Academy of Athens
4 Soranou Ephessiou Street, Athens, Greece
Tel: +302106597331
Fax: +30 210 6597345
E-mail: smichal@bioacademy.gr
Received date: November 30, 2017; Accepted date: December 27, 2017; Published date: December 31, 2017
Citation: Mallis P, Papadopoulos G, Michalopoulos E, et al. Wharton Jelly Mesenchymal Stem Cells Derived from Human Umbilical Cord Capable to Differentiate into Neural-Like Cells and their Potential Use in Neurological Disorders. Electronic J Biol, 13:4
Abstract
Worldwide, the neurological disorders are affecting a great number of patients, lowering their quality of life. Stem cell-based therapies have been applied but still need further optimization. MSCs could be used in neurological disorders via differentiation to neurallike cells. The main objectives of this study were to determine the expression of CD271 in WJ-MSCs and then to differentiate them into neural-like cells, with a combination of bFGF and forskolin. The results clearly showed the expression of CD271 and the successful differentiation of WJ-MSCs into neurallike cells that can be used for personalized medicine in patients with neurological disorders.
Keywords
Mesenchymal stem cells; CD271; Neural like cells; Basic fibroblast growth factor; Forskolin.
Introduction
Neurological disorders are a worldwide problem, accounting 20% of the total burden of diseases. Moreover, 12% of the patients with neurological disorders are dying due to severe complications such as ischemic heart disease [1]. Neurological disorders involve autoimmune diseases such as Amytrophical Lateral Sclerosis (ALS), Multilple Sclerosis and neural issues caused by traumatic injury and tumor development [2]. The common pathological outcome is the demyelination either of the central or peripheral nervous system, leading to neural necrosis, thus occurring severe health problems. In this way, the remyelination and the production of functional neural cells in the affected areas is a low process, leading most of the times to scar tissue formation [2]. Current approaches involve the use of an autologous secondary nerve as nerve conduit or stem cellbased approaches. However, the stem cell based approaches are demanding well defined conditions under good manufacturing practices (GMPs) in order to be applied to humans [2]. The induced pluripotent stem cells (iPSCs) could be used in neurological diseases but still are considered as a non-safe approach [2]. For this purpose, Mesenchymal Stem Cells (MSCs) could be an alternative source for the production of neural cells such as neurons, oligodendrocytes and astrocytes. Until now several groups are trying to differentiate the MSCs derived from Bone Marrow (BM) or Adipose Tissue (AT) to neural cells with contradictory results [3,4]. Furthermore, the isolation of BM or AT MSCs is a quite invasive procedure and the percentage of the obtained cells is 0.01-0.001% [5]. On the other hand, MSCs can be easily obtained from the Wharton Jelly (WJ) tissue, a material that can be discarded after the gestation. WJ-MSCs are characterized by increase proliferation and differentiation capacities than the cells from the aforementioned sources. WJ-MSCs have been characterized as immune privileged cells capable of differentiating into osteocytes, chondrocytes and adipocytes with high expression of surface markers CD90, CD73 and CD105 as indicated by the International Society for Cellular Therapy (ISCT) [6,7]. Until date, it has been described by several groups the expression of low-affinity nerve growth factor receptor (LNGFR), CD271, in the BM and AT MSCs, though the expression of this surface marker in the WJ MSCs needs further research [8,9]. LNGFR is highly expressed in cells of the central and peripheral nervous system, contributing to neural cell fate establishment and survivability.
Τhe objective of this study was to confirm the expression of CD271 in the WJ-MSCs and to further assess their differentiation capacity to neural like cells with the use of a two stage differentiation protocol. Under this scope, the WJMSCs were isolated from human umbilical cords, expanded and assessed for surface markers and multilineage differentiation. Finally, the welldefined WJ-MSCs were tested for differentiation on neural-like cells with the use of basic fibroblast growth factor (bFGF) and forskolin.
Methods
Isolation and culture of WJ-MSCs
Fresh human umbilical cords (n=5, l=5 to 10 cm) were collected from normal deliveries (gestational ages 38-40 weeks) by experienced midwives trained in cord blood collection after informed consent form from the mothers and has been accepted by the ethical committee of Biomedical Research Foundation Academy of Athens-Ref ID 2017/14-5 (Supplementary Information). Demographic data of each umbilical cord used in this study is provided in Table 1. The Wharton Jelly tissue then processed. Briefly, the umbilical vessels were removed; the exposed Wharton Jelly tissue was cut with scissors into small pieces (1-3 mm3), placed into 6-well plates (Costar) and cultured with growth medium in a humidified atmosphere with 5% CO2 at 37°C. Upon reaching confluency, cells were detached using 0.25% trypsin EDTA solution (Gibco), washed with PBS 1x and re-plated into the 75 cm2 flasks (Costar) with the appropriate culture medium. The growth medium that was used for the expansion of WJ-MSCs consisted of α-Minimum Essentials Medium (α-MEM, Gibco) supplemented with 15% Fetal Bovine Serum (FBS, Gibco), 10 U/ml penicillin (Gibco), 10 μg/ml streptomycin (Gibco) and 2 mM L-glutamine (Gibco). The growth medium was changed twice every week and the cultures were maintained in a humidified atmosphere with 5% CO2 at 37°C.
| No | Gestational Ages (weeks) | Gender | Birth Weight (kg) | Delivery | Nationality |
|---|---|---|---|---|---|
| 1 | 38 | Male | 3.250 | Normal | Greek |
| 2 | 40 | Female | 2.588 | Normal | Greek |
| 3 | 39 | Male | 4.100 | Normal | Greek |
| 4. | 38 | Male | 3.580 | Normal | Greek |
| 5. | 40 | Female | 3.120 | Normal | Greek |
Table 1: Demographic data of the human umbilical cords.
Phenotypic characterization of WJ-MSCs
WJ-MSCs (n=5) were analyzed for cell surface antigen phenotyping using flow cytometry. Each sample was measured in triplicate. Cells were labeled with fluorescein isothiocyanate-conjugated anti-CD90, HLA-ABC, CD29, CD19, CD31, CD45. Epitopes CD105, CD73, CD44, CD3, CD271 and CD14, HLADR and were assessed with phycoerythrin-conjugated and PC5-conjugated mouse anti-human monoclonal antibodies, respectively. All monoclonal antibodies for determining the expression of surface markers by flow cytometry were purchased from Beckman- Coulter except CD271 that was purchased from Dako. The WJ-MSCs phenotypes were analyzed in Cytomics FC 500 (Beckman Coulter) flow cytometer with the CXP Analysis software (Beckman Coulter).
WJ-MSCs multi-lineage differentiation
WJ-MSCs were characterized for their multilineage differentiation capacity towards to adipogenic, osteogenic and chondrogenic lineages. Briefly, WJ-MSCs (n=3) were plated into 6-well plates and differentiated into osteogenic, and adipogenic lineages using osteogenic and adipogenic stimulatory medium (StemCell Technologies) respectively. After 30 days of differentiation adipogenic and osteogenic lineages were assessed with Oil Red-O (Sigma-Aldrich), Alizarin Red S (Sigma-Aldrich) respectively. Chondrogenic differentiation of WJ-MSCs was induced in a spheroid cultures using chondrogenic stimulatory medium for 30 days. The pellets were fixed with 10% formalin (Sigma-Aldrich), paraffin embedded and cut into 5 μm sections. Chondrogenic differentiation was assessed with Toluidine blue (Fluka, Sigma Aldrich) staining.
Neural-like cells differentiation
WJ-MSCs were tried to differentiate into neural-like Cells with a two stage protocol, combining bFGF and forskolin. More specifically, the WJ-MSCs were plated into 24 well plates and 3 different concentrations of bFGF were applied, in order to evaluate the best effects on WJ-MSCs differentiation. Briefly, the WJMSCs plated into 24 well plates, subgrouped into 3 categories and followed by the addition of D-MEM supplemented with 100 ng/ml, 200 ng/ml and 500 ng/ml bFGF, respectively for 7 days. Then removal of the medium was applied, and addition of D-MEM supplemented with 10 mM forskolin was performed for another7 days. The whole procedure performed in a humidified atmosphere with 5% CO2 at 37°C for a time period of 14 days. Finally, in order to observe the morphological differences between undifferentiated and differentiated cells Vybrant- Dil-Dye (Invitrogen) in combination with DAPI stain (Invitrogen) was used.
Statistical analysis
Statistical analysis was performed by using Graph Pad Prism v 6.01. Indicated values are mean ± standard deviation.
Results
Phenotypic characterization of WJ-MSCs
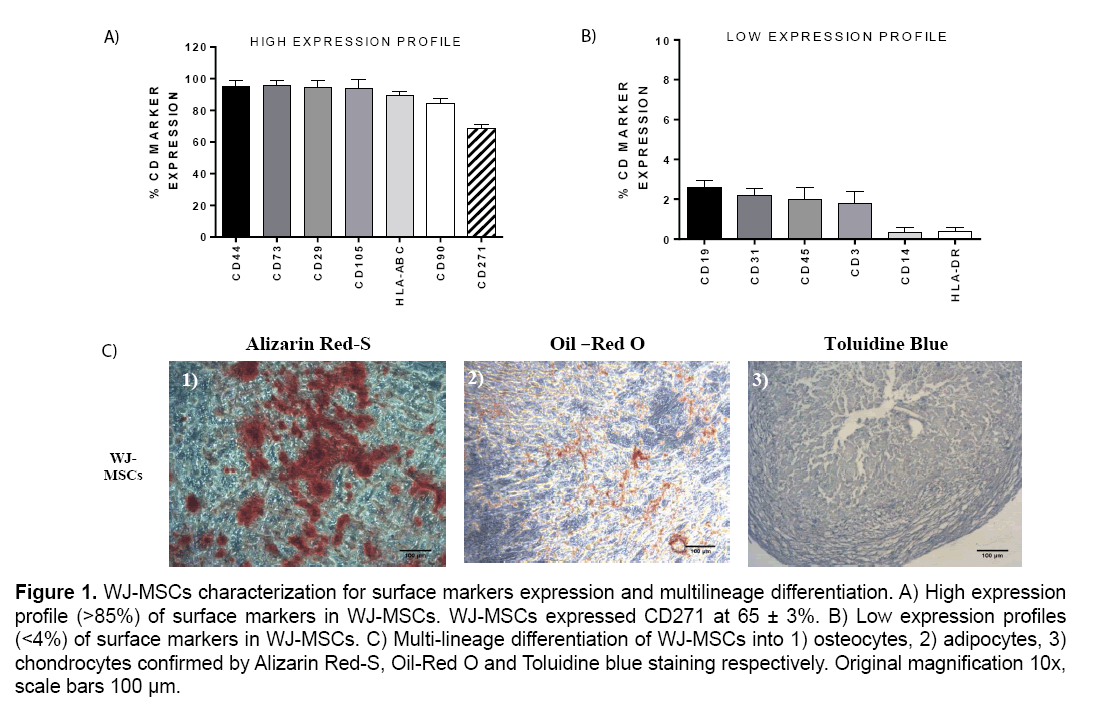
The WJ-MSCs characterized by high expression of surface markers CD44, CD29, CD73, CD105, HLAABC, CD90 (>85%) and by low expression of CD19, CD3, CD31, CD14, CD45 and HLA-DR (<4%). Furthermore, the expression of CD271 marker by WJ-MSCs was 65 ± 3% (Figure 1).
Figure 1: WJ-MSCs characterization for surface markers expression and multilineage differentiation. A) High expression profile (>85%) of surface markers in WJ-MSCs. WJ-MSCs expressed CD271 at 65 ± 3%. B) Low expression profiles (<4%) of surface markers in WJ-MSCs. C) Multi-lineage differentiation of WJ-MSCs into 1) osteocytes, 2) adipocytes, 3) chondrocytes confirmed by Alizarin Red-S, Oil-Red O and Toluidine blue staining respectively. Original magnification 10x, scale bars 100 μm.
Evaluation of multilineage differentiation of WJ-MSCs
WJ-MSCs successfully differentiated into osteocytes, adipocytes and chondrocytes as determined by the histological stains that were performed. Specifically, WJ-MSCs successfully exhibited after 30 days of cultivation, calcium depositions that were stained positively with Alizarin Red S stain, which is specific for calcium mineralization (Figure 1). Additionally, WJ-MSCs exhibited under adipogenic stimulatory conditions lipidic inclusions within 30 days and stained positively with Oil-Red –O stain (Figure 1). Finally, these cells were capable to form spheroids which were characterized by high glycosaminoglycan content as determined by Toluidine blue staining (Figure 1).
Evaluation of Neural-like cells differentiation of WJ-MSCs
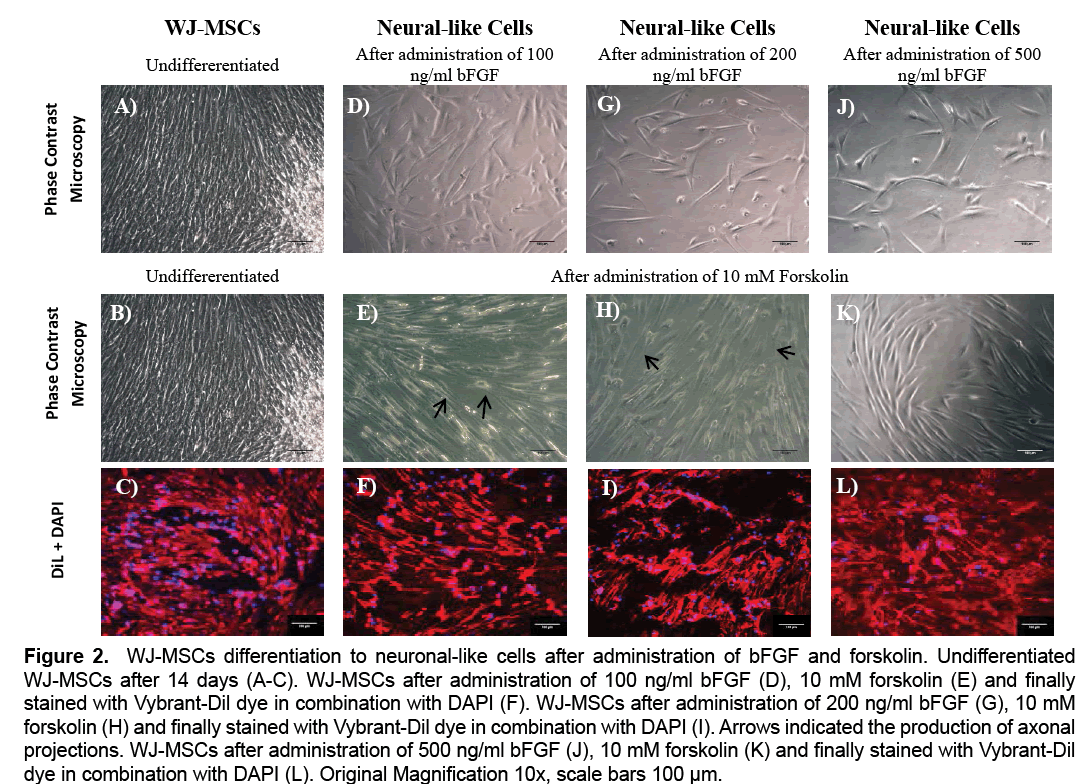
WJ-MSCs showed progressive morphological change in cell shape during the 14 days of differentiation. Moreover, WJ-MSCs gradually changed their fibroblastic-like morphology into spindle-shaped cells under 100 ng/ml, 200 ng/ml and 500 ng/ml bFGF treatment (Figure 2). Finally, after administration of 10 mM forskolin, only the WJ-MSCs treated with 100 ng/ml and 200 ng/ml of bFGF were able to produce a small number of axonal projections. On the other hand, the WJ-MSCs treated with 500 ng/ml of bFGF, weren’t able to produce axons, and retained their initial shape (Figure 2). The above results were further confirmed with the use of Vybrant-Dil dye in combination with DAPI stain, where the morphological alteration of WJ-MSCs after the administration of the above growth factors, could be observed (Figure 2).
Figure 2: WJ-MSCs differentiation to neuronal-like cells after administration of bFGF and forskolin. Undifferentiated WJ-MSCs after 14 days (A-C). WJ-MSCs after administration of 100 ng/ml bFGF (D), 10 mM forskolin (E) and finally stained with Vybrant-Dil dye in combination with DAPI (F). WJ-MSCs after administration of 200 ng/ml bFGF (G), 10 mM forskolin (H) and finally stained with Vybrant-Dil dye in combination with DAPI (I). Arrows indicated the production of axonal projections. WJ-MSCs after administration of 500 ng/ml bFGF (J), 10 mM forskolin (K) and finally stained with Vybrant-Dil dye in combination with DAPI (L). Original Magnification 10x, scale bars 100 μm.
Discussion
The aim of this study was first to confirm the expression of CD271 surface marker on the WJ-MSCs and then to evaluate a two stage differentiation protocol in order to produce Neural-like cells. Stem Cell-based therapy could be applied to a wide variety of neurological disorders including neurodegenerative diseases and those occurred from traumatic injuries. Additionally, in traumatic injuries the gold standard therapeutic strategy is to join the proximal and distal stumps and when the gap is too big, a nerve conduit is needed. In case of neurodegenerative diseases, transplantation of neural cells such as neurons, astrocytes and oligodendrocytes can be performed, derived either from iPSCs or from autologous sources [2]. However, both approaches need further establishment in order to be applied safely in patients. Under this scope, the use of WJ-MSCs might be an alternative stem cell-based therapy, without demanding huge time periods such as the iPSCs technology. WJMSCs can be efficiently isolated from the Wharton Jelly tissue of the human umbilical cord without invasive procedures, expanded and differentiated to mesodermal cells. Until now, the BM-MSCs and ATMSCs have been used for the production of neural cells with contradictory results [3,4]. These cells are expressing the LNGFR, a receptor that is needed for the development of neural cells. One of the objectives of this study was to confirm the presence of low affinity nerve growth factor, CD271, in the WJMSCs. It is known, that CD271 plays a crucial role in the development and survival of mature neurons via recruiting BDNF, NGF and NT 3/4 and leading to neural gene expression through upregulation of phosphoinositide 3-kinase (PI3K), phosphoinositidekinase 1 (PDK-1) and protein kinase B (PKB). The WJ-MSCs express the CD271 surface marker at 65 ± 3% as determined by flow cytometry analysis in the present study, indicating that this cellular population might be a good candidate for neural-like cell development. These results are in accordance with the study of Viejo et al. [8], who confirmed the presence of CD271 in BM-MSCs, under the same culture conditions as in the current project. Furthermore, the WJ-MSCs were characterized by high expression of CD90, CD73 and CD105 (>85%) and by low expression CD45 and HLA-DR (<4%) and were capable to be differentiated into osteocytes, chondrocytes and adipocytes upon stimulatory conditions as mentioned by the ISCT [7].
Then, the WJ-MSCs were tested for differentiation into neural-like cells via a two stage differentiation protocol combining bFGF and forskolin. It was observed, that only WJ-MSCs treated with 100 ng/ ml, 200 ng/ml of bFGF and 10 mM of forskolin were able to produce a few axonal projections. On the other hand, WJ-MSCs treated with 500 ng/ml failed to acquire neural shape. The produced axons are a consequence of bFGF and forskolin administration. Specifically, bFGF induces axon outgrowth through its signaling pathway that involves the activation of Mitogen Activated Protein Kinase (MAPK)/ Extracellular Regulated Kinase (ERK)1/2, thus leading to upregulation of Cofilin and GAP43 while forskolin seems to enhance this mechanism via the elevated levels of cyclic Adenoside Monophosphate [10]. On the contrary, high levels of bFGF might have a negative feedback in axon development of WJMSCs, as previously shown in this study, but further experiments must be performed in order to obtain safe conclusions. Additionally, the morphological characteristics of WJ-MSCs observed with vibrant Dil dye in combination with DAPI stain, indicated the production of elongated cells after the administration of bFGF and forskolin and this cytoplasmic alteration were in accordance with previous studies. More specifically, in the study of Yen et al., similar morphological changes were occurred in BM-MSCs, confirming the differentiation potential of these cells towards to neural lineage [11].
Conclusion
WJ-MSCs might be used as an alternative source for production of neural-like cells that can be applied in patients with neurological disorders, providing a personalized approach. Further analysis, must be performed in order to confirm that these cells are capable to retain their neural characteristics, and can further be differentiated into mature neurons, oligodendrocytes and astrocytes.
References
- Prince M, Patel V, Saxena S, et al. (2007). No health without mental health. Lancet. 370: 859-877.
- Fathi SS, Zaminy A. (2017). Stem cell therapy for nerve injury. World J Stem Cells. 9: 144-151.
- Takeda YS, Xu Q. (2015). Neural differentiation of human mesenchymal stem cells using exosomes derived from differentiating neural cells. PLoS One. 10.
- Huang T, He D, Kleiner G, et al. (2007). Neuron-like differentiation of adipose derived stem cells from infant piglets in vitro. J Spinal Cord Med. 30: 35-40.
- Akiyama K, You YO, Yamaza T, et al. (2012). Characterization of bone marrow derived mesenchymal stem cells. Stem Cell Res Ther. 3: 2-13.
- Wang HS, Hung SC, Peng ST, et al. (2004). Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 22: 1330-1337.
- Dominici M, Le Blank K, Mueller I, et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8: 315-317.
- Viejo MA, Menendez YM, Gelaz AB, et al. (2013). LNGFR (CD271) as a marker to identify mesenchymal stem cells from different Human Sources: Umbilical cord blood, Wharton’s jelly and bone marrow. J Bone Marrow Res. 1: 1-6.
- Wu Y, Hoogduijn MJ, Baan CC, et al. (2017) Adipose tissue-derived mesenchymal stem cells have a heterogenic cytokine secretion profile. Stem Cells Int. 2017. 1-7.
- Auer M, Schweigreiter R, Hausott B, et al. (2012). Rho-independent stimulation of axon outgrowth and activation of the ERK and Akt signaling pathways by C3 transferase in sensory neurons. Front Cell Neurosci. 6: 1-11.
- Ye Y, Zeng YM. (2011). Induction of human bone marrow mesenchymal stem cells differentiation into Neural-like cells using cerebrospinal fluid. Cell Biochem Biophys. 59: 179-184.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences