Evaluation of Anti-Ulcer Activity of Lagenaria siceraria Chloroform Extracts in Pylorus Ligated Rats
Prashanth Manchala*
Department of Pharmacology, KGR Institute of Technology and Management, Osmania University, Telangana, India
- *Corresponding Author:
- Prashanth Manchala
Department of Pharmacology
KGR Institute of Technology and Management
Osmania University, Telangana, India
Tel: +916300722622
E-mail: prashanthmp427@gmail.com
Received date January 09, 2019; Accepted date March 21, 2019; Published date March 28, 2019
Citation: Manchala P, Evaluation of Anti-ulcer activity of Lagenaria siceraria chloroform extracts in pylorus ligated rats. Electronic J Biol, 15:1
Abstract
The present investigation is to assess the antiulcer activity by utilizing natural remedy Lagenaria siceraria. The chloroform extract of Lagenaria siceraria treated groups demonstrates a huge impact when related to controller group animals. The acute toxicity study for chloroform extract of Lagenaria siceraria indicates that, it is harmless till 400 mg/kg body weight. The chloroform extract of Lagenaria siceraria at 250 mg/ kg dose has shown mucosal erosion, the partial healing of ulcer with few inflammatory cells and the dose 400 mg/kg has shown healing ulcer, mucosa and no inflammation of cells. Lagenaria siceraria extracts reported to own antioxidant activity and to contain various types of compounds such as flavonoids, saponins and tannins. The gastro protective effect exhibited by chloroform extract Lagenaria siceraria is speculated to be recognized for its antioxidant property, which in turn could be linked to existence of flavonoids and polyphenolic compounds, saponins and tannins. These compounds most likely inhibit gastric mucosal injury. The chloroform extract of Lagenaria siceraria treated groups illustrate a major effect when related to control group animals which shows that the plant containing antiulcer action.
Keywords
Lagenaria siceraria; Antiulcer; Actue toxicity.
1. Introduction
Ulcer is characterize as a break in the mucosa of the nutritious tract, which stretches out through the muscularis mucosa into the submucosa or more profound. It usually happens within the abdomen and proximal small intestine, and generally less it happens in the lower throat, the distal duodenum, or the jejunum.
There were two kinds of peptic ulcer: gastric ulcer, which caused because of harm to the coating of the stomach, and duodenal ulcer, related with inordinate corrosive emission by the stomach. Peptic ulcer is caused by a nonattendance of harmony between the stomachic forceful components and furthermore the layer cautious elements.
Peptic ulcer illness happens fundamentally because of utilization of NSAIDs (Nonsteroidal Anti- Inflammatory Drugs), smoking, liquor and disease by H. pylori, stress or because of pathological condition like Zollinger-Ellison Syndrome. Pain in the upper abdomen below the sternum (breastbone), it may occur most before meal or when we feel hungry other symptom bloating, dyspepsia, nausea, vomiting, poor appetite, weight loss and burping (belching) [1]. Previous works/literature provided information that, Lagenaria siceraria can decrease the mucosal ulcers and peptic ulcers in alcohol, ethanol induced animals. In those works methanolic and ethanolic extracts of the plant leaves are used.
In current work I intended to extract the material with chloroform (different solvents give different chemical constituents, as some Chemical constituents have more solubility in chloroform) and anti-ulcer activity will be screened in pylorus ligated animals these methods are never tried in the research work for antiulcer activity of Lagenaria siceraria.
1.1 Plant profile
Lagenaria siceraria
The calabash or lauki, Lagenaria siceraria, conjointly commonplace by a few distinct names that include: Opo squash (from Tagalog: Upo), long melon, suzza melon New Guinea bean and Tasmania bean could be a vascular plant huge for its natural product, which may either be collected youthful and utilized as a vegetable, or gathered develop, dried, and utilized as a container, utensil or pipe [2]. The contemporary natural product includes a greenish smooth skin and a white tissue. Rounder assortments are called calabash gourds. Leaves have a smooth surface inferable from the fine hairs, especially on the underside (Figure 1). The gourd blooms are borne exclusively on the axils of the leaves, the guys on long peduncles and accordingly the females on short peduncles. The blossoms are white and connecting with, up to four crawls in distance across, with spreading petals.
1.2 Morphology
Lagenaria siceraria is an incredible yearly herb. Stems territory unit prostrate or rising, precise, ribbed, thick, weak, delicately fuzzy, upto 5 m long, cut stems radiate no sap. Leaves zone unit clear, up to four hundred millimeter long and four hundred millimeter expansive, since a long time ago petioled, 5-lobed, cordate, pubescent, in a matter of seconds and delicately textured, extensively egg, kidney, or cordate in characterize, 3-7 lobed, flaps adjusted, edges shallowly toothed, pounded leaves nonaromatic [2]. Blooms territory unit pedunculate (female blossom stalks shorter than male), lone, unisexual, axillary, monoecious; petals five, crisped, cream or white hued with darker veins, light yellow at the base, obovate, up to forty five millimeter long, hole inside the nights, by and by wither [3]. Organic products zone unit huge, variable, round and hollow, flagon molded or ball-formed with a choking over the center; substantial, thickly shaggy, indehiscent, green, creating yellowish or pale darker, squash drying out on maturing, leaving a thick, hard, unfilled. Seeds district unit a couple, embedded in an astoundingly light crush, compacted, with 2 level facial edges, in a couple of varieties rather unusual and unsmooth.
1.3 Traditional uses
The fruits, leaves, oil, and seeds square measure edible and employed by native individuals as people medicines within the treatment of jaundice, diabetes, ulcer, piles, colitis, insanity, cardiovascular disease, and skin diseases [3]. The fruit pulp is employed as associate remedy, sedative, purgative, cooling, diuretic, antibilious, and pectoral. The flowers are an antidote to poison. Leaf juice is widely used for baldness [3, 4]. A wrapping of the crushed leaves has been applied to the head to treat headaches.
2. Materials and Methods
2.1 Accumulation and verification of plant material
The whole plant of lagenaria siceraria was collected from local fields and authenticated by Dr. K. Madhava Chetty, Department of Botany, Sri Venkateswara University, Tirupati, India.
2.2 Extraction of plant material
The plant leaves are shade dried for 2 weeks then crushed in to a coarse powder with the assistance of a mixer (LABCOMPARE motor grinder-RM-200) (Figure 2).
2.3 Hot continuous extraction (Soxhlet):
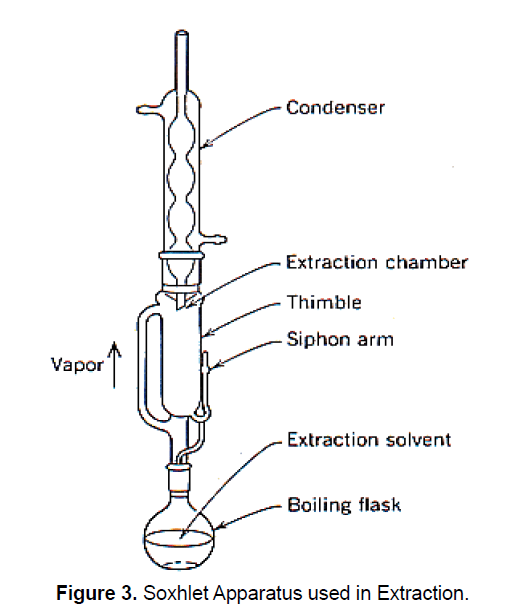
In this method, the finely ground crude drug is placed in a porous bag or “thimble” made of strong filter paper, which is placed in Soxhlet chamber. The extracting solvent in bottom flask is heated, and its vapours rise up and are condensed by condenser. The condensed extract leaks into the thimble containing the crude drug, and extracts it by contact. When the level of liquid in thimble rises up, the liquid content, automatically siphons back into bottom flask containing organic solvent. This process continuous and it is carried out until a drop of solvent from the siphon tube does not leave residue when evaporated. The Main advantage over other methods is that large amounts of drug can be extracted with a smaller quantity of solvent (Figure 3).
The filtrates (chloroform extract) extricate) got were evaporated utilizing Rotary evaporator in a porcelain dish. They rendered a sticky think of greenish dark. The concentrate was put aside in vaccum dissecator for 7 days.
% Yield value of chloroform extract from aerial parts of Lagenaria siceraria
Powder taken for extraction=200 gm
Load of the vacant china dish=59.70 gm
Load of the china dish with concentrate=93.28 gm
Load of the concentrate got=(93.28-59.70)=33.58 gm
% yield of chloroform extract=(load of concentrate) / (powder taken for extraction) × 100
= 33.58/200 × 100=16.79%.
2.4 Preliminary phytochemical screening of concentrates
Qualitative chemical tests were directed for chloroform concentrates to recognize the different phytoconstituents by utilizing regular screening tests [5, 6].
Test for Carbohydrates
Molisch’s test: To 2-3 ml of concentrate, few drops of α-naphthol arrangement in liquor were included, shaken and conc. H2So4 was included from the side of the test tube. It was watched for violet ring at the intersection of two fluids.
Tests for glycosides
Liebermann-Buchard’s, reaction: Mixed 2 mL of concentrate with chloroform. Included 1-2 mL of acidic anhydride and 2 drops conc. H2So4 from the side of the test tube. Watched for red, then blue lastly green.
Fehling’s, test: 1 ml of Fehling An and 1 ml of Fehling B arrangements was blended and heated for 1 min. measure up to volume of test arrangement was included. Warmed in bubbling water shower for 5-10 min and watched for a yellow and afterward brick red precipitate.
Benedict’s test: Equal volume of Benedict's official and test arrangement in test tube were blended. Warmed in bubbling water shower for 5 min. Arrangement may seem green yellow or red relying upon measure of decreasing sugar present in test arrangement.
2.4 Test for amino acids
Ninhydrin tests: 3 ml of test solution and 3 drops of ninhydrin were heated in boiling water bath for 10 min. observed for purple or bluish color.
2.4 Test for proteins
Millon’s test: Mixed 3 ml of test arrangement with 5 ml of Millon's reagent, white ppt. got. Ppt warmed turns brick red or Ppt breaks down given red arrangement.
2.4 Test for flavonoids
• To little amount of buildup, included lead acetic acid derivation arrangement watched for yellow Ppt.
• To the test arrangement, included couple of drops of Fecl2 arrangement watched for serious green.
2.4 Tests for tannins and phenolic compounds
• To 2-3 ml of test arrangement included couple of drops of following arrangements was searched for individual coloration or precipitate.
• 5% ferric chloride solution-Deep blueblack colored. Lead acetate solution-white precipitate. Gelatine solution-white precipitate. Acetic acid solution: Red colour solution.
Materials
Drugs: Standard drug ranitidine.
Animals: The animals utilized in the present investigation were adult male Wistar rats (10-12 weeks old with body weight 150-200 g), got from the animal house. The creatures were housed in state confines, underneath typical research facility conditions (12 h light, 12 h dull cycle), with free access to common business diet and water [7]. Every single trial method utilized in the present examination was endorsed by IAEC (Institutional Animal Ethics Committee).
3. Acute Toxicity Studies
3.1 Objective of performing acute toxicity studies
The point of performing acute toxicity investigations is to build up Therapeutic Index (TI) of a specific drug and to ensure the wellbeing in vivo. Intense danger examine dependent on OECD (Organisation for Economic Co-operation and Development) rule 423 is made for the assurance of LD50 (Lethal Dose) esteem in test creatures.
3.2 Requirements
Animal: Wistar rats, 150-200 gm.
Drugs/Extracts: Extracts of Lagenaria siceraria.
4. Procedure
• The overnight not eat rats were weighed up and selected.
• The extracts were dosed in a stepwise procedure, with the initial dose being selected as the dose expected to produce some signs of toxicity and were observed for a time of 14 days.
• The toxic doses were selected based on the Guideline 423.
• The Wistar rodents of single sex, weighing between 150 to 200 g were chosen and separated in to 5 bunches each comprising of 5 creatures. They were kept up under standard conditions (room temperature at 22 ± 3̊C, 12 h light/dull) and enabled free access to water alongside standard pelleted diet for multi week before the investigation. The creatures were oppressed for intense danger think about utilizing each concentrate at a portion of 2000 mg/kg orally in 5 gatherings and seen at regular intervals of 1, 2, 4, 8, 12 and 24 h for skin changes, horribleness, forcefulness, increase oral secretion, affectability to the sound and pain and respiratory developments and mortality.
4.1 Design of pylorus-ligation induced gastric ulcer
The Wister rats were unsystematically divided into 4 groups of 6 animals each, as given in table 1. Creatures were fasted for 24 h prior experiment, with access to water (Table 1).
| S. No | Groups | Treatment |
|---|---|---|
| 1 | I | control (saline) |
| 2 | II | Ranitidine (50 mg/kg) |
| 3 | III | CELS 250 mg/kg |
| 4 | IV | CELS 400 mg/kg |
Table 1: Experimental design of Pylorus-ligation induced gastric ulcer.
4.2 Experimental procedure
The control assembles treated with ordinary saline as it were. Second, third, fourth gatherings of creatures were treated with ranitidine through p.o, low and high portions of concentrates separately 1 h before pylorus ligation upon the arrival of analysis at around 10 AM. Following 1 h of medications treatment, creatures were anesthetized with the assistance of analgesic ether the stomach area was opened by a little midline cut underneath the xiphoid process. The stomach area was replaced thoroughly and in this manner the divider was shut by interfered with sutures. The abdomen was opened, viscus finish of the abdomen was cleft out and therefore the substances were taken into a glass tube. The volume of the stomach related liquid was estimated and centrifuged at 2000 rate for 10 min.
From the supernatant, aliquots (1 ml of each) were engaged for the assurance of pH scale, total and free acidity. Each abdomen was analyzed for sores inside the fore stomach area divide and ranked based on the severity.
4.3 Estimated Parameters
Estimation of gastric volume, pH: The gastric substance that was moved into rotator tubes was utilized for estimation of gastric volume, pH. The cylinders were centrifuged at one thousand cycles for each moment for ten min and furthermore the stomachic volume was specifically discussed from the graduation on the cylinders [8-10]. The supernatant was then gathered and pH scale resolve by utilizing a computerized pH scale meter.
Determination of total acidity: An aliquot of 1 ml gastric juice weakened with 1ml of refined water was taken into a 50 ml cone shaped flask and two drops of phenolphthalein marker was added to it and titrated with 0.01N NaOH till lasting pink shading was recognized. The volume of 0.01N NaOH spent was noted.
Determination of free acidity: As a substitute of phenolphthalein marker, the Topfer's reagent was utilized. Aliquot of gastric juice was titrated with 0.01N NaOH until the point when canary yellow shading was watched. The volume of 0.01N NaOH expended was noted. The free corrosiveness was determined by a similar equation for the assurance of absolute sharpness. Acridity was communicated as underneath,

Determination of Ulcer Index (UI):
The ulcer index was considered by severity of stomachic tissue layer and sorted as follows:
0=no ulcer
1=superficial ulcerssss
2=deep ulcer
3=perforation
UI=UN+US+UP × 10-1
Where, UN=average of no. of ulcers per animal
US=average of severity score
UP=Percentage of animals with ulcers
% gastro protection was calculated according to,
% gastro protection=(UIC-UIT)/UIC*100
Where, UIC-Ulcer Index of Control.
UIT-Ulcer Index of Test.
4.4 Histopathological evaluation
The stomach tissue was mounted in 10% ethanol cushion formalin and prepared however arranged ethanol, xylene and impregnated with paraffin wax, areas were made by microtome. In the wake of recoloring with hematoxylin and eosin recolor, the segments were inspected under an examination magnifying instrument by an individual who knew about exploratory conventions [11, 12]. The diverse histopathological files screened were: Congestion, discharge, oedema, rot, provocative and dysplastic changes, disintegrations and ulcerations.
4.5 Histopathological approach to rat stomach Procedure
The rodents were executed by cervical disengagement and separated. The stomachs were expelled from the staying gastrointestinal tract, leaving 2 cm of the throat and duodenum appended. The duodenum was ligated with careful string and each midsection delicately loaded up with 0.9% NaCl. This caused the expansion of the stomach avoiding contact of the neighbouring dividers. The stomachs were then ligated at the throat, at that point solidified in icebox and put away at 20°C [13-15]. Sequential cryostat cross-segments of 15 to 20 μm of the full stomach area were cut inside the plane from the lesser to the greater curvature.
• This segment thickness was picked as it was adequately enough to hold the bodily fluid layer and empower the representation of the mucosal structure.
• Then place the tissue into Hematoxylin recolor for 1-2 min.
• Now, expel it from Hematoxylin recolor and again hold it under faucet water for 1-2 min.
• Plunge the slides containing tissue areas into 1N HCl pursued by Scott's water (Sodium Bicarbonate 3.5 g, Magnesium sulfate 20 g, refined water 1 L) for 1 min each.
• Dip the tissue in Eosin recolors for 30 sec.
• Dehydrate the tissue logically with 80%, 90%, 100% isopropyl alcohol ultimately with Xylene for 20-30 min.
• Place coverslip on the slides using one drop of DPX, taking consideration to leave no air pockets and dry medium-term to make the enduring slide.
Statistical analysis: The qualities are communicated as mean esteem ± standard deviation (SD). The information was assessed by methods for the SPSS (form 12.0) and one-way ANOVA, trailed by bonferroni t-test. Measurable criticalness was viewed as when estimation of P 0.05.
5. Results
5.1 Phytochemical Analysis
% yield of chloroform concentrate of Lagenaria siceraria was observed to be 16.79% (Table 2).
| Phytoconstituents | Present or Absent |
|---|---|
| Carbohydrates | +ve |
| Glycosides | +ve |
| Fats | +ve |
| Gums and mucilage’s | +ve |
| Proteins and amino acids | +ve |
| Saponins | +ve |
| Flavonoids | +ve |
| Phytosterols | +ve |
| Tannins and Phenolic compounds | +ve |
Table 2. Phytochemical analysis.
6. Pharmacological Studies
Acute toxicity studies: The chloroform extract of Lagenaria siceraria was underwent acute toxicity study to determine the healing dose utilizing Wister rats in controlled condition. Acute oral toxicity study was executed according to OECD-423 guidelines. Acute toxicity study completed on CELS up to dose of 250 mg/kg demonstrated that the extract did not show any sign of harmfulness and mortality. Thus, 250 and 400 mg/kg dose of the concentrate chosen for examination of anti-ulcer activity.
6.1 Effect of chloroform extract of Lagenaria siceraria in pylorus ligation induced gastric ulcer
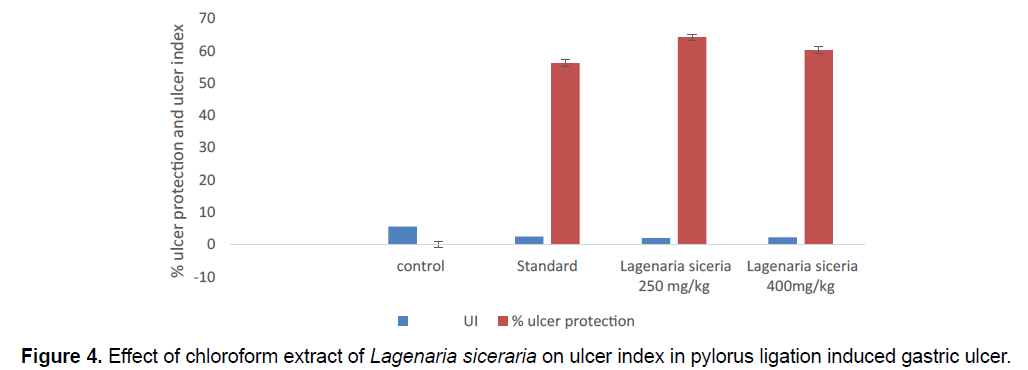
The Ulcer index of toxic control group 5.56 ± 0.6007. The creatures treated with chloroform extract of Lagenaria siceraria at 400 mg/kg dose displayed significant (P<0.01) drop in the no. of ulcers and ulcer index is 2.21 ± 0.6668. Lagenaria siceraria extract produced a dose reliant on and major decrease in the ulcer index. Extract at 250 mg/kg shows the safety against the pylorus ligation induced gastric ulcer, ulcer index 1.990 ± 1.199. Here also, maximum protection was seen in the ranitidine treated group. The volume of gastric secretion and total acidity, free acidity was significantly reduced in all extract treated groups when contrasted with toxic control. Lagenaria siceraria in the highest dose tested (400 mg/kg) was quite significant to ranitidine in reducing the gastric volume, and total acidity. Gastric pH was also observed to be essentially increased in all extract treated groups when contrasted with control (Table 3; Figure 4).
| S.No | Groups (n=5) | Treatment | UI | % ulcer protection |
|---|---|---|---|---|
| 1 | I | Toxic control | 5.56 ± 0.6007 | 0 |
| 2 | II | Standard | 2.435 ± 0.4943** | 56.2 |
| 3 | III | Lagenaria siceraria 250 mg/kg | 1.990 ± 1.199ns | 64.208 |
| 4 | IV | Lagenaria siceraria 400 mg/kg | 2.21 ± 0.6668** | 60.25 |
*Values express as mean ± SEM; n=6 in each group, statistical comparisons as follows: Significant at P<0.01** compared to control group, P>0.05 ns: non-significant.
Table 3. Effect of chloroform extract Table 3. Effect of chloroform extract of Lagenaria siceraria on ulcer index and % ulcer protection in pylorus ligation induced gastric ulcer.
7. Pylorus Ligation Parameters
7.1 Volume of gastric content
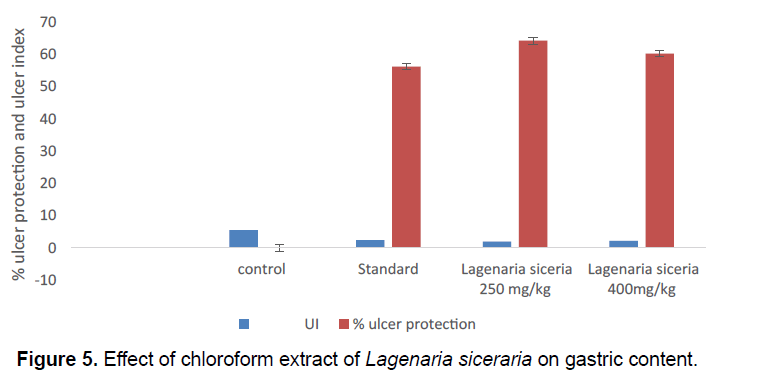
In the present model, the gastric content volume high (4.2 ± 0.1132) in control group. Gastric content volume significantly decreases in chloroform extract of Lagenaria siceraria at 250 (2.653 ± 0.234) and 400 mg/kg (2.195 ± 0.1946) doses. Gastric content volume significantly decreases in standard group (2.093 ± 0.195) compared control group (Table 4; Figure 5).
| S.No. | Groups | Treatment | Volume of gastric content |
|---|---|---|---|
| 1 | I | Control | 4.2 ± 0.1132 |
| 2 | II | Standard | 2.093 ± 0.195** |
| 3 | III | Lagenaria siceraria250 mg/kg | 2.653 ± 0.234** |
| 4 | IV | Lagenaria siceraria 400 mg/kg | 2.195 ± 0.1946** |
*Values express as mean ± SEM; n=6 in each group, statistical comparisons as follows: significant at P<0.01** compared to control group.
Table 4. Effect of chloroform extract of Lagenaria siceraria on gastric content volume.
7.2 Volume of gastric juice
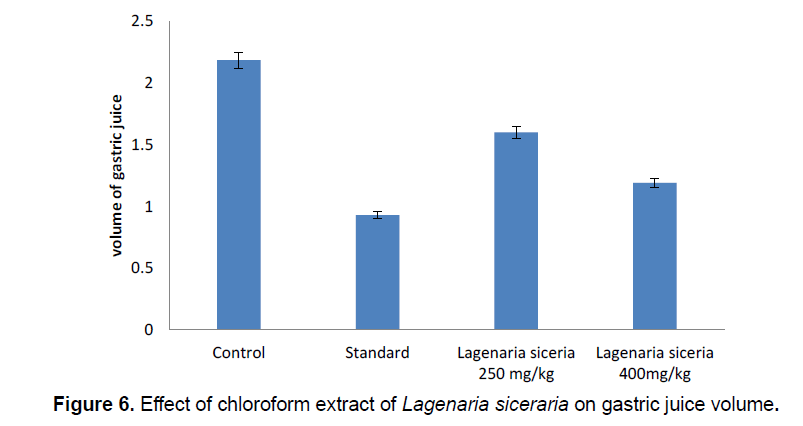
In pylorus ligation induced gastric ulcer model the Gastric juice volume high (2.285 ± 0.1022) in control group. Gastric juice volume significantly decreases in chloroform extract of Lagenaria siceraria at 250 (1.6 ± 0.2816) and 400 mg/kg (2.292 ± 0.1326) doses. Gastric juice volume significantly decreases in standard group (1.03 ± 0.09465) compared control group (Table 5; Figure 6).
| S.No. | Groups | Treatment | Volume of Gastric juice |
|---|---|---|---|
| 1 | I | Control | 2.285 ± 0.1022 |
| 2 | II | Standard | 1.03 ± 0.09465** |
| 3 | III | Lagenaria siceraria 250 mg/kg | 1.6 ± 0.2816* |
| 4 | IV | Lagenaria siceraria 400 mg/kg | 1.292 ± 0.1326** |
*Values express as mean ± SEM; n=6 in each group, statistical comparisons as follows: significant at P<0.01**, p<0.05* compared to control group.
Table 5. Effect of chloroform extract of Lagenaria siceraria on gastric juice volume.
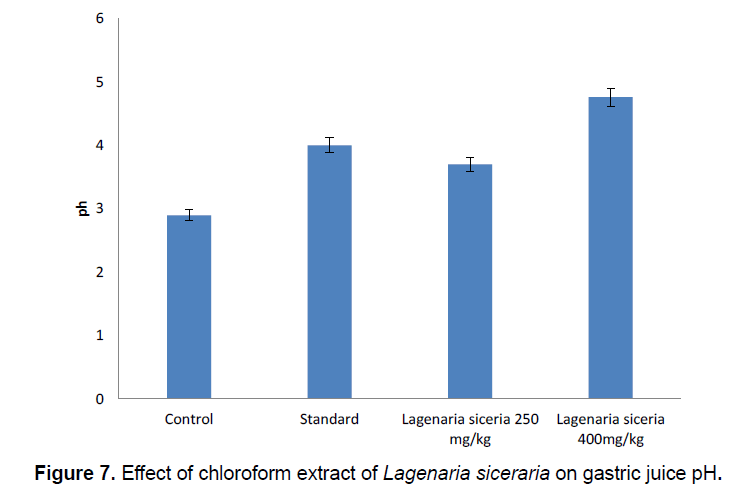
| S.No | Groups | Treatment | pH |
|---|---|---|---|
| 1 | I | Control | 2.865 ± 0.1018 |
| 2 | II | Standard | 4.15 ± 0.1766** |
| 3 | III | Lagenaria siceraria 250 mg/kg | 3.75 ± 0.1993** |
| 4 | IV | Lagenaria siceraria 400 mg/kg | 4.76 ± 0.1995** |
*Values express as mean ± SEM; n=6 in each group, statistical comparisons as follows: significant at P<0.01** compared to control group.
Table 6. Effect of chloroform extract of Lagenaria siceraria on gastric juice pH.
pH: In pylorus ligation induced gastric ulcer model the Gastric juice pH low (2.865 ± 0.1018) in control group. Gastric juice pH significantly increases in chloroform extract of Lagenaria siceraria at 250 (3.75 ± 0.1993) and 400 mg/kg (4.76 ± 0.1995) doses. Gastric juice pH significantly increases in standard group (4.15 ± 0.1766) compared control group (Table 6; Figure 7).
7.3 Total acidity (mEq/lit)
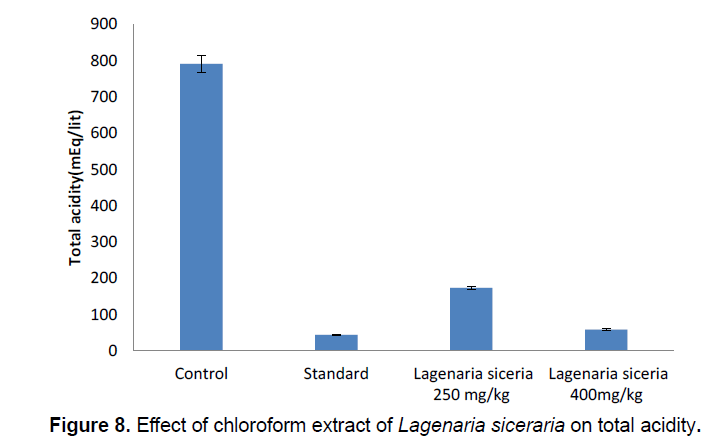
In pylorus ligation induced gastric ulcer model the total acidity high (791 ± 1.417) in control group. Total acidity significantly decreases in chloroform extract of Lagenaria siceraria at 250 (173.5 ± 1.416) and 400 mg/kg (58.8 ± 0.2259) doses. Total acidity significantly decreases in standard group (44.6 ± 0.2198) compared toxic control group (Table 7; Figure 8).
| S.No | Groups | Treatment | Total acidity(mEq/lit) |
|---|---|---|---|
| 1 | I | Control | 791 ± 1.417 |
| 2 | II | Standard | 44.6 ±0.2198** |
| 3 | III | Lagenaria siceraria 250 mg/kg | 173.5 ± 1.416** |
| 4 | IV | Lagenaria siceraria 400 mg/kg | 58.8 ± 0.2259** |
*Values express as mean ± SEM; n=6 in each group, statistical comparisons as follows: significant at P<0.01** compared to toxic control group.
Table 7. Effect of chloroform extract of Lagenaria siceraria on total acidity.
7.4 Free acidity (mEq/lit)
In pylorus ligation induced gastric ulcer model the free acidity high (322 ± 2.162) in control group. Free acidity significantly decreases in chloroform extract of Lagenaria siceraria at 250 (68.3 ± 0.4988) and 400 mg/kg (68.3 ± 0.4988) doses. Free acidity significantly decreases in standard group (35 ± 0.1982) compared control group (Table 8; Figure 9).
| S.No | Groups | Treatment | Free acidity(mEq/lit) |
|---|---|---|---|
| 1 | I | Control | 322 ± 2.162 |
| 2 | II | Standard | 35 ± 0.1982** |
| 3 | III | Lagenaria siceraria250 mg/kg | 68.3 ± 0.4988** |
| 4 | IV | Lagenaria siceraria400 mg/kg | 99.98 ± 0.2679** |
*Values express as mean ± SEM; n=6 in each group, statistical comparisons as follows: significant at P<0.01** compared to toxic control group.
Table 8. Effect of chloroform extract of Lagenaria siceraria on free acidity.
7.5 Histopathological evaluation
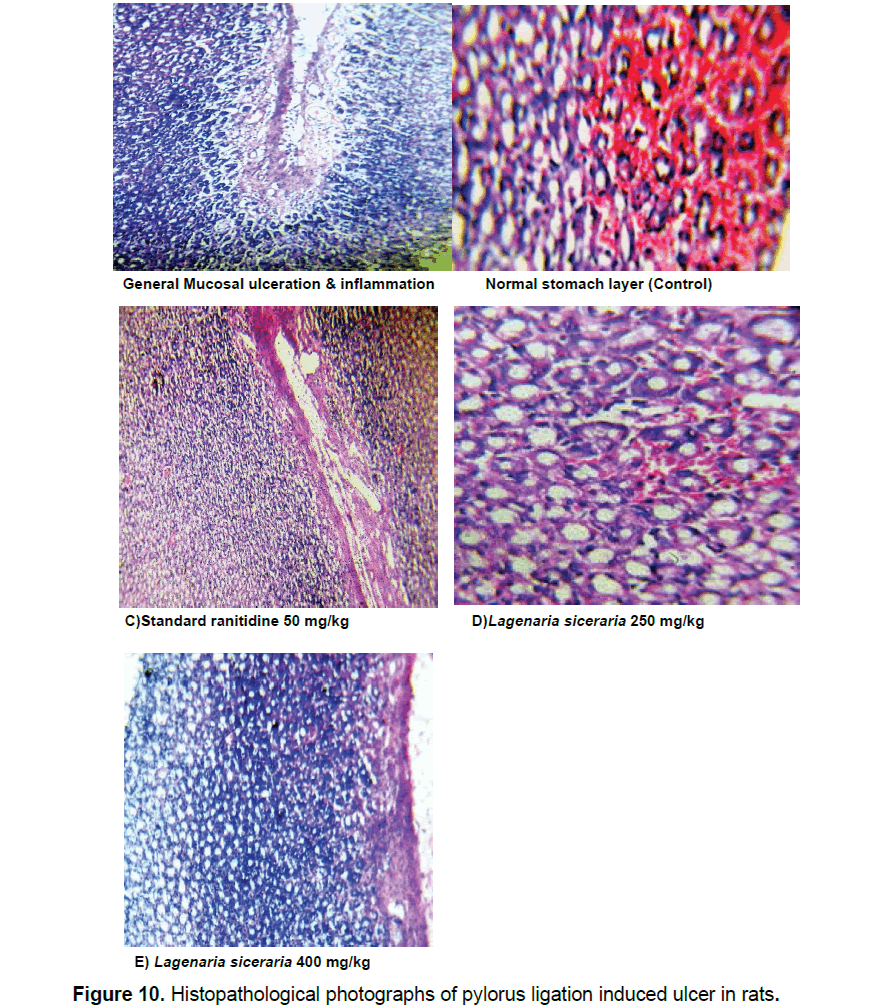
Microscopic evaluation of rodents, executed by cervical disengagement, was carried out using standard and test drug with various doses (250 and 400 mg/kg). Upon microscopic examination, animal tissue shows considerable decrease in ulceration at 400 mg/kg, which is cited in Figure 10.
8. Discussion
The majority of studies show the significance of regular items in medication disclosure. The utilization of phytoconstituents as medication treatment to treat real illnesses has ended up being clinically compelling and less dangerous than the current medications. Chemical substances got from plant have gotten an extremely long history in treatment of human sicknesses [16].
9. Conclusion
In the present work, the acute toxicity carried out based on OECD-423 rules for chloroform extract of Lagenaria siceraria prove that, the doses of 250 and 400 mg/kg did not indication any sign of toxicity and mortality. Hence these doses of the concentrate chosen for assessment of anti-ulcer activity. Lagenaria siceraria in the highest dose tested (400 mg/kg), shows increased in Gastric pH and Gastric juice pH, whereas decrease in Gastric content, Gastric juice volume and Total acidity. Therefore, as per histopathological evaluation studies, it was concluded that, Lagenaria siceraria, at the highest dose of 400 mg/kg, found to be safe and more effective in eradicating gastric ulceration. In Conclusion, based on the results obtained the chloroform extract of Lagenaria siceraria treated groups demonstrates a critical impact when contrasted with control group animals which showing that the plant having the having the anti-ulcer activity.
The anti ulcer action of Lagenaria siceraria was assessed by pylorus ligation instigated ulcer models. These models cause the gastric ulcer in people. Numerous variables and instruments are associated with the ulcerogensis and gastric mucosal harm [17- 19].
Pylorus ligation induced ulcer was utilized to note the impact of Lagenaria siceraria extract on gastric acid secretion and bodily fluid emission [19, 20]. The ligature of the opening finish of the abdomen causes accumulation of internal organ acid within the abdomen. This increase within the internal organ acid secretion causes ulcers within the abdomen. Ligation of pyloric end of the stomach is made in 24 h fasted rats, the UI is resolved 4 h after pylorus ligation [21]. The lesions created by this methodology are placed inside the lumen area of abdomen. The Chloroform extract of Lagenaria siceraria and ranitidine altogether diminished the complete acidity, free acidity and significantly enhance the pH, this proposes it is having an anti-secretory effect [22]. Its antiulcer activity is any supported by histopathological study demonstrates that protection of tissue layer layer from ulceration and inflammation.
Pylorus ligature induced lesion management rats shown perforated lesion, deep ulceration of granular epithelial tissue and nearly reducing the submucosa. The chloroform extract of Lagenaria siceraria at 250 mg/kg dose has shown mucosal erosion, the partial healing of ulcer with few inflammatory cells and the dose 400 mg/kg has shown the healed ulcer, normal mucosa and no inflammatory cells. Lagenaria siceraria extracts have been reported to possess antioxidant activity and to contain various types of compounds such as flavonoids and polyphenolic compounds, saponins and tannins [23]. The gastroprotective effect exhibited by chloroform extract Lagenaria siceraria is speculated to be attributed to its antioxidant property, which in turn could be linked to the presence of flavonoids and polyphenolic compounds, saponins and tannins [16]. These compounds most likely inhibit gastric mucosal injury.
Acknowledgment
With a deep sense of gratitude, i wish to express my sincere thanks to my honorific guide Y. Sridhar, Professor, Dept. of Pharmacology and Dr. Vijay kumar G, Professor and Head, Dept. of Pharmacy, for their helpful suggestions and comments throughout my study. I also express my heartfelt thanks to my teacher, S Murali, other teaching and non-teaching staff of my institution for their assistance to carry out the research work.
References
- Maury PK, Jain SK, Lal N, et al. (2012). A Review on Antiulcer Activity.IJPSR. 3: 2487-2493.
- Upaganlawar A, Balaraman R. (2009). Bottle Gourd (Lagenaria siceraria)-A vegetable food for human health”- A Comprehensive Review. Pharmacologyonline. 1: 209-226.
- Prashar Y, Gill NS, Perween A. (2014). An Updated Review on Medicinal Properties of Lagenaria siceraria. Int Universal Pharmacy Bio Sci. 3: 362-376.
- Prajapati RP, Kalaria MV, Karkare VP, et al. (2011). Effect of methanolic extract of Lagenaria siceraria (Molina) Standley fruits on marble-burying behavior in mice: Implications for obsessive-compulsive disorder. Pharmacognosy Res. 3: 62–66.
- Khalid S, Shahzad A, Basharat N, et al. (2018). ‘Phytochemical Screening and Analysis of Selected Medicinal Plants in Gujrat’. J Phytochemistry Biochem 2: 108.
- Prajapati RP, Kalariya M, Parmar SK, et al. (2010). Phytochemical and pharmacological review of Lagenaria sicereria, J Ayurveda Integr Med. 1: 266–272.
- Rao NV, Venu K, Sowmya U, et al. (2011). ‘Evaluation of anti -ulcer activity Of Momordica Charantia in rats’. Int J Int J Pharm Bio Sci.1: 1-16.
- Gupta1 J, Kumar D, Gupta A. (2012). Evaluation of gastric anti-ulcer activity of methanolic extractof Cayratia trifolia in experimental animals. Asian Pac J Trop Dis. 99-102.
- Khan MSA, Hussain SA, Jais AMM, et al. (2011). Anti-ulcer activity of Ficus religiosa stem bark ethanolic extract in rats. J Med Plant Res 5: 354-359.
- Amandeep K, Robin S, Ramica S, et al. (2012). Peptic ulcer: A review on etiology and pathogensis. IRJP 3: 34-38.
- Vella V. (2005). Drug-induced peptic ulcer disease. J Pharmacy Prac. 10: 15-19.
- Wallace JL. (2000). How do NSAIDs cause ulcer disease?. Baillieres Clin Gastroenterol. 14: 147-159.
- Wallace JL. (2001). Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 15: 691-703.
- https://www.protocol-online.org/prot/Protocols/Histopathological-Approach-to-Rat-Liver-Tissue-3441.html.
- Khomenko T, Szabo S, Deng X, et al. (2009) Role of iron in the pathogenesis of cysteamine-induced duodenal ulceration in rats. Am J Physiol Gastrointest Liver Physiol 296: 1277-1286.
- Rahman AS. Bottle Gourd. (2003). A vegetable for good health. Nat Prod Rad. 2: 249-250.
- Susumu OKABE, Kikuko AMAGASE. (2005) An Overview of Acetic Acid Ulcer Models The History and State of the Art of Peptic Ulcer Research. Biol Pharm Bull. 28: 1321-1341.
- Tuorkey MJ, Abdul-Aziz KK, Webmed Central. (2011). Gastric Ulcer's Diseases Pathogenesis, complications and Strategies for Prevention. Gastroenterology. 2: 1-24.
- M Khushtar, V Kumar, K Javed, et al. (2009). Protective effect of ginger oil on aspirin and pylorus ligation-induced gastric ulcer model in rats. Indian J Pharm Sci. 71: 554-558.
- Kapil Patel. (2007). A study on antiulcer activity of acacia leaucophloea bark extract in pylorus ligated rats. Pharmatutor.
- Desahpande JR, Mishra MR, Meghre VS, et al. (2007). Free radical scavenging activity of Lagenaria siceraria (Mol.) standl Fruit. Nat Prod Rad 6: 127–130.
- Chopra RN, Chopra IC, Verma BS. (1992). Supplement of Glossary of Indian Medicinal plants. New Delhi, India. CSIR. 51.
- Ghule BV, Ghante MH, Saoji AN, et al. (2007) Diuretic activity of Lagenaria siceraria fruit extract in rats. Int J Pharm Sci 69: 817–819.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences