Assessment of the Polyphenol Profile and Antioxidant Properties of Leaves, Stem and Root Barks Of Khaya senegalensis (Desv.) A.Juss
Atawodi, S.E., Atawodi, J.C., Pala, Y. , Idakwo P.
1Department of Biochemistry, Ahmadu Bello University, Zaria, Nigeria
2Department of Veterinary Public Health & Preventive Medicine, Ahmadu Bello University, Zaria, Nigeria
- Corresponding Author:
- Tel: +234 (0)8033 850613
E-mail: atawodi_se@yahoo.com
Abstract
The leaves, stem and root barks Khaya senegalensis (Desv.) A.Juss were evaluated for polyphenol composition and antioxidant properties. The identifiable phenolics in the leaf extract of Khaya senegalensis include catechin, rutin and quercetin rhamnoside, while the stem barks contain catechin and procyanidins. Using the hypoxanthine/xanthine assay model, the plant displayed antioxidant potential with IC50 values of 46, 37 and 64 μl for the leaves, stem bark and roots, respectively. Using the 2-deoxyguanosine assay model system the leaves, stem bark and roots showed radical scavenging capacity with IC50 values of 178, 91 and 122 μl, respectively. These observed activities are therapeutically relevant in Nigeria where the plant is part of herbal recipes in treating diseases with oxidative stress as a major etiological factor.
Keywords
Polyphenol; antioxidant potential; Khaya senegalensis; Mahogany.
1. Introduction
Khaya senegalensis (Desv.) A.Juss (family: Meliaceae) commonly called mahogany, is also known as ‘madaci’ and ‘ago’ by the Hausa and Igala ethnic groups of northern and central Nigeria, respectively. It is a large tree native to sub-saharan savannah area from Senegal to Uganda, and one of the most popular medicinal Meliaceous plants in African traditional remedies. The decoction of the bark is extensively used as a febrifuge and antimalarial [1]
In northern Nigeria, the decoction of the stem bark is also used for treatment of stomach disorders, urinogenital diseases, worm infestation, and in the treatment of trypanosomiasis [2]. It is reported to be an effective agent as a gastrointestinal nematocide [3], antisickling [4], antimicrobial and as an antiprotozoal agents [5].
The stem bark has been demonstrated to contain a bitter principle called ‘calicedrin’ which consist of a mixture of triterpenes with a lactone or epoxide function and a furan ring. It has alsobeen reported that the bark contains 2,6-dimethoxy-p-benzo-tannins, quinone, B-sitosterol and its B-D-glucoside, catechin, tannins, saponins and polysaccharides. Recently, it has been shown that African mahogany as a close relative of the South American genus, Swietenia, is also one of the main sources of B-D-secolimonoids such as methylangolensates, mexicanolides and phragmalins limonoids [6,7], and rearranged phragmalin limonoids called khayanolides [8] and seneganolide [9]. The antifeeding [10,11] and antifungal [10] effects of these limonoids have been demonstrated.
Because of these widespread use of Khaya senegalensis in Nigeria [2,3] and elsewhere in Africa [8] and other continents [10], it was considered worthwhile to evaluate the different parts of the plant for its polyphenol content, as well as the antioxidant properties.
Polyphenols are secondary metabolites of plants believed to be generally involved in their defense against ultraviolet radiation and aggression by pathogens [12] Because polyphenols such as flavonoids, lignans and phenolic acids have been widely demonstrated for capacity to scavenge reactive oxygen species [13], thereby chemopreventing diseases like neurodegenerative disorders [14], asthma [15], diabetes [16,17], cardiovascular disorders [18] and different forms of cancer [19], which have oxidative damage as the underlying etiological factor, interest in them have grown astronomically in the last few decades.
2. Materials and Methods
Plant materials and chemicals
The leaves, stem and root barks of Khaya senegalensis were obtained in May 2004, from the same plant growing in Samaru-Zaria, Kaduna State, Nigeria. The plant was identified by the Department of Biological Sciences, Ahmadu Bello University, Zaria, Nigeria. The parts collected were first dried in open air in the laboratory within the country of collection until brittle, and later subjected to freeze-drying in the laboratory of analysis at DKFZ, Heidelberg, Germany. Prior to drying, the leaves were washed in three portions of distilled water while the outer coatings of the root and stem barks were scrapped off.
Acetic acid, ethylene diaminetetraacetic acid (EDTA), hypoxanthine, methanol, xanthine and xanthine oxidase were obtained from Merck (Darmstadt, Germany). K2HPO4 and KH2PO4 were obtained from Serva (Heidelberg, Germany). Formic acid, salicylic acid and FeCl3.6H2O were obtained from Aldrich Chemie (Steinheim, Germany). N-methyl-N-(trimethylsilyl)-trifluoroacetamide (BSTFA) was obtained from Fluka (Buchs, Switzerland), while tetrabutylammonium hydroxide was obtained from Sigma Chemie (Deisenhofen, Germany). Standard phenolic compounds were obtained from laboratory stock, acquired from commercial sources or isolated, purified and characterized from natural sources. All solutions were made in double-distilled water.
Extraction
Freeze-dried sample (5g) was defatted with hexane through a 3-hr Soxlex extraction. The defatted material was allowed to dry under atmospheric air or under nitrogen, and then the phenolic component was extracted through a 3-hr reflux with methanol (X3). The methanol extracts were evaporated to constant weight in vacuo at 35°C, and the residue was then dissolved in 10 ml methanol. The three extracts were combined with rinsing, their methanol content evaporated in vacuo, and residues taken up again in 10 ml methanol [20]. This was used with or without dilution for further analyses.
Analytical High Performance Liquid Chromatography (HPLC)
Analytical HPLC was conducted on a Hewlett-Packard (HP) 10980 Liquid chromatograph fitted with a C-18, reverse phase (5μm column (25 cm X 4 mm I.D) Latex, Eppelheim, Germany). For separation of individual compounds in the extract, 2% acetic acid in water (solvent A) and methanol (solvent B) were used as mobile phase when 20μl of the extract solution was injected.
The solvent gradient consisted of 95% A for 2 min, 75%A in 8 min, 60 % A in 10min, 50A in 10min and 0%A until completion of the run at 45 min (Owen et al. 2000c). The flow rate of the mobile phase was maintained at 1ml/min, and phenolic compounds in the eluate were detected with a UV dual-array detector (HP1040M) set at 278 and 340 nm. Instrument control and data handling was by means of a HP Chemstation operating in the Microsoft Windows software environment. The amount of phenolic compounds in the extracts was estimated by the external standard method [21,22].
Hypoxanthine/ Xanthine Oxidase Assay
To assess the total antioxidant potential due partly to the scavenging of reactive oxygen specie and the inhibition of the enzyme, xanthine oxidase, the Hypoxanthine/Xanthine oxidase assay system was utilized. In this assay, the extent of diphenol (2,5 dihydroxybenzoic acid and 2,3 dihydroxybenzoic acid) produced by hydroxyl radical (HO*) attack on salicylic acid was measured from standard curves of their respective diphenols [20-23].
The assay involves the re-suspension of different extract residues (prepared in duplicates by drying 0-500 μl of extract solution) in 1ml phosphate buffer (pH6.6). After addition of 5μl xanthine oxidase 20mu/1.09ml), the tubes were incubated at 37 °C for 3 hrs, following which reaction was stopped by addition of 5 μl of conc. HCl. Where necessary, the reaction mixture was centrifuged at 10,000 rpm for 2 min. in a Fico Biofuge, Hareaus Instruments), and 20 μl of the mixture was analysed by HPLC using the mobile phase and gradient condition earlier mentioned. The hydroxylation of hypoxanthine was monitored at 278nm, while the hydroxylation of salicylic acid was monitored at A325nm. The end products of the enzyme or free radical reaction were quantified against standard curves measured at the same wavelength.
2-Deoxyguanosine Assay for radical Scavenging Potential
To evaluate the radical scavenging capacity of the extract, the 2-deoxyguanosine-assay model was adopted. The buffer system is similar to that of the hypoxanthine/xanthine oxidase system, except that salicylic is replaced with 2-deoxyguanosine (2mM). The generation of ROS was initiated by addition of ascorbic acid (500 μM). Dried residues (of 0-500μl extract solution prepared in duplicates) were re-suspended in buffer and incubated at 37 °C for 24 hrs. The assay of the 8-oxo-2-deoxyguanosine resulting from the ROS attack on 2-deoxyguanosine was analysed using an isocratic system consisting of 5% methanol and 95% aqueous buffer (5mM tetrabutylammonium hydroxide, adjusted to pH 4.3 with 6% formic acid). The UV detector was set at A293nm [20,21].
Determination of IC50
The amount of extracts producing 50% inhibition of oxidation (IC50) using the hypoxanthine/xanthine oxidase model system as well as the 2-deoxyguanosine assay methods were determined using the Table Curve Program (Jandel Scientific, Chicago, IL, USA).
Gas Chromatography – Mass Spectrometry
Analyses were performed on a HP 5973 mass spectrometer coupled to a HP 6890 gas chromatograph. Prior to GC-MS analysis, dried methanolic extracts (1μl) were derivatized by addition of BSTFA (100 μl) at 37°C for 30 min. Separation of the analytes was achieved using a HP 5MS capillary column, (30 m X 0.25 mm I.D., 0.25 um film thickness). Helium was used as the carrier gas with a linear velocity of 0.9 ml/s. The oven temperature program was: initial temperature 100 °C, 100-270 °C at 4 °C/min, and maintained at 270°C for 20 min. The GC injector temperature was maintained at 250 °C; the transfer line temperature was held at 280 0C. The mass spectrometer parameters for EI mode were: ion source temperature: 230 °C; electron energy: 70 eV; filament current; 34,6 uA; electron multiplier voltage: 1200V [22].
Liquid-Chromatography Electrospray – Ionization Mass Spectrometry (LC-ESI)
LC-ESI was conducted on an Agilent 1100 HPLC coupled to an Agilent LC/MSD (HP1101). Chromatographic separation was conducted using a C-18, reversed phase (5 um column (25 cm X 2mm I.D.; Latex Eppelheim, Germany) utilizing the same mobile phase and gradient as described for analytical HPLC, except that the flow rate was maintained at 0.5ml / min. The analyses were conducted in the negative ion mode under the following conditions: dry gas (nitrogen) flow rate 10L/min.; nebulizer pressure= 30psi, drying gas temperature= 350 °C; capillary voltage = 2500V; fragmenter voltage=100V; mass range=50-3000D.
3. Results
3.1 Phenolic compounds in K. senegalensis
The identifiable phenolics in the leaf extract of Khaya senegalensis include catechin, rutin and quercetin rhamnoside, while the stem barks contain catechin and procyanidins. In the root extract, only peaks of procyanidins of unknown identity were detectable.
3.2 Antioxidant potential of K. Senegalensis
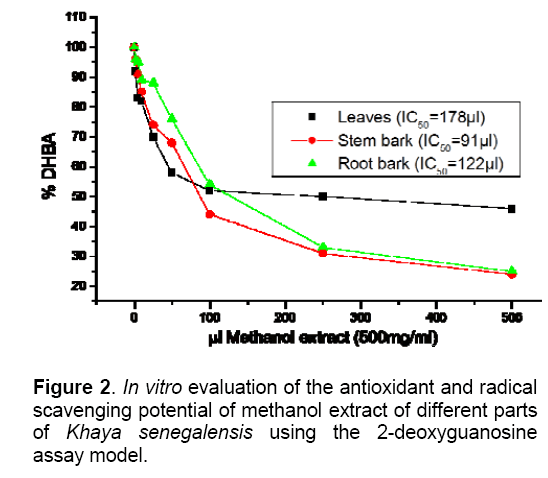
The results for the assessment of antioxidant potential is presented in figures 1, while that of the radical scavenging capacity of methanol extract of the plant parts are presented in Fig. 2. All parts of the plant displayed significant antioxidant potential with IC50 values of 46, 37 and 64 μl for the leaves, stem bark and roots, respectively (Fig.1). Similarly, the parts exhibited a fairly good radical scavenging capacity with IC50 values of 178, 91 and 122 μl, respectively for the leaves, stem bark and roots when extracts were tested In vitro using the 2-deoxyguanosine assay model system (Fig.2). Thus, the stem bark possesses both the highest antioxidant potential and the highest radical scavenging capacity.
4. Discussions and Conclusions
Although the exact identity of the peaks are yet to be established, the preponderance of procyanidin peaks appear to conflict with earlier reports that suggest the stem bark mainly contain the tetranortriterpenoids seneganolide of the mexicalinolide type rings, β-D-Secolimonoid and re-arranged phragmalin limonoid, khayanolides [6,9]. However, the discrepancy may be explained by the fact that these earlier workers analysed fractionated chloroform and ether extracts, respectively, while this report investigated the more polar, defatted, polyphenol-enriched methanol extracts.
The high antioxidant/radical scavenging capacity observed (Fig. 1&2) is consistent with the presence of many procyanidin peaks in all parts of the plants, in addition to catechin in the leaves and stem bark. Catechin and catechin-related compounds like epicatechin and epigallocatechingallate have been shown to be powerful antioxidants which can inhibit a number of tumour cell proliferation- and survival-related proteins [24], delay tumour onset in transgenic mouse model [25], inhibit the growth and invasion of oral carcinoma cells [26], and are involved in the modulation of xenobiotic metabolizing enzymes as well as inhibition of tumour promotion [27].
The roles of procyanidin in the chemoprevention of many oxidative damage-induced diseases have been a subject of many investigations in the last decade [28]. In addition to other biological activities like anti-inflammatory effects and modulation of cyclooxygenase and lipoxygenase activities [29], procyanidins have been reported to possess potent free radical scavenging and antioxidant activity [30]. Cycloxygenase, a key enzyme in prostaglandin biosynthesis, catalyses oxygenation of arachidonic acid to prostaglandin G2 (PGG2) and reduction of PGG2 to Prostaglandin H2(PGH2), an immediate precursor for production of eicosanoids. Arachidonic acid metababolites derived from PGH2 are believed to be important mediators of inflammatory responses, immunological effects and tumor development [13,31]. Chronic inflammation is thought to play an important role in the etiology of a number of cancers, and COX-2 inhibition by flavonoids such as procyanidins and catechins may therefore play significant role in the chemoprevention of such cancers [32].
Besides, the presence of limonoids in these samples cannot be ruled out, since earlier workers [4,8,10] have detected these compounds in some parts of the plant. Certain limonoids have been found to inhibit fore-stomach, colon and buccal pouch carcinogenesis in rodents, increase GST activity by 3-4 fold in benzo (o)pyrene-induced forestomach neolplasia with ICR/Ha mice, reduce tumour burden in DMBA-induced buccal pouch epidermoid carcinomas by 60%, and significantly inhibited ACF formation in the initiation and post-initiation stages [13,33,34].
Based on the above, it can be concluded that the observed potent antioxidant/radical scavenging activities of extracts of Khaya senegalensis may be responsible for its therapeutic efficacy in traditional medicine for treatment of diseases, such as sickle cell anaemia [4,35].
The possibility that the flavonoids identified here are acting in concert with limonoids reported by other workers to be present in these plant parts [6], to exert the above mentioned chemotherapeutic effects warrant serious consideration. Hence, studies are underway to establish the identity of all peaks in extracts of the different plant parts by different spectroscopic methods, and conduct antioxidant assays as well as In vitro short-term cancer chemopreventive tests on purified components.
Acknowledgements
SEA would like to thank Alexander von Humboldt Foundation (AvH), Bonn, Germany for grant of a research fellowship tenable at the Institute for Toxicology and Cancer Risk Factors, German Cancer Research Centre, Heidelberg, Germany in 2004/2005, and the subsequent material support award in respect of his scientific efforts. We thank Prof Dr H Bartsch and members of his group particularly Drs B Spiegelhalder, R.W Owen and B.Pfundstein for hosting the analytical aspect.
References
- Atawodi, S. E., Ameh, D. A., Ibrahim, S., et al. (2002) Indigenous knowledge system for treatment of trypanosomiasis in Kaduna state of Nigeria", J.Ethnopharmacol., 79 (2): 279-282.
- Omar, S., Zhang, J., MacKinnon, Set al. (2003) Traditionally-used antimalarials from the Meliaceae. Curr.Top.Med.Chem., (3)2: 133-139.
- Ademola, I. O., Fagbemi, B. O., Idowu, S. O. (2004) Evaluation of the anthelmintic activity of Khaya senegalensis extract against gastrointestinal nematodes of sheep: In vitro and in vivo studies", Vet.Parasitol., 122 (2): 151-164.
- Fall, A. B., Vanhaelen-Fastre, R., Vanhaelen, M., et al. (1999) In vitro antisickling activity of a rearranged limonoid isolated from Khaya senegalensis, Planta Med., (65) 3: 209-212.
- Abreu, P. M., Martins, E. S., Kayser, O., et al. (1999) Antimicrobial, antitumor and antileishmania screening of medicinal plants from Guinea-Bissau", Phytomedicine 6(3): 187-195.
- Khalid, S. A., Friedrichsen, G. M., Kharazmi, A., et al. (1998) Limonoids from Khaya senegalensis. Phytochemistry, 49 (6): 1769-1772.
- Olmo, L. R. V., daSilva, M. F. D, Fo, E. R., et al. (1996) Rearranged limonoids from Khaya senegalensis", Phytochemistry, 42 (3): 831-837.
- Abdelgaleil, S. A., Iwagawa, T., Doe, M. et al. ( 2004). Antifungal limonoids from the fruits of Khaya senegalensis", Fitoterapia, 75, (6): 566-572.
- Nakatani, M., Abdelgaleil, S. A., Kassem, S. M., et al. (2002) Three new modified limonoids from Khaya senegalensis", J.Nat.Prod., 65 (8): 1219-1221.
- Nakatani, M., Abdelgaleil, S. A., Kurawaki, J., (2001a) Anti-feedant rings β and α opened limonoids from Khaya senegalensis", J.Nat.Prod., 64 (10): 1261-1265.
- El Aswad, A. F., Abdelgaleil, S. A., Nakatani, M. (2004) Feeding deterrent and growth inhibitory properties of limonoids from Khaya senegalensis against the cotton leafworm, Spodoptera littoralis", Pest.Manag.Sci, 60 (2): 199-203.
- Manach, C., Scalbert, A., Morand, C., et al. (2004) Polyphenols: food sources and bioavailability. Am. J. Clin. Nutri, 79 (5): 727-747.
- Park, E. J., Pezzuto, J. M.(2002) Botanicals in cancer chemoprevention. Cancer Metastasis Rev., 21 (3-4): 231-255.
- Mandel, S., Weinreb, O., Amit, T., et al. (2004) Cell signaling pathways in the neuro-protective actions of the green tea polyphenol (-) epigallo-catechin-3-gallate: implications for neuro-degenerative diseases, J.Neurochem, 88 (6):1555-1569.
- Arts, I. C., Hollman, P. C. (2005) Polyphenols and disease risk in epidemiologic studies, Am.J.Clin.Nutr., 81(1): 317S-325S.
- Adhami, V. M., Siddiqui, I. A., Ahmad, N., et al. (2004) Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer", Cancer Res., 64 (23): 8715-8722.
- Anderson, R. A., Broadhurst, C. L., Polansky, M. M., et al.(2004) Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity, J.Agric.Food Chem., 52 (1): 65-70.
- Park, D., Huang, T., Frishman, W. H. (2005) Phytoestrogens as Cardioprotective Agents. Cardiol.Rev, 13 (1): 13-17
- Araki, R., Inoue, S., Osborne, M. P., et al. (1995) Chemoprevention of mammary pre-neoplasia. In vitro effects of a green tea polyphenol, Ann.N.Y.Acad.Sci., 768 : 215-222.
- Owen, R. W., Giacosa, A., Hull, W. E., et al. (2000) The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur.J.Cancer, 36(10): 1235-1247.
- Owen, R. W., Haubner, R., Hull, et al. ( 2003) Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem.Toxicol., 41(12): 1727-1738.
- Owen, R. W., Haubner, R., Mier, et al. (2003) Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds in brined olive drupes. Food Chem.Toxicol. 41(5): 703-717.
- Wiseman, S. A., Mathot, J. N., de Fouw, N. J., et al. (1996). Dietary non-tocopherol antioxidants present in extra virgin olive oil increase the resistance of low density lipoproteins to oxidation in rabbits. Atherosclerosis, 120 (1-2): 15-23.
- Kazi, A., Smith, D. M., Daniel, K et al. (2002) Potential molecular targets of tea polyphenols in human tumor cells: significance in cancer prevention", In Vivo, 16 (6): 397-403.
- Ebeler, S. E., Brenneman, C. A., Kim, G. S., et al. (2002) Dietary catechin delays tumor onset in a transgenic mouse model, Am.J.Clin.Nutr., 76 (4): 865-872.
- Hsu, S. D., Singh, B. B., Lewis, J. B., et al. (2002) Chemoprevention of oral cancer by green tea, Gen.Dent, 50( 2): 140-146.
- Lin, J. K. (2002) Cancer chemoprevention by tea polyphenols through modulating signal transduction pathways, Arch.Pharm.Res, 25 (5): 561-571.
- Carnesecchi, S., Schneider, Y., Lazarus, S. A., et al (2002) Flavanols and procyanidins of cocoa and chocolate inhibit growth and polyamine biosynthesis of human colonic cancer cells, Cancer Lett, 175 (2):147-155.
- Cos, P., De Bruyne, T., Hermans, N., et al. (2004) Proanthocyanidins in health care: current and new trends. Curr.Med.Chem, 11 (10): 1345-1359.
- Torres, J. L., Lozano, C., Julia, L., et al. (2002) Cysteinyl-flavan-3-ol conjugates from grape procyanidins. Antioxidant and antiproliferative properties, Bioorg.Med.Chem, 10 (8): 2497-2509
- Park, W. C. Jordan, V. C. (2002) Selective estrogen receptor modulators (SERMS) and their roles in breast cancer prevention, Trends Mol.Med., 8(2): 82-88.
- Le Marchand, L. (2002) Cancer preventive effects of flavonoids—a review", Biomed. Pharmacother., 56 (6): 296-301.
- Miller, E. G., Porter, J. L., Binnie, W. H., et al. (2004) Further studies on the anticancer activity of citrus limonoids J.Agric.Food Chem., 52 (15): 4908-4912.
- Tian, Q., Miller, E. G., Ahmad, H., et al. (2001) Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutr.Cancer, 40 (2): 180-184.
- Thioune, O., Pousset, J. L., Lo, I. (1999) Anti-inflammatory activity of the bark of Khaya senegalensis (A Juss). Preliminary research of structure/activity relationship, Dakar Med., 44 (1): 12-15.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences