Significance of the Expression of CD 90, 96, 117 and 123 in Egyptian Patients with Acute Myeloid Leukemia, Relation to Prognosis and Response to Treatment
Sahar K Hussein, Ahmed A Shams El Deen, Nohair Soliman, Kareeman G Mohammed, Marwa T Ashour, Noha Y Ibrahim, Ahmed A Mohammed, Aml S Nasr
1Clinical and Chemical Pathology Department, Faculty of Medicine, Cairo University, Cairo, Egypt
2Medical Oncology Department, Faculty of Medicine, Cairo University, Cairo, Egypt
3Internal Medicine Department, Faculty of Medicine, Cairo University, Cairo, Egypt
Received date: July 11, 2017; Accepted date: August 18, 2017; Published date: August 25, 2017
Citation: Hussein SK, El Deen AAS, Soliman N, et al. Significance of the Expression of CD 90, 96, 117 and 123 in Egyptian Patients with Acute Myeloid Leukemia, Relation to Prognosis and Response to Treatment. Electronic J Biol, 13:4
Abstract
Objective: Investigating the expression pattern of CD 90, 96,117 and 123 in bone marrow or peripheral blood from adult patients with acute myeloid leukemia and their use as markers for diagnosis and prognosis. Patients and methods: Using Muli-color flowcytometry, we analyzed the expression of CD90, 96, 117 and 123 among [CD34+/CD38-] cell population in AML patients at initial diagnosis. Results: It was found that percentage of CD90+ cells was lower among [CD34+/CD38-] cells in of AML cases than control group, however this difference didn't reach any statistical significance (p value=0.06), the percentage of CD96+ cells among CD34+CD38- cells was higher in AML cases than for control group (that show a highly statistically significant difference (p value<0.001), the percentage of CD117 cells was higher among CD34+ CD38- cells in AML cases than for control group, however this difference didn't show statistical significance (p value=0.079), While percentage of CD123 was significantly higher among [CD34+/CD38-] cells in AML cases than the control group and this difference shows a high statistical significance (P value<0.001).
Keywords
Acute myeloid leukemia; Leukemic stem cell; CD 90, 96, 117, 123.
1. Introduction
Acute Myeloid Leukemia (AML) is a life-threatening haematopoietic disease that is characterized by clonal growth and accumulation of myelopoietic progenitor cells. Many data have shown that each AML clone consists of Leukemic Stem Cells (LSC) and their progeny and that AML stem cells differ from more mature cells in several aspects, including survival and target antigen profiles [1].
Stem cells possess two defining characteristics: the ability to self-renew and the capacity to differentiate. A number of major cancers including acute myeloid leukemia have been shown to follow a cancer stem cell model in which cancer cells are hierarchically organized [2].
CD34 is a cell surface transmembrane protein that is expressed primarily on the surface of immature hematopoietic normal progenitor cells. It has been widely used as a marker to assist in the identification and isolation of Hematopoietic Stem Cells (HSCs) and progenitors. Cell surface expression of CD34 is developmentally regulated in hematopoiesis and is inversely related to the stage of differentiation, as CD34 expression is lost beyond the committed progenitor stage. The functional significance of CD34 expression on hematopoietic progenitor cells and developing blood vessel is unknown, except that CD34 on vascular endothelial cells binds to L-selectin [3]. It is not lineage restricted and thus not useful for distinguishing AML from acute lymphoblastic leukemia (ALL). In addition, CD34 is involved in cellular adhesion and mediates resistance to apoptosis [4].
CD90 or Thymocyte differentiation antigen 1 (THY-1) is a cell surface glycoprotein expressed on some early T and B lymphocytes, fibroblasts and neural cells [5]. It is also expressed on primitive hematopoietic cells. In this latter cell population, in normal BM, about 5-25% of CD34+ cells co-express CD90. It seems to be involved in proliferation and expansion processes. A higher expression level of CD90 in HSCs than in LSCs was further emphasized in another study [6].
In non-hematopoietic tissue, CD96 is expressed in the convoluted tubular epithelium of the kidney, the mucosal epithelium of the small and large intestines and the vascular endothelium [7,8].
A possible function of this receptor in natural killer (NK) cell mediated killing activities were suggested recently [9]. Moreover, CD96 was described as a tumor marker for T-cell acute lymphoblastic leukemia and acute myeloid leukemia [8,10]. It has been shown to be expressed at high levels on hematopoietic cells from adult AML patients, whereas its expression in cells from hematologically normal subjects is significantly reduced [8].
The c-kit proto-oncogen (CD 117) has been shown to be present in several cell types including normal and neoplastic hemopoietic cells. Among normal Bone Marrow (BM) cells, CD117 expression has been found in about half of the CD34+ precursors including progenitors committed to the erythroid, granulomonocytic and megakaryocytic cell lineages [11].
In addition, strong CD117 expression is detected in bone marrow mast cells as well as in a small subset of NK cells displaying strong reactivity for CD56, and in a relatively important proportion of CD3/CD4/CD8 prothymocytes. These results suggest that CD117 expression can be detected in both myeloid and lymphoid lineages although for the lymphoid lineage it would be restricted to a small NK-cell subset and early T-cell precursors [12].
In acute leukemias, CD117 expression was initially associated with AML. Nevertheless, at present it is well established that CD 117 expression may also be found in a relatively important proportion of T-ALL while it is usually absent in B-lineage ALL [11].
CD123, the α-subunit of the inerleukin-3 (IL-3) receptor, has generated considerable interest as a cell-surface antigen with potential clinical application because it is highly expressed on stem/progenitor cells from adult AML, whereas it is practically absent on their normal hematopoietic counterparts [13].
The aim of this study was to investigate the expression pattern of CD, 90, 96, 117 and 123 in bone marrow or peripheral blood from adult patients with acute myeloid leukemia and their value as markers for diagnosis.
2. Patients and Methods
The current study was conducted on 40 patients with de novo acute myeloid leukemia at the time of diagnosis (De novo AML was defined if the patient had no history of prior treatment with chemotherapy and had no prior diagnosis of myelodysplastic syndrome or chronic myeloid leukemia), 24 males (60%) and 16 females (40%). Their ages ranged between 18 and 65 years with a (mean ± SD: 37.9 ± 12.6 years). Patients were diagnosed and selected among cases referred to medical oncology department at El Kasr Al-Aini hospital, Cairo University over the period from October 2014 to June 2015.
In addition, this study included 40 bone marrow samples with non-haematological malignancy (as a control group). These patients were selected among cases referred to Kasr AL-Aini hospital. They were diagnosed as idiopathic thrombocytopenic purpura, hypersplenism and some normal bone marrow samples from donors within the bone marrow transplantation unit. Among the control group, there were 24 males (60%) and 16 females (40%). Their ages ranged between 17 and 60 years with a (mean ± SD: 38 ± 11.32 years).
Consent was obtained from all the participants prior to the study accompanied by detailed explanation of the procedure and its outcome, this study was carried out in accordance to the guidelines approved by the Ethics Committee, Kasr AL-Aini hospital, Cairo University. The data were collected from patients' files after permission.
2.1 All the participants included in the study was subjected to
Clinical evaluation including: Complete history taking including: Age, Complaints, onset, course, duration, present history, past medical history, drug history and family history, clinical examination for the presence of: hepatomegaly, splenomegaly, lymphadenopathy, anaemic manifestations.
2.2 Laboratory investigations included
Complete blood picture for assessment of anemia, thrombocytopenia and leucocyte count, Bone marrow examination to reach the diagnosis, cytochemistry, immunophenotyping to establish the FAB classification and cytogenetic studies, special investigations (for patients and controls): Flow-cytometric analysis using CD34, CD38,CD 90, CD 96,CD117 and CD123 antibodies using Multicolor flow cytometry (Coulter EPICS XL-MCL) from Beckman coulter. All reagents purchased from Beckman coulter Company, California, United States, except Anti-Human CD96 (TACTILE) PE purchased from affymetrix eBioscience, San Diego, California, United States).
Phycoerythrin-cyanine 5 (PC5)-conjugated mouse monoclonal anti human CD34 antibodies (IgG1 subclass); Contain 1 ml PC5-labeled antibodies (Cat No: PN A07777). Fluorescein isothiocyanate (FITC)- conjugated mouse monoclonal anti human CD38 antibodies (IgG1 subclass); Contain 1 ml FITClabeled antibodies (Cat No: PN A07778).
Phycoerythrin (PE)-conjugated mouse monoclonal anti human CD90 antibodies (IgG1 subclass); Contain 1 ml PE-labeled antibodies (Cat No: IM1840U).
Phycoerythrin (PE) mouse monoclonal (IgG1 kappa subclass) Anti-Human CD96 (TACTILE) PE; (purchased from affymetrix eBioscience, United States); contain 1 ml PE-labeled monoclonal antibodies (Cat No: 12-0969).
Phycoerythrin (PE) mouse monoclonal antibody CD117 purchased from (Beckman coulter; United States). Contain 1 ml PE-labeled antibody of mouse IgG1 subclass (Cat No: IM1360U).
Phycoerythrin (PE)-conjugated mouse monoclonal anti human CD123 antibodies (IgG1 kappa subclass); Contain 1 ml PE-labeled antibodies (Cat No: PN A32535).
1 ml of peripheral blood/bone marrow was collected from AML patients at initial diagnosis under complete aseptic conditions and 1 ml bone marrow sample was collected from control groups through bone marrow aspiration under complete aseptic conditions. Samples were delivered to a vacutainer tube containing EDTA for Flow-cytometric analysis using CD34, CD38, CD90, CD96, CD117 and CD123 antibodies.
The analysis was performed using direct staining method by the following monoclonal antibodies: anti CD34, CD38, CD90, CD96, CD117 and CD123 antibodies.
Samples were measured using EPICS XLMCL Beckman coulter, a logarithmic scale was implemented for forward scatter signal, side scatter signal and each fluorescent channel. Data analysis was performed as follows: for each specimen a minimum of 10,000 events were studied, primary gate was constructed on CD34+ CD38- cells and measuring CD123+ and CD90+ percent within the primary gate using an appropriate isotypic control. Data recorded as percentage and mean fluorescence intensity (MFI).
2.3 Statistical analysis
Data was analysed using IBM SPSS advanced statistics version 21 (SPSS Inc.). Numerical data were expressed as mean and standard deviation or median and range as appropriate. Qualitative data were expressed as frequency and percentage. Chi-square test was used to examine the relation between qualitative variables. For not normally distributed quantitative data, comparison between two groups was done using Mann-Whitney test (nonparametric t-test). Spearman-rho method was used to test correlation between numerical variables. P values<0.05 were considered significant. According to Pearson correlation, r value between 0.5-1 signifies strong correlation, 0.3-0.5 signifies moderate correlation, 0.1-0.3 signifies weak correlation, 0.0-0.1 signifies no correlation. Correlation may be whether positive or negative.
3. Results
The current study was conducted on 40 patients with de novo AML, 24 males (60%) and 16 females (40%). Their ages ranged between 18-65 years with a mean ± SD 37.9 ± 12.6. Forty age and sex matched participants presented with non-hematological malignancies as a control group, 24 males (60%) and 16 females (40%). Their ages ranged between 17-60 years with a mean ± SD 38.3 ± 11.32.
Demographic data of patients and controls are summarized in Table 1.
| Cases (no=40) |
Control (no=40) |
||
|---|---|---|---|
| No (%) | No (%) | ||
| Gender | Male | 24 (60.0%) | 24 (60.0%) |
| Female | 16(40.0%) | 16 (40.0%) | |
| Age (years) | Range | 18-65 | 17-60 |
| Mean ± SD | 37.9 ± 12.6 | 38.3 ± 11.32 | |
Table 1: Demographic data of the AML and control groups.
3.1 Clinical presentations of the control group
Cases among the control group were diagnosed as: 20/40 cases (50%) as idiopathic thrombocytopenic purpura, 15/40 cases (37.5%) as hypersplenism, 5/40 cases (12.5%) normal bone marrow (Table 2).
| Clinical Presentation | No (%) |
|---|---|
| •Organomegaly: | 26 (65.0%) |
| •Hepatomegaly •Lymphadenopathy •Splenomegaly |
15 (37.5%) 13 (32.5 %) 10 (25.0%) |
| Constitutional symptoms | 16 (40.0%) |
| Anemic manifestations | 15 (37.5%) |
Table 2: Clinical presentations of the AML group.
3.2 Laboratory data of the control group
Laboratory data of AML patients are summarized in Table 3.
| Parameter | Mean ± SD | Range |
|---|---|---|
| TLC (× 109/L) | 85.4 ± 29.5 | 2.8-356.7 |
| Hb (g/dl) | 8.3 ± 1.9 | 4.5-11.8 |
| PLT (× 109/L) | 63.5 ± 19.5 | 11-115 |
| PB blasts (%) | 45.6 ± 15.7 | 13-88 |
| BM blasts (%) | 59.8 ± 18.6 | 21-90 |
Hb: Hemoglobin; TLC: Total Leucocytic Count; PLT: Platelets, PB: Peripheral Blood; BM: Bone Marrow
Table 3: Laboratory data of the AML group.
Complete blood picture: Total leucocytic count ranged beween 0.5-20.4 × 109/L, with a mean ± SD: 7.3 ± 2, hemoglobin level ranged between 4.1-16.8 g/dl with a mean ± SD: 9.2 ± 2.4), platelet count: ranged between 5-259 × 109/L with a mean ± SD 60 ± 31.7).
French-American-British subtypes of patients : Among AML cases, 1 case (2.5%) was M0, 16 cases (40%) were M1, 8 cases (20%) were M2, 3 cases (7.5%) were M3, and 7 cases (17.5%) were M4, 4 cases (10%) were M5 and 1 case (2.5%) was M7.
Immunophenotyping results of studied markers in AML group are summarized in Table 4.
| Parameter | Patients (no=40) | Controls (no=40) | P value | ||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| [CD34+/CD38-] Cells % | 12.75 | 0.2-32.4 | 0.33 | 0.01-1.53 | <0.001(HS) |
| [CD34+/CD38-/CD90+] MFI | 1.97 | 0.98-3.34 | 1.98 | 0.42-4.23 | 0.32(NS) |
| [CD34+/CD38-/CD90+] % | 0.43 | 0.01-4.27 | 0.63 | 0.03-4.55 | 0.06(NS) |
| [CD34+/CD38-/CD96+] MFI | 3.30 | 0.12-18.50 | 1.78 | 0.11-4.10 | <0.001(HS) |
| [CD34+/CD38-/CD96+] % | 6.53 | 0.13-35.20 | 0.49 | 0.01-2.98 | <0.001(HS) |
| [CD34+/CD38-/CD117+] MFI | 0.96 | 0.07-6.95 | 0.94 | 0.05-3.78 | 0.855 (NS) |
| [CD34+/CD38-/CD117+] % | 0.58 | 0.01-4.17 | 0.36 | 0.01-2.13 | 0.079 (NS) |
| [CD34+/CD38-/CD123+] MFI | 3.55 | 1.21-8.8 | 2.11 | 1.01-3.75 | <0.001(HS) |
| [CD34+/CD38-/CD123+] % | 3.68 | 0.05-31.6 | 0.71 | 0.01-3.53 | <0.001(HS) |
MFI: Mean Fluorescence Intensity; SD: Standard Deviation; %: Percentage
P value<0.05 is statistically significant*
Table 4: Immunophenotyping results of studied markers in AML group.
In AML cases, correlation between studied markers' expression and gender, age, organomegaly, lymphadenopathy, laboratory data and FAB subtypes, none of them revealed statistically significant correlations.
Although the results of our study were reported and published by other researches, but what is different concerning our work is that to our knowledge, it is the first study performed on the Egyptian patients to rule out the role of the studied markers in the pathogenesis of AML, relation to prognostic markers and response to treatment.
Correlations between the studied markers (expression of CD90, CD96, CD117, CD123) and bad prognostic markers(old age more than 60 years, presence of bulky tumor, presence of organomegaly or lymphadenopathy, high LDH) as well as correlation with FAB subtypes and different laboratory parameters were done, but none of them were proved to be statistically significant, i.e., CD96 and CD123 among CD34+CD38- were significantly increased in AML patients compare to controls, so they are considered as independent risk factors in the pathogenesis of AML.
All patients included in the study were treated according to the protocol of the nuclear medicine and oncology department, Cairo University, using ongoing induction and consolidation regimens for treatment of adult AML cases.
Induction of remission: Patients were subjected to 7-3 protocol for induction of remission [14]: Novantrone: 12 mg/m2, IV on day 1 and 3. ARA–C: 100 mg/m2, continuous IV infusion, from day l-7. If remission is not achieved, this protocol was repeated again. If no or minimal response, patients were shifted to high dose chemotherapy. Induction therapy for acute promyelocytic leukemia (PML) included oral administration of all-trans-retinoic acid (ATRA) 45 mg/m2/day until CR induces remission in 70% to 90% of patients with M3 AML. ATRA induces terminal differentiation of the leukemic cells followed by restoration of non-clonal hematopoiesis.
Consolidation [15]: High dose ARA-C for 4 cycles. ARA-C: 2 g/m2, over 2 h infusions, every 12 h on days 1-4.
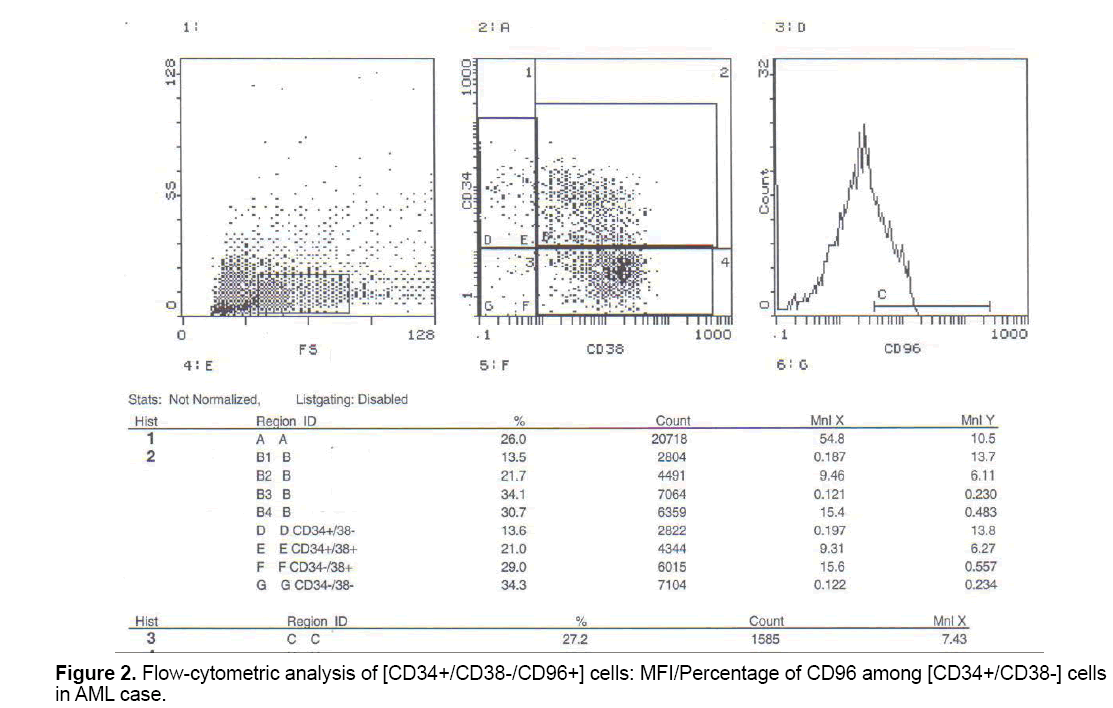
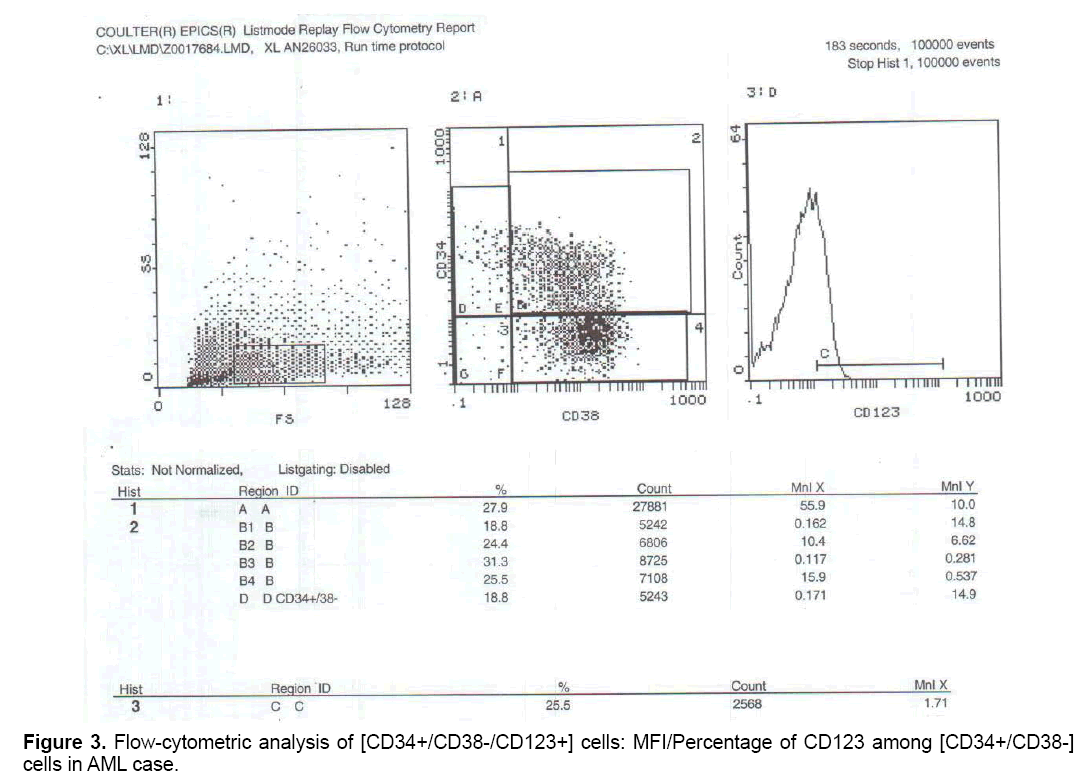
But when correlation between the studied markers and response to treatment were done, no statistically significant positive or negative correlation could be proved (Figures 1-3).
4. Discussion
AML is one of the most life threatening and aggressive hematological malignancies and despite the advancement in treatment options for this disease, its prognosis is still very poor and many patients die either from complications of intensive chemotherapy, resistance to the current treatment options or they experience relapse after initial response to traditional chemotherapy [16].
Most patients with AML will achieve a remission but many will relapse and die from the disease. Due to the disease heterogeneity, classification is critical to provide prognostic data informing risk-adapted therapeutic strategies, which have improved outcome dramatically in some subtypes of AML [17].
Due to the high incidence of relapse and resistance to conventional chemotherapy in AML, it is hypothesized that there is a rare subset of malignant cells that could evade the effect of the current treatment regimens [18]. These cells are termed LSCs. LSCs share the normal stem cells their self-renewal capacity as well as extensive proliferative capacity. The property of self- renewal helps initiation of leukemic cells growth and establishing the hierarchical organization of AML [19]. In addition, these LSCs usually reside in a stem cell niche in the bone marrow and are in quiescent state, remaining in G0, so they could survive the conventional chemotherapeutic drugs and this leads to the incidence of high relapse rates among AML patients [20]. The lack of durable response in a high percentage of AML patients suggests that current treatments do not effectively target LSCs [19].
One of the major challenges in the design of new therapies to eradicate LSCs is to achieve high therapeutic specificity. To this end, it is important to distinguish LSCs from the concomitantly present HSCs and to assess whether this distinction is of prognostic value, since it would underline the clinical importance of LSCs [20].
In similarity to normal hematopoietic stem cell, many authors assumed that LSCs were enriched within [CD34+/CD38-] cells [2]. This specific initial phenotype [CD34+/CD38-] was assigned to the AML stem cell and the characteristics of this population were defined by transplantation studies using the xenogeneic NOD/SCID mouse model system [21].
Although the exact phenotypes and genotypes of LSCs remain controversial, an enormous effort has been taken to identify specific surface markers in order to differentiate between normal and leukemic stem cells [22].
LSC immunophenotype has been also tested for its utility at detecting MRD. LSCs can be identified in most patients by the cell presence or absence of the cell surface antigens that include: CD34+, CD38-, CD123+, CD96+, CD90-, CD47dim and CD99+ [23]. Furthermore, it has been shown that patients presenting higher proportion of LSCs (defined as CD34+CD38−) demonstrate significantly lower relapse-free survival than patients with less LSCs [23].
CD90 is expressed on the immature hemopoietic cells in the BM. CD90 is shown to be highly expressed on cord blood primitive cells, especially which are with high proliferation capacity; It is suggested that CD90 is involved in HSC development by mediating a negative signal which inhibits the proliferation of primitive cells. It is observed that LSCs with high proliferation capacity in vivo and in vitro lack the expression of CD90 [24].
CD96 is also expressed at higher levels in normal progenitors than HSC. Expression was elevated in the CD34+ CD38- LSC compartment when compared to normal HSC in 65% of AML patients [8]. These indicate that AML-LSC could be distinguished from normal HSC by the presence of CD96 expression. This finding suggested that CD96 may be an excellent candidate target for antibody therapy against LSC.
Expression of CD117 has been demonstrated in approximately 2-5% of normal bone marrow cells [25]. Up to 60-70% of CD34+ bone-marrow progenitor cells co-express CD117 [26].
CD123 is the α-subunit of the IL-3R, which together with its β subunit (CD131) form the functional IL-3R. The binding of IL-3 to CD123 leads to activation of the receptor that accelerates survival and proliferation of the cells [27]. Expression of CD123 might be indicative of poor prognosis and might identify patients requiring aggressive therapeutic schedules [28].
In the current study, we analyzed the expression of 4 cell surface antigens relevant to human hematopoiesis (CD90, CD96, CD117 and CD123) on the primitive cell population [CD34+/CD38-] cells in 40 patients with de novo AML compared to 40 age and sex matched subjects with non-hematological malignancies as a control group and to test the efficiency of this surface markers to characterize LSCs in AML.
Regarding the percentage of [CD34+/CD38-], It was found that this cell compartment was higher in AML cases at diagnosis when compared to the control group. This finding was in agreement with Van Rhenen et al. [29]. who found high [CD34+/CD38-] frequency at time of diagnosis in AML patients, and also in concordance with Du et al. [30] who observed that the proportion of the CD34+CD38- population in nucleated cells in healthy volunteers was much lower compared to the AML patients (P<0.01).
Also, Terwijin et al. [31] reported that the frequency of CD34+/CD38- (putative stem cell compartment in AML patient) at diagnosis has a strong prognostic impact and by multivariate analysis showed that CD34+/ CD38- frequency after first and second treatment cycles was an independent prognostic factor for RFS and OS while the neoplastic part of the CD34+/CD38+ and CD34- putative stem cell compartment has no prognostic impact at all.
Also, Van Rhenen et al. [29] reported a significant correlation between CD34+/CD38- frequencies and all survival parameters (overall survival, relapse-free survival and disease-free survival). He also found correlation between stem cell frequency at diagnosis MRD frequency after the third cycle of chemotherapy in CR, with borderline significance for CD38-/CD34+ (P=0.05).
CD90 was found to be expressed in a low proportion among [CD34+/CD38-] cells in AML group at diagnosis in comparison to control group and there was no statistically significant difference observed between both groups. This result was in agreement with Chavez-Gonzalez et al. [6] who found that CD90 and CD117 were expressed in low proportion on [CD34+/CD38-] at diagnosis and also had no impact on survival in AML patients.
These findings were also in agreement with a larger study including 142 adult AML patients, Wuchter et al. [32] observed a reduced frequency of CD90+ cells in bone marrow at time of diagnosis, Blair et al. [33] included bone marrow samples from 15 AML patients and found that the vast majority of AML blasts as well as more primitive CD34+ cell populations including cells with in vivo repopulating capacity, lacked expression of CD90.
The present study included the association between the expression of [CD34+/CD38-/CD90+] and different clinical and laboratory data of AML patients where no association or correlation was found, as regards the age, the findings were consistent with Petrovici et al. [34] who reported no significant association between expression of [CD34+/CD38-/CD90+] cells and age. In a study including 148 patients (12 to 89 years old) with de novo AML, Buccisano et al. [35] reported that CD90 was expressed in 17% of the patients. In those de novo AML patients, CD90 was expressed only in elderly patients. Interestingly, in those CD90+ patients, 21-92% of CD34+ cells co-expressed CD90, which is in contrast to our study.
Also, according to our study, the expression (mean fluorescence intensity) CD96 (Tactile) among CD34+CD38- cells was significantly higher in AML cases at initial diagnosis compared to control group. This in agreement with Hosen et al. [8] who found that CD96 is selectively expressed in AML-LSC and demonstrates that CD96 is expressed on the majority of CD34+ CD38- AML cells in many cases (74.0-25.3% in 19 of 29 cases), whereas only a few (4.9-1.6%) cells in the normal HSC-enriched population (Lin− CD34+ CD38- CD90+) expressed CD96 weakly. Also in keeping with the study by Chavez et al. [6] who observed that in AML samples at diagnosis, levels of CD96 cells were significantly increased compared to the levels found in normal marrow. And also in concordance with Du et al. [30] who investigated the expression of cluster of differentiation 96 (CD96), a potential marker for LSCs, in CD34+CD38- cells of 105 acute leukemia (AL) patients (87 AML, 15ALL, 3MAL patients) by flow cytometry, and found that all the CD34+, CD34+ CD38- and CD34+ CD38- CD96+ proportions were much higher in AL compared to the normal control (P<0.01). Also Du et al. [30] who examined correlation between CD96 expression in CD34+CD38- cells and the response for chemotherapy in AML patients observed that a higher expression of CD96 (>10%) may promote a poor response for chemotherapy, which may be closely associated with primary resistant. Many studies have suggested that CD96 could be used as a valuable therapeutic target in AML.
According to our study, there was no significant impact of CD117 at diagnosis; this is in agreement with Blair et al. [36] who reported that primitive AML cells capable of long-term proliferation in vitro and NOD/SCID repopulation differ from primitive normal progenitor cells in their lack of surface expression of c-kit. And also in concordance with Blair and Sutherland [37]. who demonstrated that in adult AML patients, CD117 is not expressed on the most primitive CD34+ CD38- cell population that includes HSC and also in agreement with Chavez et al. [6], who found that the majority of CD34+ CD38- cells from pediatric AML marrow were negative for CD117.
MFI/Percentage of CD123 was significantly higher among [CD34+/CD38-] cell population in AML patients when compared to control.
The higher expression of CD123 on [CD34+/CD38-] cell population in the current study on AML patients was consistent with several studies which stated that CD123 was overexpressed on myeloid leukemia cells in comparison with normal HSCs [1,38-43] Also in agreement with a study Florian et al. [43], who measured the expression levels of CD123 on LSCs of 14 AML patients, found that 99.1% of LSCs were CD123+.
In this study, the expression of CD123 among [CD34+/ CD38-] cells was evaluated against other clinical and hematological parameters such as patients' age, sex, organomegaly, lymphadenopathy, initial TLC, Hb level, platelets, peripheral blood blasts, bone Marrow blasts percentage and different FAB subtypes. Regarding the relation with different FAB subtypes; no significant difference was found, this finding was also reported by Testa et al. [44] and Zhao et al. [45] who found that there is no significant difference in CD123 expression levels detected with respect to the different FAB subtypes. No significant association between the expression of [CD34+/CD38-/CD123+] and other previous patients' data was encountered. Up to the best of our knowledge, no previous studies addressed this issue.
In another study, in which follow up of AML patients was done, Hwang et al. [16] observed a statistically non-significant higher level of CD123 expression on LSCs in the non-CR AML group and relapsed group than in CR AML group at initial treatment. Further studies with repeated assessment of CD123 expression throughout the course of the treatment would help better assessment of the influence of CD123 expression on the clinical outcome and disease progression of AML.
Many studies have emphasized CD123 as a valuable therapeutic target in AML and several phase І studies assessing anti-CD123 monoclonal antibodies targeting LSCs in AML are ongoing [22,46].
5. Conclusion
In conclusion, CD90 is expressed a little lower among [CD34+/CD38-] cells in AML group than the control group, although this difference did not reach any statistical significance; so CD90 isn't considered a reliable marker in the detection of LSCs in AML patients. CD 96 is strongly expressed among [CD34+/ CD38-] cell in AML patients and may be used as a marker for detection of leukemic stem cells and in combination with CD34+/CD38- may be LSCs specific. While c-KIT 117 (CD117) is not a reliable marker for LSCs.CD123) is strongly expressed among [CD34+/ CD38-] cell population in AML patients at diagnosis compared to control group and this difference show a high statistical significance; CD123 in the combination [CD34+/CD38-] may be used as a useful marker for detection of LSCs in AML.
6. Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research board committee of Kasr Al Ainy School of Medicine, Cairo University and with 1964 Helsinki declaration and its later amendments.
Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
References
- Jordan CT, Upchurch D, Szilvassy SJ, et al. (2000). The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 14: 1777-1784.
- Bonnet D, Dick JE. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 3: 730-737.
- David CW, Stuart MC, Nathan CB, et al. (2007). CD150 negative side population cells represent a functionally-distinct population of long-term hematopoietic stem cells. Blood. 1: 90-115.
- Oyan AM, Bø TH, Jonassen I, et al. (2005). CD34 expression in native human acute myelogenous leukemia blasts: Differences in CD34 membrane molecule expression are associated with different gene expression profiles. Cytometry B Clin Cytom. 64: 18-27.
- Manz MG, Miyamoto T, Akashi K, et al. (2002). Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci U S A. 99: 11872-11877.
- Chavez-Gonzalez A, Dorantes-Acosta E, Moreno-Lorenzana D, et al. (2013). Expression of CD90, CD96, CD117 and CD123 on different hematopoietic cell populations from pediatric patients with acute myeloid leukemia. Arch Med Res. 45: 343-350.
- Gramatzki M, Ludwig WD, Burger R, et al. (1998). Antibodies TC-12 ("unique") and TH-111 (CD96) characterize T-cell acute lymphoblastic leukemia and a subgroup of acute myeloid leukemia. Exp Hematol. 26: 1209-1214.
- Hosen N, Park CY, Tatsumi N, et al. (2007). CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci U S A. 104: 11008-11013.
- Meyer D, Seth S, Albrecht J, et al. (2008). CD96 interaction with CD155 via its first Ig-like domain is modulated by alternative splicing or mutations in distal Ig-like domains. J Biol Chem. 284: 2235-2244.
- Wang PL, Clayberger C, Krensky AM. (1992). Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J Immunol. 148: 2600-2608.
- Miettinen M, Lasota J. (2005). KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 13: 205-220.
- Escribano L, Ocqueteau M, Almeida J, et al. (1998). Expression of the c-kit (CD117) molecule in normal and malignant hematopoiesis. Leuk Lymphoma. 30: 459-466.
- Rollins-Raval M, Pillai R, Warita K, et al. (2013). CD123 immuno-histochemical expression in acute myeloid leukemia is associated with underlying FLT3-ITD and NPM1 mutations. Appl Immunohistochem Mol Morphol. 21: 212-217.
- Preisler H, Davis RB, Kirshner J, et al. (1987). Comparison of three remission induction regimens and two post induction strategies for the treatment of acute non-lymphocytic leukemia: A cancer and leukemia group B study. Blood. 69: 1441-1449.
- Mayer RJ, Davis RB, Schiffer CA, et al. (1994). Intensive post remission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 331: 896-903.
- Hwang K, Park CG, Jang S. (2012). Flow cytometric quantification and immunophenotyping of leukemic stem cells in acute myeloid leukemia. Ann Hematol. 91: 1541-1546.
- Sanz MA, Montesinos P, Rayón C, et al. (2010). Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: Further improvements in treatment outcome. Blood. 115: 5137-5146.
- Roboz CJ, Guzman MA. (2009). Acute myeloid leukemia stem cells: seek and destroy. Expert Rev Hematol. 2: 663-672.
- Zhi L, Wang M, Rao Q. (2010). Enrichment of N-Cadherin and Tie2-bearing CD34+/CD38_/CD123+ leukemic stem cells by chemotherapy-resistance. Cancer Lett. 296: 65-73.
- Lane SW, Scadden DT, Gilliland DG. (2009). The leukemic stem cell niche: Current concepts and therapeutic opportunities. Blood. 114: 1150-1157.
- Jordan CT. (2002). Unique molecular and cellular features of acute myelogenous leukemia stem cells. Leukemia. 16: 559–562.
- Jin L, Hope KJ, Zhai Q, et al. (2009). Targeting of CD44 eradicates human acute myeloid leukemic stem cells. NatMed. 12: 1167–1174.
- Van Rhenen A, Moshaver B, Kelder A, et al. (2007). Aberrant marker expression patterns on the CD34+ CD38- stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia. 21: 1700-1707.
- Hoang VT, Hoffmann I, Borowski K, et al. (2013). Identification and separation of normal hematopoietic stem cells and leukemia stem cells from patients with acute myeloid leukemia. Methods Mol Biol. 1035: 217-230.
- Ashman LK, Bühring HJ, Aylett GW, et al. (1994). Epitope mapping and functional studies with three monoclonal antibodies to the c-kit receptor tyrosine kinase, YB5.B8, 17F11 and SR-1. J Cell Physiol. 158: 545-554.
- Strobl H, Takimoto M, Majdic O, et al. (1992). Antigenic analysis of human haemopoietic progenitor cells expressing the growth factor receptor c-kit. Br J Haematol. 82: 287-294.
- Bagley CJ, Woodcock JM, Stomski FC, et al. (1997). The structural and functional basis of cytokine receptor activation: Lessons from the common beta subunit of the granulocyte-macrophage colony-stimulating factor, interleukin-3 (IL-3) and IL-5 receptors. Blood. 89: 1471-1482.
- Mohty M, Szydlo RM, Yong AS, et al. (2008). Association between BMI-1 expression; acute graft-versus-host disease; and outcome following allogeneic stem cell transplantation from HLA-identical siblings in chronic myeloid leukemia. Blood. 112: 2163-2166.
- Van Rhenen A, Feller N, Kelder A, et al. (2005). High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res.11: 6520-6527.
- Du W, Hu Y, Lu C, et al. (2015). Cluster of differentiation 96 as a leukemia stem cell-specific marker and a factor for prognosis evaluation in leukemia. Mol Clin Oncol. 3: 833-838.
- Terwijn M, Kelder A, van Putten WLJ, et al. (2010). High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: Prospective data from the HOVON/SAKK AML 42a study. Blood ASH Annual Meeting Abstracts. 116: 760-761.
- Wuchter C, Ratei R, Spahn G, et al. (2001). Impact of CD133 (AC133) and CD90 expression analysis for acute leukemia immunophenotyping. Haematologica. 86: 154-161.
- Blair A, Hogge DE, Ailles LE, et al. (1997). Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 89: 3104-3112.
- Petrovici K, Graf M, Reif S, et al. (2010). Expression profile of the progenitor cell markers CD34, CD38 and CD90 in acute myeloid leukemia and their prognostic significance. J Cancer Mol. 5: 79-86.
- Buccisano F, Rossi FM, Venditti A, et al. (2004). CD90/Thy1 is preferentially expressed on blast cells of high risk acute myeloid leukaemias. Br J Haematol. 125: 203-212.
- Blair A, Hogge DE, Sutherland HJ. (1998). Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34/CD71-/HLA-DR. Blood. 92: 4325-4335.
- Blair A, Sutherland HJ. (2000). Primitive acute myeloid leukemia cells with long term proliferative ability in vitro and in vivo lack surface expression of c-Kit (CD117). Exp Hematol. 28: 660- 670.
- Luz M, Josep FN, Olga L, et al. (2001). Interleukin-3 receptor α chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 86: 1261-1269.
- Muñoz L, Nomdedéu JF, López O, et al. (2001). Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 86: 1261-1269.
- Testa U, Riccioni R, Militi S, et al. (2002). Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity and poor prognosis. Blood. 100: 2980-2988.
- Riccioni R, Rossini A, Calabrò L, et al. (2004). Immunophenotypic features of acute myeloid leukemias overexpressing the interleukin 3 receptor alpha chain. Leuk Lymphoma. 45: 1511-1517.
- Sperr WR, Piribauer M, Wimazal F, et al. (2004). A novel effective and safe consolidation for patients over 60 years with acute myeloid leukemia: intermediate dose cytarabine (2 × 1 g/m2 on days 1, 3 and 5). Clin Cancer Res. 10: 3965-3971.
- Florian S, Sonneck K, Hauswirth AW, et al. (2006). Detection of molecular targets on the surface of CD34+/CD38-/- stem cells in various myeloid malignancies. Leuk Lymphoma. 47: 207-222.
- Testa U, Riccioni R, Diverio D, et al. (2004). Interleukin-3 receptor in acute leukemia. Leukemia. 18: 219-226.
- Zhao M, Zhu H, Sajin R, et al. (2013). Clinical significance of leukemic stem cells immunophenotype expression in patients with acute leukemia. Life Sci J. 10: 2543-2548.
- Kugler M, Stein C, Kellner C, et al. (2010). A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol. 150: 574-586.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences