Recent Status on Enzymatic Saccharification of Lignocellulosic Biomass for Bioethanol Production
Meysam Madadi, Yuanyuan Tu, Aqleem Abbas
1National Key Laboratory of Crop Genetic Improvement, Huazhong Agricultural University, Wuhan, China
2College of Plant Science and Technology, Huazhong Agricultural University, Wuhan, China.
Received date: April 04, 2017; Accepted date: April 18, 2017; Published date: April 25, 2017
Citation: Madadi M, Tu Y, Abbas A. Recent Status on Enzymatic Saccharification of Lignocellulosic Biomass for Bioethanol Production. Electronic J Biol, 13:2
Abstract
During the past decades, bioethanol becomes the best alternative to fossil fuels. Ethanol production by using edible feedstocks like sugarcane and grains became a point of concern in terms of the food supply and demand. Lignocellulosic biomass comprises non-edible feedstock opened a new method for the second-generation bioethanol production. Bioethanol production from lignocellulosic biomass is comprised of three main processes; pretreatment, enzymatic saccharification and fermentation. The major parameter to preventing the commercialization of bioethanol depends on the improvement of the enzymatic hydrolysis process. Through the enzymatic saccharification process carbohydrates (cellulose and hemicellulose) polymers get transformed into free monomeric sugars. The main issues connected with enzymatic saccharification are high incubation time for carbohydrates degradation, the price of the enzyme, prevent of enzyme activity in the attendance of phenolic compounds and thermal inactivation of cellulase and hemicellulase enzyme. This review describes recent tendency and improvement of the enzymatic saccharification step for cheaper bioethanol production. In this article, the authors discuss the following views: the process of biomass to ethanol production, enzymes for lignocellulosic hydrolysis, factors affecting enzymatic hydrolysis and cellulase intervened hydrolysis and the improvement or enhancement of enzymatic saccharification and its future prospects for commercial lignocellulosic bioethanol production.
Keywords
Second-generation bioethanol; Lignocellulosic biomass; Cellulose and hemicellulose enzyme; Enzymatic hydrolysis.
1. Introduction
As countries develop and living standards improve, energy demand grows rapidly. Another side, depletion of fossil fuel creating energy gap which proposes a considerable need for alternative energy resources [1]. The best theory to fill this energy gap is the use of sustainable and renewable resources like lignocellulosic biomass [2]. Bioethanol due to high energy density, reduction of CO2 emission, greater air-fuel ratio, and more heat of vaporization is one of the promising renewable energy which has a high potential for the replacement of fossil fuels [3,4]. Bioethanol is differentiated as first- and secondgeneration ethanol, based on the raw material. Firstgeneration bioethanol is produced mainly from C6 sugars such as sugar beets, cereals, and sugarcane while second-generation delivered from renewable lignocellulosic biomass and industrial by-products or residues [5,6]. But because of disputation of food versus energy, ethanol production from lignocellulosic residues has earned noticeable attention as a wide variety of feedstocks can be used as materials with no significant competition with the food chain [5- 7]. The majority of the procedure cost of ethanol production relies on the cost of raw material and in such a scenario; lignocellulosic biomass had made the procedure commercially feasible [8].
Production of bioethanol from lignocellulose particularly rely on two desirable steps: (1) pretreatment, and (2) hydrolysis [9,10]. Pretreatment is the crucial step of removing the lignin because the extent to which the biomass becomes easily obtainable to the enzyme for hydrolysis extremely relies on the type of pretreatment occupied [11]. Beside the pretreatment step, another important process is effective saccharification during hydrolysis of lignocellulosic biomass as it is the rate limiting process towards the techno-economic feasibility of lignocellulosic bioethanol [12,13]. Enzyme cellulase catalyzes the hydrolysis of cellulose by breaking the 1, 4-β-glycosidic bonds in between the cellulose chain of biomass [14]. Among the hemicellulase enzymes, xylan is one of the crucial ones which are engaged in the enzymatic saccharification [15]. Whole consumption of carbohydrate components in lignocellulosic biomass is dependent on the development of cheaper methods of technologies for cellulase and hemicellulase production [16,17], and also the improvement of enzymatic saccharification of carbohydrate component to monomeric sugars such as hexoses and pentoses [18]. It was reported by many researchers that enzyme production is the most expensive step in the production of ethanol from lignocellulosic biomass. It covers about 40% of the total cost [19,20]. Then, for bioethanol production from commercial companies improvement of cheaper/cost-effective hemicellulase and cellulase production technology is necessary. Hence, this review describes widely about the current status of enzymatic saccharification to provide insight into hydrolysis process.
2. Process of Biomass to Ethanol Production
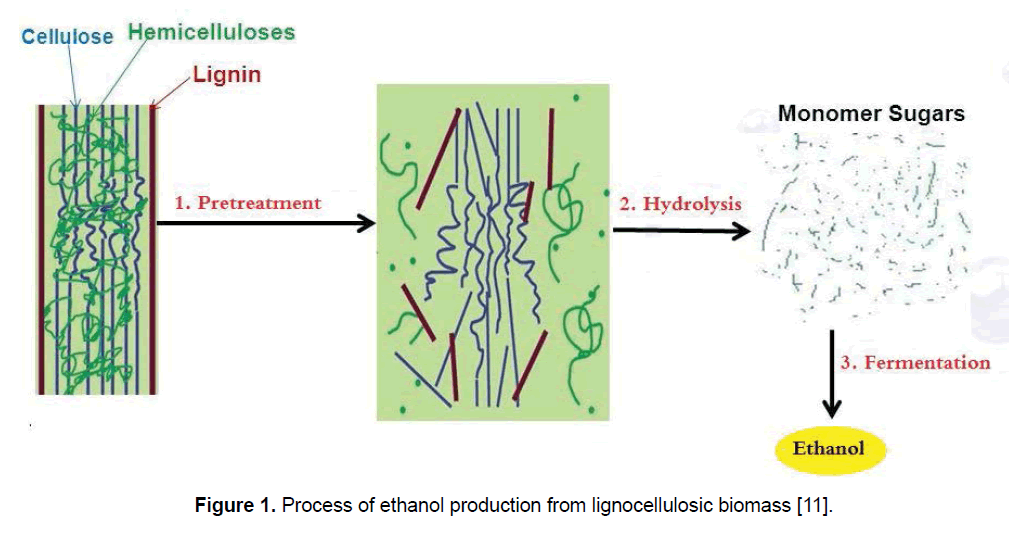
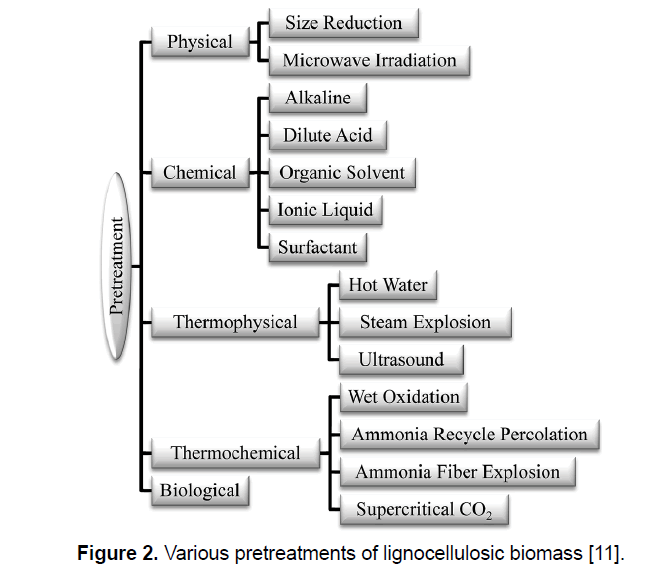
Production of ethanol from lignocellulosic biomass includes three main steps: (1) pretreatment, (2) enzymatic saccharification/hydrolysis, and (3) fermentation [21]. Figure 1 presents the process of ethanol production from lignocellulosic biomass. For alteration of biomass structure as well as its overall chemical component a suitable pretreatment is needed to facilitate rapid and effective enzyme access and hydrolysis of carbohydrates to fermentable sugars [12,22]. Pretreatment is responsible for a notable proportion of process cost, and as a result, various pretreatment technologies have been identified during the last three decade; though these technologies are mainly determined to the biomass and enzymes. Figure 2 shows the most common pretreatment methods used on lignocellulosic biomass [9,10,23,24]. Enzymatic hydrolysis relates to the procedure that alters polysaccharides into monomeric sugars. The fermentable sugars obtained from hydrolysis can be fermented into ethanol and other products by microorganisms, which can be either naturally obtained or genetically modified [18,25].
3. Enzymes for Lignocellulosic Hydrolysis
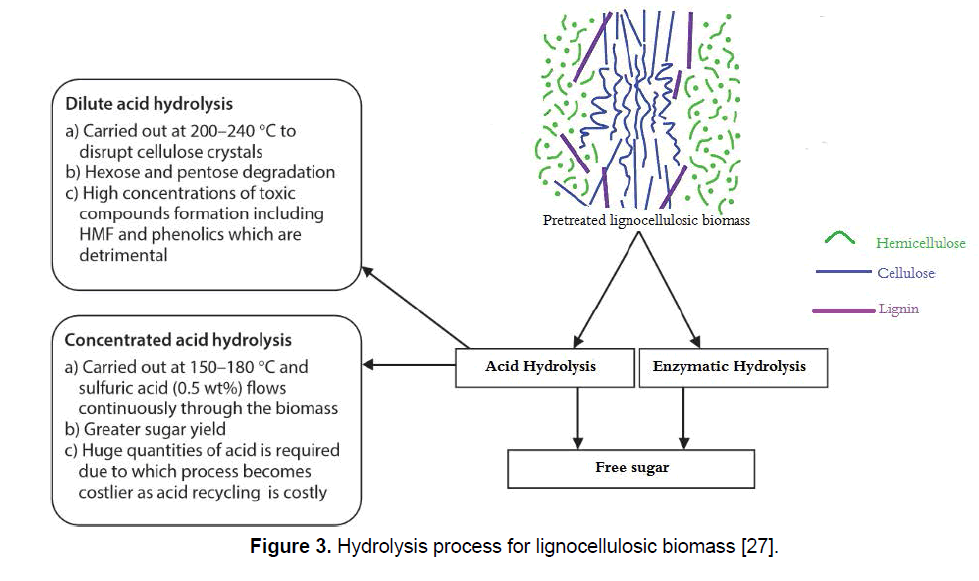
The saccharification procedure can be mainly carried out in two ways; enzymatically (biological) by (hemi) cellulolytic enzymes or chemically (acidic) by sulfuric or other acids [26]. The chemical reaction is done by using either dilute or concentrated acid. However, enzymatic hydrolysis is becoming a suitable process due to several benefits like low toxic compound generation, high product yield, less chemical requirements, less energy and mild environment conditions, and generation of fewer fermentation inhibitor products (Figure 3) [27,28]. Enzymatic decomposition of lignocellulose because of many structural features is extremely complicated and make it very recalcitrant. In addition to the complex network formed by cellulose, hemicellulose, and lignin, some enzymes can be absorbed by condensed lignin which decreases the hydrolysis yield by nonspecific linkages of these enzymes [29].
3.1 Cellulases
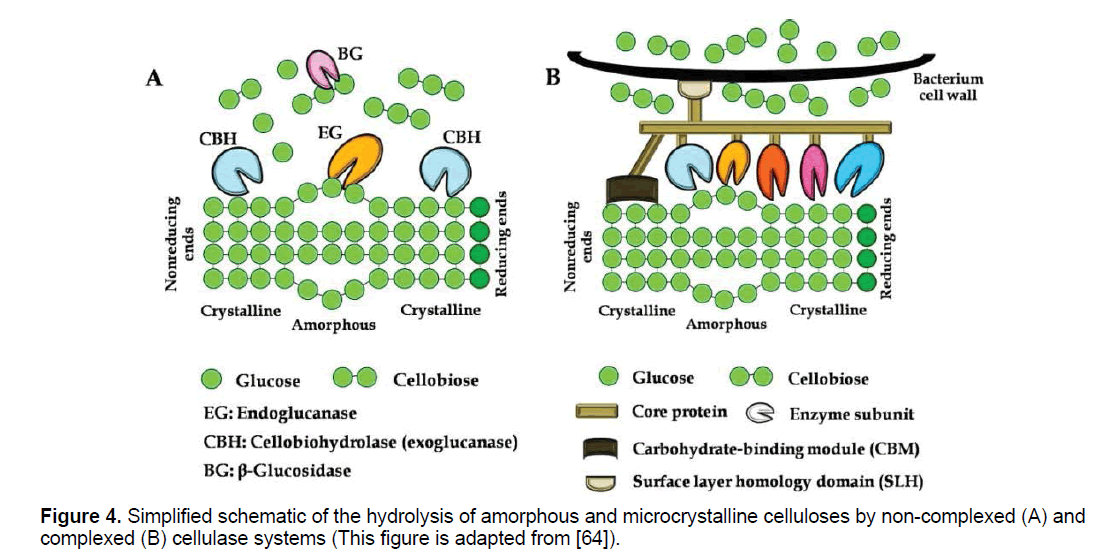
Cellulases represent the primary family needed to depolymerize lignocellulosic substrates. Cellulose complex consists of endo-β-glucanase (EC 3.2.1.4), exo-β-glucanase (EC 3.2.1.91) and β-glucosidase (EC 3.2.1.21). Cellulase acts on cellulose in the following manner: endoglucanases act randomly on internal glucosidic linkages, in the amorphous portion of cellulose, releasing oligosaccharides with several polymerization degrees. Cellobiohydrolases degrade cellulose by removing cellobiose molecules; they can act on the crystalline portion of cellulose and attack from the reducing and non-reducing ends of the glucose chain [14,30]. Exoglucohydrolases are responsible for removal of glucose units from the non-reducing ends of cyclodextrins. Finally, β-glucosidases hydrolyze cellobiose into glucose and also remove glucose units from non-reducing ends of small cyclodextrins (Figure 4a) [14,30]. Individual enzymes are not effective for cellulose chain degradation to a monomeric unit, then synergistic action leads to a suitable hydrolysis. Major synergism has been observed firstly between endo and exo-βglucanase and secondly between exo-β-glucanases which act from both reducing and nonreducing end. βglucosidase overcomes catabolic repression by preventing accumulation of cellobiose [14].
3.2 Hemicellulases
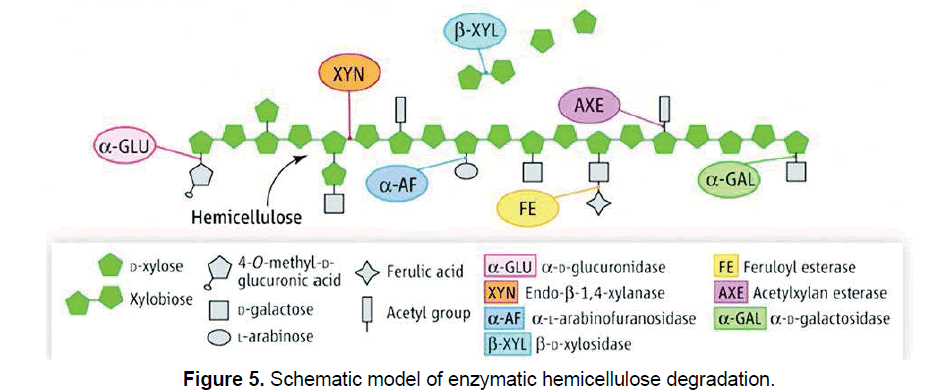
Hemicellulases are the enzymes involved in the degradation of hemicellulose and it requires a more complex group of enzymes. In hemicelluloses, xylan is one of the major important enzymes which is involved in the enzymatic hydrolysis [15]. It demands endo-β-1,4-xylanase (EC 3.2.1.8), which acts randomly on the internal bond of xylan to release xylo-oligosaccharides, β-xylosidase (EC 3.2.1.37) which hydrolyzes the non-reducing ends of xylose chains to release xylose, and several supplementary enzymes including α-L-arabinofuranosidase (EC 3.2.1.55), α-D-glucuronidase (EC 3.2.1.139), α-Dgalactosidase (EC 3.2.1.22), acetyl xylan esterase (EC 3.1.1.72) and ferulic acid esterase (EC 3.1.1.73) [15,31,32]. Schematic view of a hemicellulolytic system, degradation of arabinoxylan is depicted in Figure 5.
The notion of supplementary enzymes has developed in the past years since most are regarded critical in enzymatic cocktails to improve sugar yields during biomass saccharification [33,34]. Furthermore, it has reported by Hu et al. [35] that supplementation of cellulase combination with hemicellulases can increase the rate and yield of glucan conversion since the reduction of hemicellulose exposes the cellulose fibrils and improve substrate accessibility. Synergism between the enzymes is a widely identified phenomenon in biomass hydrolysis and it relies on several parameters including the nature of the substrate and the source of enzymes.
3.3 Producing cellulase and hemicellulase by microorganisms
There are numerous bacteria and fungi microorganisms which are involved in the cellulase production for digestibility. Bacteria demand anaerobic growth condition and have a very low growth rate, hence fungal cellulase have been mainly used for this purpose (Table 1). The system of fungal cellulases production works on the restrainer/inducer phenomena where glucose easily metabolized carbon sources act as restrainers while cellulose or other oligosaccharide act as inducers [36,37]. For cellulase production, the most examined fungi microorganisms are Trichoderma spp. and Aspergillus spp., native or genetically modified. These fungi produce a crude enzyme which can be used in the industry [38,39].
Endo-β-glucanase and exo-β-glucanase generate in high quantity and β-glucosidase generates in the low quantity by Trichoderma generate. However, Aspergillus is one of the fungi mostly produces endo- β-glucanase and β-glucosidase in high quantity and generally lacks exo-β-glucanase [38,39]. Therefore, various researchers have presented blending enzymes from these two fungi as a technique to maximize the conversion of lignocellulose to monomer sugars. One of the noticeable fungal strain for high cellulase production is Aspergillus niger. It has a group of nine genera, but only some of them dominate for the production of cellulase [40,41].
| Lignocellulosic Biomass | Source of Cellulase | ÃÆââ¬Å¡ÃâàTotal Sugar Yield Reduction (mg/g dry substrate) |
Incubation Time (h) |
References |
|---|---|---|---|---|
| Rice straw | Aspergillus niger | 624 | 24 | [65] |
| Rice straw | Commercial cellulase | 567 | 48 | [66] |
| Wheat straw | Trichoderma longibrachiatum | 294 | 72 | [67] |
| Wheat straw | Trichoderma reesei | 270 | 48 | [68] |
| Wheat straw | Trichoderma reesei NCIM 1186 | 371.44 | 24 | [69] |
| Pinus roxburghii | Locally isolated microorganism | 334 | 24 | [70] |
| Sorghum straw | Coriolus versicolour TD17 | 440 | 5 days | [71] |
| Sargassum sp. | Commercial cellulase | 326 | 72 | [72] |
Table 1. Total sugar yield reduction from different types of biomass by various microorganisms.
Different media have investigated for the production of cellulase by using Aspergillus niger [38,40,42]. Isolation of five prospective Aspergillus sp. for cellulase production reported [43]. They reported the highest production of endo-β-glucanase in the case of Aspergillus MAM-F23. Moreover, it was reported that sorghum straw has a great potential substrate for cellulase production. In this study, by using A. niger under submerged fermentation, maximum (about 0.77 IU/ml) and minimum (about 0.28 IU/ ml) cellulase production was belong to sorghum and wheat straws as substrate. Under solid state fermentation used Trichoderma reesei NCIM 992 for the production of cellulase [44]. Aspergillus niger produced maximum xylanase activity (approximately 14.41 FPU/mg) under solid state fermentation [45]. It was reported by Kumar et al. [46] that the highest CMCase production from Paenibacillus polymyxa is around 7.814 U/mg. In a study 34 fungal strains isolated for xylanase and cellulase production, the maximum xylanase and cellulase production obtained using Aspergillus sydowii SBS45 and Trichoderma sp. SBS60, relatively [47].
4. Production of Cellulase
The first cellulose production endeavored on liquid water but because of gathering free sugar catabolic repression took place, that hindered the cellulase synthesis during the microbial growth. By nourished batch or continuous mode culturing can overcome this challenge but increase the final cost [48,49]. One of the favorable technical methods for reduction of processing cost is the production of cellulase on the agro-industrial residues through solid state fermentation (SSF) [50]. In these cheap residues carbohydrate act as a carbon source for fungal growth [48,50].
It has been reported various substrates for cellulase production by different researchers, like rice straw, wheat straw, sorghum straw, corn cob, cotton flower, cassava residue and groundnut shell [43-47,51]. The cellulase production under SSF by various fungal strains presented in Table 2.
5. Factors Affecting Enzymatic Hydrolysis and Cellulase Intervened Hydrolysis
5.1 Factors affecting enzymatic hydrolysis
Enzymatic saccharification of lignocellulosic biomass is disturbed by different hindrances that limit the enzyme's action. Despite, the factors that affect the productivity of saccharification of lignocellulose have been revised by many authors to obtain a comprehensive, rapid and effective conversion of cellulosic substrates remains a challenging goal [52]. Main factors influencing enzymatic hydrolysis can be divided into substrate features and enzyme-related factors. A summary of factors that influence enzymatic hydrolysis is shown in Table 3. The percentage and level of cellulose saccharification by cellulase enzymes are affected by two main chemical and physical parameters of the substrate: (1) CrI and its DP that decrease enzyme efficiency, and (2) the matrix polysaccharides and lignin coat the cellulose fibril that act as a physical barrier preventing enzymes from reaching the cellulose [54-56].
| ÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàFungal strains | ÃÆââ¬Å¡ÃâàCMCase activity (IU/gds) | References |
|---|---|---|
| Trichoderma citrinoviride AUKAR04 | 375 | [73] |
| Penicillium oxalicum EU2106 | 225 | [51] |
| Trichoderma sp. RCK65 | 145 | [74] |
| Aspergillus oryzae | 123.64 | [75] |
| Fungal strains CG-10 | 29.04 | [76] |
| Bacillus licheniformis | 2.11 | [77] |
| Trichoderma atroviride | 90.43 | [78] |
| Aspergillus niger HN-1 | 416.3 | [40] |
| Aspergillus fumigatus Z5 | 526.30 | [56] |
| Humicola insolens TAS-13 | 18.98 | [77] |
Table 2. Cellulase (Carboxymethylcellulose cellulase) production by various fungal strains under SSF condition.
| Parameters | References |
|---|---|
| Substrate-related factors | |
| Lignin and hemicellulose content | [20,55,56,80,81] |
| Particle size | [80,81] |
| Accessible surface area | [82ÃÆâÃâââ¬Ãâââ¬Å84] |
| Cellulose crystallinity (CrI) and degree of polymerization (DP) | [53,54] |
| Enzyme-related factors | |
| Cellulase activity | [85,86] |
| ÃÆââ¬Å¡ÃâàCost Reduction | [16,17] |
| Composition of cocktail | [87,88] |
Table 3. Summary of factors influencing enzymatic saccharification of lignocelluloses.
5.2 Factors affecting cellulase intervened hydrolysis
Cellulase intervened hydrolysis include mainly three steps: Adsorption of cellulase enzymes onto the surface of the cellulose (i) Bioconversion of cellulose to fermentable sugars (ii) Desorption of cellulase (iii) The controlling factors for these steps are mainly substrate concentration, enzyme dosage and reaction conditions. At intense substrate concentration, the decreasing sugar yield and reaction rates are reduced and it could be because of end product inhibition of cellulose enzyme but at low concentration, the decreasing sugar yield and reaction rates are improved [57,58].
It has reported avoiding substrate inhibition, lower substrate concentrations are more appropriate. Moreover, in this study, the authors realized at 16% stopping of corn flour the glucose yield was 76%, while at 40% stopping was just 50.2% [59]. High enzyme dosage considerably improves the procedure cost but it increases the sugar yield reduction at the same time. Hence, one of the best approaches to conquering the challenges is finding of optimum factors like pH, temperature and, incubation time at low enzyme dosage. Wang et al. [59] reported that sugarcane bagasse under alkali pre-treatment by using crude Trichoderma can have the highest rate of hydrolysis (37.29%) at 50°C. Enzymatic hydrolysis of alkali treated sugarcane bagasse quickly improved (more than 8 h) and at the later stages, the level of this improvement was significantly decreased [60]. Ahmed et al. [61] presented the highest sugar yield reduction (about 343 mg/g dry substrate) from wheat straw under NaOH pre-treatment at 55°C for 30 h by using cellulose which produced from Penicillium waksmanii. The differences in temperature were because of various species for the production of cellulose. Furthermore, the duration of hydrolysis procedure affects the hydrolysis rate [62,63].
6. Future Prospects of Enzymatic Hydrolysis
Generally, hydrolysis process faces different hindrances which are economical and technical. Economic problems associated with the cost of raw material, cellulase enzyme, while technical issues are ineffective cellulase adsorption and value because of minimal specific substrate area, end product inhibition and lignin [11,28]. Then, hemicellulase and cellulase saccharification issue need to be taken care of for more improvement of lignocellulosic bioethanol technology. For enhancement of cellulase efficiency and yield under stress conditions, using genetically modified cellulolytic microorganisms by cellulase coding sequences into fungi, plants, and bacteria is suggested [78-80]. Although, a genetically engineered raw material with low lignin level and high carbohydrate content could decrease the cost. In addition, Solid-State Fermentation (SSF) also can be cost-effective by overcoming the end product inhibition. There is an important research to find out the mode of action of the crucial parameters that maintain interactions between biomass, hemicellulase, cellulase and prohibitive compounds [89-91]. This knowledge will supply a new approach to recognize better pretreatment and hydrolysis methods as industrial demand.
References
- Höök M, Tang X. (2013). Depletion of fossil fuels and anthropogenic climate change-A review. Energy Policy. 52: 797-809.
- Anwar Z, Gulfraz M, Irshad M. (2014). Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J Radiat Res Appl Sci. 7: 163-173.
- De Oliveira MED, Vaughan BE, Rykiel EJ. (2005). Ethanol as fuel: Energy, carbon dioxide balances and ecological footprint. Bioscience. 55: 593-602.
- Bansal A, Illukpitiya P, Tegegne F, et al. (2016). Energy efficiency of ethanol production from cellulosic feedstock. Renew Sustain Energy Rev. 58: 141-146.
- Naik SN, Goud VV, Rout PK, et al. (2010). Production of first and second generation biofuels: A comprehensive review. Renew Sustain Energy Rev. 14: 578-597.
- Ho DP, Ngo HH, Guo W. (2014). A mini review on renewable sources for biofuel. Bioresour Technol. 169: 742-749.
- Miret C, Chazara P, Montastruc L, et al. (2016). Design of bioethanol green supply chain: Comparison between first and second generation biomass concerning economic, environmental and social criteria. Comput Chem Eng. 85: 16-35.
- Kulkarni SJ, Shinde NL, Goswami AK. (2015). A review on ethanol production from agricultural waste raw material. Int J Sci Res Sci Eng Technol. 1: 231-233.
- Xu Z, Huang F. (2014). Pretreatment methods for bioethanol production. Appl Biochem Biotechnol. 174: 43-62.
- Putro JN, Soetaredjo FE, Lin SY, et al. (2016). Pretreatment and conversion of lignocellulose biomass into valuable chemicals. RSC Adv. 6: 46834-46852.
- Madadi M, Tu Y, Abbas A. (2017). Pretreatment of lignocellulosic biomass based on improving enzymatic hydrolysis. Int J Appl Sci Biotechnol. 5: 1-11
- Maitan-Alfenas GP, Visser EM, Guimarães VM. (2015). Enzymatic hydrolysis of lignocellulosic biomass: Converting food waste in valuable products. Curr Opin Food Sci. 1: 44-49.
- Rodhe AV, Sateesh L, Sridevi J, et al. (2011). Enzymatic hydrolysis of sorghum straw using native cellulase produced by T. reesei NCIM 992 under solid state fermentation using rice straw. Biotech. 1: 207-215.
- Benoit I, Culleton H, Zhou M, et al. (2015). Closely related fungi employ diverse enzymatic strategies to degrade plant biomass. Biotechnol Biofuels. 8: 107.
- Kumar D, Murthy GS. (2013). Stochastic molecular model of enzymatic hydrolysis of cellulose for ethanol production. Biotechnol Biofuels. 6: 63
- Tolan JS. (2006). Iogen’s demonstration process for producing ethanol from cellulosic biomass. Clean Technologies and Environmental Policy. 3: 339-345.
- Singhania RR, Sukumaran RK, Pandey A. (2007). Improved cellulase production by Trichoderma reesei RUT C30 under SSF through process optimization. Appl Biochem Biotechnol. 142: 60-70.
- Harner NK, Wen X, Bajwa PK, et al. (2015). Genetic improvement of native xylose-fermenting yeasts for ethanol production. J Ind Microbiol Biotechnol. 42: 1-20.
- Zhao X, Zhang L, Liu D. (2012). Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels, Bioprod Biorefining. 6: 465-482.
- Chang VS, Holtzapple MT. (2000). Fundamental factors affecting biomass enzymatic reactivity. Twenty-First Symp Biotechnol Fuels Chem. 5-37.
- Madadi M, Chen P, Abbas A. (2017). Advances in genetic manipulation of lignocellulose to reduce biomass recalcitrance and enhance biofuel production in bioenergy crops. J Plant Chem Physiol. 5: 1-15.
- Njoku SI, Ahring BK, Uellendahl H. (2012). Pretreatment as the crucial step for a cellulosic ethanol biorefinery: Testing the efficiency of wet explosion on different types of biomass. Bioresour Technol. 124: 105-110.
- Madadi M, Abbas. A. (2017). Lignin degradation by fungal pretreatment: A review. Plant Pathol Microbiol. 8: 398.
- Alvira P, Ballesteros M, Negro MJ. (2010). Bioresource technology pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis : A review. Bioresour Technol. 101: 4851-4861
- Casey E, Mosier NS, Adamec J, et al. (2013). Effect of salts on the co-fermentation of glucose and xylose by a genetically engineered strain of Saccharomyces cerevisiae. Biotechnol Biofuels. 6: 83.
- Zhang Z, Liu B, Zhao ZK. (2012). Efficient acid-catalyzed hydrolysis of cellulose in organic electrolyte solutions. Polym Degrad Stab. 97: 573-577.
- Kuila A, Sharma V, Garlapati VK, et al. (2016). Present status on enzymatic hydrolysis of lignocellulosic biomass for bioethanol production. Adv Biofeedstocks Biofuels. 1: 85.
- Brummer V, Jurena T, Hlavacek V, et al. (2014). Enzymatic hydrolysis of pretreated waste paper-Source of raw material for production of liquid biofuels. Bioresour Technol. 152: 543-547.
- Poovaiah CR, Nageswara-Rao M, Soneji JR, et al. (2014). Altered lignin biosynthesis using biotechnology to improve lignocellulosic biofuel feedstocks. Plant Biotechnol J. 12: 1163-1173.
- Van den Brink J, de Vries RP. (2011). Fungal enzyme sets for plant polysaccharide degradation. Appl Microbiol Biotechnol. 91: 1477.
- Van Dyk JS, Pletschke BI. (2012). A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-factors affecting enzymes, conversion and synergy. Biotechnol Adv. 30: 1458-1480.
- Ivetić DŽ, Omorjan RP, DJor djević TR, et al. (2017). The impact of ultrasound pretreatment on the enzymatic hydrolysis of cellulose from sugar beet shreds: Modeling of the experimental results. Environ Prog Sustain Energy. 1: 1944-7450
- Robl D, da Silva Delabona P, Mergel CM, et al. (2013). The capability of endophytic fungi for production of hemicellulases and related enzymes. BMC Biotechnol. 13: 94.
- Corrêa RCG, Rhoden SA, Mota TR, et al. (2014). Endophytic fungi: Expanding the arsenal of industrial enzyme producers. J Ind Microbiol Biotechnol. 41: 1467-1478.
- Hu J, Arantes V, Pribowo A, et al. (2013). The synergistic action of accessory enzymes enhances the hydrolytic potential of a “cellulase mixture” but is highly substrate specific. Biotechnol Biofuels. 6: 112.
- Pandiyan K, Tiwari R, Singh S, et al. (2014). Optimization of enzymatic saccharification of alkali pretreated Parthenium sp. using response surface methodology. Enzyme Res. 1-8.
- Belosevic M. (2014). Degradation of Alizarin Yellow R using UV/H2O2 advanced oxidation process. Environ Sci Technol. 33: 482-489.
- de Almeida MN, Falkoski DL, Guimarães VM, et al. (2013). Characteristics of free endoglucanase and glycosidases multienzyme complex from Fusarium verticillioides. Bioresour Technol. 143: 413-422.
- Amore A, Giacobbe S, Faraco V. (2013). Regulation of cellulase and hemicellulase gene expression in fungi. Curr Genomics. 14: 230-249.
- Sandhu SK, Oberoi HS, Babbar N, et al. (2013). Two-stage statistical medium optimization for augmented cellulase production via solid-state fermentation by newly isolated Aspergillus niger HN-1 and application of crude cellulase consortium in hydrolysis of rice straw. J Agric Food Chem. 61: 12653-12661.
- Mahalakshmi N, Jayalakshmi S. (2016). Research Article Cellulase production by Aspergillus niger under solid state fermentation using agro industrial wastes. Int J Adv Multidiscip Res. 3: 78-83.
- Umsza-Guez MA, Díaz AB, Ory ID, et al. (2011). Xylanase production by Aspergillus awamori under solid state fermentation conditions on tomato pomace. Brazilian J Microbiol. 42: 1585-97.
- Swelim M, Hammad AI, Gannam RB, et al. (2010). Some critical factors affecting cellulase (S) production by Aspergillus terreus Mam-F23 and Aspergillus flavus Mam-F35 under solid-state fermentation of wheat straw. World Appl Sci J. 9: 1171-1179.
- Maurya DP, Singh D, Pratap D, et al. (2012). Optimization of solid state fermentation conditions for the production of cellulase by Trichoderma reesei. J Environ Biol. 33: 5-8
- Amira D, Roshanida AR, Rosli MI. (2012). Effects of xylanase and cellulase production during composting of EFB and POME using fungi. Intl J Biol Biomol Agric Food Biotechnol Engr. 6: 340-343.
- Kumar D, Ashfaque M, Muthukumar M, et al. (2012). Production and characterization of carboxymethyl cellulase from Paenibacillus polymyxa using mango peel as substrate. J Environ Biol. 33: 81-84.
- Nair SG, Sindhu R, Shashidhar S. (2008). Fungal xylanase production under solid state and submerged fermentation conditions. Afr J Microbi Res. 2: 82-86.
- Cavka A, Alriksson B, Rose SH, et al. (2014). Production of cellulosic ethanol and enzyme from waste fiber sludge using SSF, recycling of hydrolytic enzymes and yeast, and recombinant cellulase-producing Aspergillus niger. J Ind Microbiol Biotechnol. 41: 1191-1200.
- Biswas R, Persad A, Bisaria VS. (2014). Production of cellulolytic enzymes. Bioprocess Renew Resour to Commod Bioprod John Wiley Sons. 105-132.
- Yoon LW, Ang TN, Ngoh GC, et al. (2014). Fungal solid-state fermentation and various methods of enhancement in cellulase production. Biomass and Bioenergy. 67: 319-338.
- Mohiet V, Magar J. (2010). Use of agricultural waste for cellulase production by Aspergillus niger with submerged and solid state fermentation. Bionano Frontier. 3: 189-192
- Su LH, Zhao S, Jiang SX, et al. (2017). Cellulase with high β-glucosidase activity by Penicillium oxalicum under solid state fermentation and its use in hydrolysis of cassava residue. World J Microbiol Biotechnol. 33: 37.
- Zhu L, O’Dwyer JP, Chang VS, et al. (2008). Structural features affecting biomass enzymatic digestibility. Bioresour Technol. 99: 3817-3828.
- Cateto C, Hu G, Ragauskas A. (2011). Enzymatic hydrolysis of organosolv Kanlow switchgrass and its impact on cellulose crystallinity and degree of polymerization. Energy Environ Sci. 4: 1516-1521.
- Zhang Y-HP, Lynd LR. (2004). Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Non-complexed cellulase systems. Biotechnol Bioeng. 88: 797-824.
- Fockink DH, Urio MB, Chiarello LM, et al. (2016). Principles and challenges involved in the enzymatic hydrolysis of cellulosic materials at high total solids. In: Green Fuels Technol. 147-173.
- Liu ZH, Qin L, Pang F, et al. (2013). Effects of biomass particle size on steam explosion pretreatment performance for improving the enzyme digestibility of corn stover. Ind Crops Prod. 44: 176-184.
- Nikolić S, Mojović L, Pejin D, et al. (2010). Production of bioethanol from corn meal hydrolyzates by free and immobilized cells of Saccharomyces cerevisiae var. ellipsoideus. Biomass and Bioenergy. 34: 1449-1456.
- Wang W, Kang L, Wei H, et al. (2011). Study on the decreased sugar yield in enzymatic hydrolysis of cellulosic substrate at high solid loading. Appl Biochem Biotechnol. 164: 1139-1149.
- Mojović L, Nikolić S, Rakin M, et al. (2006). Production of bioethanol from corn meal hydrolyzates. Fuel. 85: 1750-1755.
- Ahmed FM, Rahman SR, Gomes DJ. (2012). Saccharification of sugarcane bagasse by enzymatic treatment for bioethanol production. Malays J Microbiol. 8: 97-103.
- Han L, Feng J, Zhang S, et al. (2012). Alkali pretreated of wheat straw and its enzymatic hydrolysis. Brazilian J Microbiol. 43: 53-61.
- Li Y, Zhang AR, Luo HF, et al. (2015). In vitro and in vivo digestibility of corn starch for weaned pigs: Effects of amylose: Amylopectin ratio, extrusion, storage duration and enzyme supplementation. J Anim Sci. 93: 3512-3520.
- Sun T, Larke HN, Jørgensen H, et al. (2006). The effect of extrusion cooking of different starch sources on the in vitro and in vivo digestibility in growing pigs. Anim Feed Sci Technol. 131: 67-86.
- Lynd LR, Weimer PJ, Van Zyl WH, et al. (2002). Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol Mol Biol Rev. 66: 506-577.
- Hashem M, Ali EH. (2013). Recycling rice straw into biofuel “Ethanol” by Saccharomyces cerevisiae and Pichia guilliermondii. J Agric Sci Technol. 15: 709-721.
- Wi SG, Choi IS, Kim KH, et al. (2013). Bioethanol production from rice straw by popping pretreatment. Biotechnol Biofuel. 6: 1-7.
- Barakat A, Rouau X. (2014). New dry technology of environmentally friendly biomass refinery : Glucose yield and energy efficiency. Biotechnology for Biofuels. 7: 138.
- Monlau F, Barakat A, Trably E, et al. (2013). Lignocellulosic materials into biohydrogen and biomethane: Impact of structural features and pretreatment. Crit Rev Environ Sci Technol. 43: 260-322.
- Kuila A, Rao PVC, Choudary N V, et al. (2015). Novel natural supplement for the production of fungal cellulases and application for enzymatic saccharification of wheat straw. Environ Prog Sustain Energy. 34: 1243-1248.
- Vats S, Maurya DP, Jain A, et al. (2013). Mathematical model-based optimization of physico-enzymatic hydrolysis of Pinus roxburghii needles for the production of reducing sugars. Indian J Exp Biol. 51: 944-953.
- Phuengjayaem S, Poonsrisawat A, Petsom A, et al. (2014). Optimization of saccharification conditions of acid-pretreated sweet sorghum straw using response surface methodology. J Agric Sci. 6: 120-133.
- Jelynne PT, Rosario EJDR. (2014). Chemical analysis and utilization of Sargassum sp. as substrate for ethanol production. Iranica Journal of Energy and Environment. 5: 202-208.
- Periyasamy K, Santhalembi L, Mortha G, et al. (2017). Production, partial purification and characterization of enzyme cocktail from Trichoderma citrinoviride AUKAR04 through solid-state fermentation. Arab J Sci Eng. 42: 53-63.
- Chakraborty S, Gupta R, Jain KK, et al. (2016). Cost-effective production of cellulose hydrolysing enzymes from Trichoderma sp. RCK65 under SSF and its evaluation in saccharification of cellulosic substrates. Bioprocess Biosyst Eng. 39: 1659-1670.
- Pirota RDPB, Tonelotto M, Delabona PS, et al. (2016). Bioprocess developments for cellulase production by Aspergillus oryzae cultivated under solid-state fermentation. Brazilian J Chem Eng. 33: 21-31.
- Gupta C, Jain P, Kumar D, et al. (2015). Production of cellulase enzyme from isolated fungus and its application as efficient refining aid for production of security paper. Int J Appl Microbiol Biotechnol Res. 3: 11-19.
- Dave BR. (2015). Optimization of process parameters for cellulase production by Bacillus licheniformis MTCC 429 using RSM and molecular characterization of cellulase gene. J Bioprocess Biotech. 5: 1-5.
- Sangwan P, Mor V, Dhankhar R, et al. (2015). Optimization of process parameters for cellulase and xylanase production using rice husk. Int J Pharm Biosci. 6:755-762.
- Ul-Haq I, Javed MM, Khan TS. (2006). Sugar cane bagasse pretreatment: An attempt to enhance the production potential of cellulases by Humicola insolens TAS-13. Biokemistri. 18: 83-88.
- Varga E, Réczey K, Zacchi G. (2004). Optimization of steam pretreatment of corn stover to enhance enzymatic digestibility. In: Proc Twenty-Fifth Symp Biotechnol Fuels Chem Held May 4-7, 2003, Breckenridge, CO. 509-523.
- Chandra RP, Bura R, Mabee WE, et al. (2007). Substrate pretreatment: The key to effective enzymatic hydrolysis of lignocellulosics? Biofuels. 67-93.
- Timilsena YP, Abeywickrama CJ, Rakshit SK, et al. (2013). Effect of different pretreatments on delignification pattern and enzymatic hydrolysability of miscanthus, oil palm biomass and typha grass. Bioresour Technol. 135: 82-88.
- Draude KM, Kurniawan CB, Duff SJB. (2001). Effect of oxygen delignification on the rate and extent of enzymatic hydrolysis of lignocellulosic material. Bioresour Technol. 79: 113-120.
- Torr KM, Love KT, Simmons BA, et al. (2016). Structural features affecting the enzymatic digestibility of pine wood pretreated with ionic liquids. Biotechnol Bioeng. 113: 540-549.
- Grethlein HE, Converse AO. (1991). Common aspects of acid prehydrolysis and steam explosion for pretreating wood. Bioresour Technol. 36: 77-82.
- Fougere D, Clarke K, Zhao Y, et al. (2015). Chemical--mechanical pretreatment of wood: Reducing downsizing energy and increasing enzymatic digestibility. Biomass and Bioenergy. 80: 17-29.
- McFarland KC, Ding H, Teter S, et al. (2007). Development of improved cellulase mixtures in a single production organism. ACS Publications. 2: 19-31.
- Kuhad RC, Deswal D, Sharma S, et al. (2016). Revisiting cellulase production and redefining current strategies based on major challenges. Renew Sustain Energy Rev. 55: 249-272.
- Rosgaard L, Pedersen S, Langston J, et al. (2007). Evaluation of minimal Trichoderma reesei cellulase mixtures on differently pretreated barley straw substrates. Biotechnol Prog. 23: 1270-1276.
- Chylenski P, Forsberg Z, Ståhlberg J, et al. (2017). Development of minimal enzyme cocktails for hydrolysis of sulfite-pulped lignocellulosic biomass. J Biotechnol. 246: 16-23.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences