Production of Cellulases and Hemicellulases from Cellulytic Fungal Cultures in Submerged Fermentation Using Agricultural Wastes
Muhammad Irfan, Muhammad Nadeem, Quratualain Syed
Food & Biotechnology Research Center (FBRC),Pakistan Council of Scientific and Industrial Research (PCSIR) Laboratories Complex,Ferozepure road Lahore 54600,Pakistan
- Corresponding Author:
- Muhammad Irfan
Food & Biotechnology Research Center (FBRC)
Pakistan Council of Scientific and Industrial Research (PCSIR) Laboratories Complex
Ferozepure road Lahore 54600,Pakistan
Tel: +92(0)4299231834
E-mail: mirfanashraf@yahoo.com
Abstract
Untreated, Chemically treated and enzyme treated (Saccharified) sugarcane bagasse, wheat straw and rice straw were evaluated in submerged fermentation for the production of cellulases and hemicellulase by monocultures and mixed cultures of Aspergillus niger and Trichoderma viride in submerged fermentation. Maximum CMCase activity of 6.8 ± 0.5 U/mg and xylanase activity of 105± 2.8 U/mg was obtained in enzyme treated sugarcane bagasse and pretreated wheat straw with mixed culture respectively. However, monocultures of Aspergillus niger and Trichoderma viride showed less enzyme production as compared to mixed cultures in rest of the experimentation.
Keywords
CMCase; Xylanase; Co-culture; Aspergillus niger; Trichoderma viride; Agricultural wastes.
1. Introduction
Lignocelluloses are major components of biomasses which come from different industries,forestry,agriculture and municipalities. The biodegradation of this lignocellulosic biomass is limited by several factors like crystallinity of cellulose,available surface area,and lignin content. Among these lignocellulosic biomasses agricultural wastes are more important which can be converted into products that are of commercial interest such as ethanol,glucose,and single cell protein [1]. Hydrolysis of these materials is the first step for either digestion to biogas or fermentation to ethanol [2]. Pre-treatment is a potential techniques which is used to degrade these lignocellulosic biomasses. The techniques involves the use of acid and alkaline pretreatments,liquid hot water treatment,pH controlled hot water and flow-through liquid hot water treatments [3]. Pretreatment of cellulose opens up the structure and removes secondary interaction (lignin and hemicellulose) between glucose chains [4,5].
For conversion of these agricultural products into fuels like ethanol,pretreatment is the first step to make the substrates greater exposiure to enzymes.
Second step is the hydrolysis of these pretreated substrates by the use of cellulases. These cellulases are produced by a variety of microorganisms. Fungi that have been reported to produce cellulases include Sclerotium rolfsii,P. chrysosporium and species of Trichoderma,Aspergillus,Schizophyllum and Penicillium [5-7]. Among all these fungal genera,Trichoderma and Aspergillus has been most extensively used for cellulase production [8].
In the whole process of converting lignocellulosic biomass into ethanol,the most critical part is the enzyme production cost. Mostly cellulases are produced widely by submerged culture processes yielding high cost enzyme production which ultimately stuck the industrial application of cellulose bioconversion [9]. Since the production of cellulase enzyme is a major factor in the hydrolysis of cellulosic materials,it is important to make the process economically viable. The final target of the whole research is to produce economically acceptable enzymatic conversion of cellulosic biomass to glucose for fermentation to ethanol or other products.
In this paper we reported here the production of cellulases and hemicellulases from untreated,pretreated and enzyme treated (saccharified) bagasse,wheat straw and rice straw by pure culture and mixed cultures of Aspergillus niger and Trichoderma viride in submerged fermentation.
2. Materials and Methods
2.1 Fungal Cultures
Fungal strains of Aspergillus niger and Trichoderma viride were obtained from Microbiology laboratory of Food & Biotechnology Research Center (FBRC),Pakistan Council of Scientific and Industrial Research (PCSIR) Laboratories complex,Ferozpur road Lahore,Lahore Pakistan. These strains were cultured and maintained on potato dextrose agar (PDA) slants and stored at 4°C for further use.
2.2 Substrates
In this investigation three different substrates like sugarcane bagasse,wheat straw and rice straw were used. These substrates were used in three different conditions which are as fallows;
2.2.1 Untreated Substrates
In this condition,the substrates were used in untreated forms (without any treatment)
2.2.2 Chemically Pretreated Substrates
In this condition,all the three substrates were treated with 5% (v/v) H2O2 + 3% (w/v) NaOH with substrate to liquid ratio of 1:10 followed by heating at 130°C,30psi for 60min. After heating the material was filtered and washed several times up to neutrality [10].
2.2.3 Enzyme Treated (Saccharified) Substrates
In this condition the chemically pretreated samples were treated with commercial cellulase enzyme for 8hr at 50°C [10]. After the completion of the reaction material was filtered and the residues were washed and used as a substrate for the production of enzymes by tested fungi.
2.3 Enzyme production
For enzyme production,Vogel’s media was used for the growth of Aspergillus niger and Trichoderma viridi in submerged fermentation. In 250ml conical flask 25 ml of the media with 2% substrates were sterilized at 121°C,15psi for 15 min. After sterilization,the media was allowed to cooled and inoculated with 2% of spore suspension of tested fungi and incubated at 30±1°C with the agitation speed of 120rpm. After termination of fermentation period the culture filtrate was centrifuged at 8000 x g for 10 min at 4°C to remove unwanted particles and spores. The supernatants obtained after centrifugation were used as the crude extracellular enzyme source.
2.4 Analytical Methods
Enzyme activities (CMCase and Xylanase),glucose and total proteins were estimated as described earlier [11]. 500 μL of the enzyme sample along with 500 μL of 1% (w/v) CMC in 50 mM acetate buffer pH 5 was incubated,in a water bath at 50°C,for 30 min. After incubation 1.5 mL of DNS was added and boiled for 5 minutes and absorbance was taken spectrophotometrically at 550nm. The reducing ends liberated were then measured with DNS. Xylanase activity in the culture filtrate was measured by adding 0.5 ml of 1% birch wood xylan prepared in 0.05 M sodium citrate buffer pH (5.0),and 0.5 ml of appropriately diluted enzyme were incubated at 50°C for 30 min. The reaction was terminated by adding 1.5ml of DNS [12]. After this the test tubes containing reaction mixtures were boiled for 5 minutes and absorbance was taken spectrophotometrically at 550nm. One unit (U) of xylanase and CMCase was defined as the amount of enzyme releasing 1 micromole of xylose or glucose per minute under the assays conditions. Total protein in the culture filtrate was determinedby the method as described by Lowery [13] using BSA as standard.
2.5 Statistical Analysis
Data was statistically analyzed by ANOVA using Microsoft Excel program with P<0.05.
3. Results and Discussion
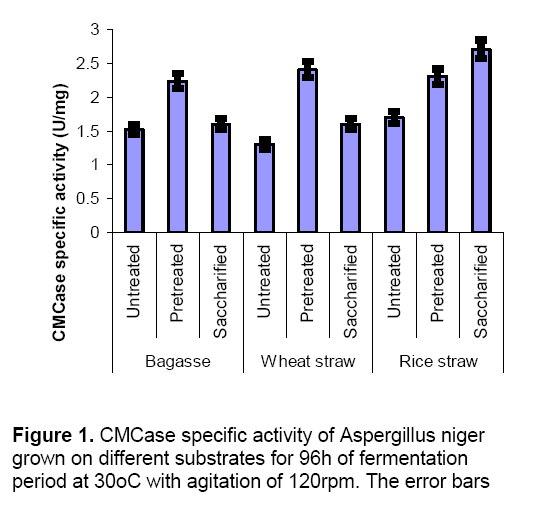
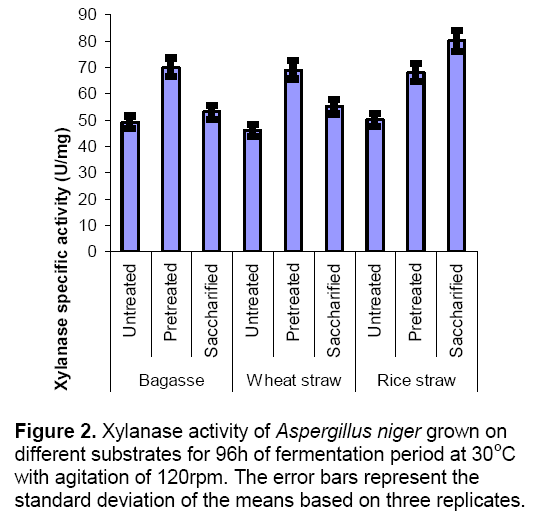
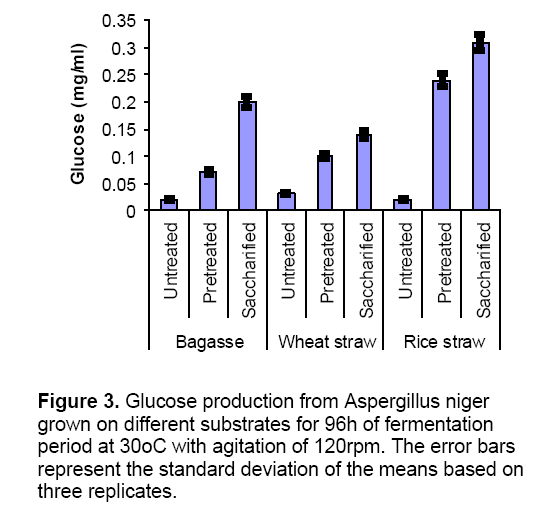
In the present investigation,different experiments were conducted to check the enzyme production by mono cultures and co-cultures using different condition of substrates i.e. untreated,chemically treated and enzyme treated. When mono cultures were grown on untreated substrates enzyme production was lowered due to the presence of hard covering (lignin) on substrates which inhibit the microbial attack. When the substrates were subjected to chemical treatment,the enzyme production was increased as compared to untreated substrates. This chemical treatment ruptures the upper covering and provides a greater surface area for microbial attack. Figure 1 and 2 explains the results of CMCase and xylanase specific activites produced by Aspergillus niger in submerged fermentation. Results indicated that pretreated substrates (Bagasse and wheat straw) showed highest enzyme activities (CMCase and Xylanase) as compared to untreated and enzyme treated substrates. But in case of rice straw enzyme production (CMCase specific activity 2.7 ± 0.3 U/mg,xylanase specific activity 80 ± 2.3 U/mg) was found maximum on saccharified (enzyme treated) conditions. In saccharified wheat straw and bagasse enzyme production was reduced which might be due to the accumulation of reducing sugars which inhibits the cell biomass [14]. Wheat straw and bagasse are the best inducer of cellulytic enzyme like ß–glucosidiase [15]. Glucose production was higher in saccharified substrates (Figure 3 ) as compared to untreated and pretreated substrates.represent the standard deviation of the means based on three replicates.
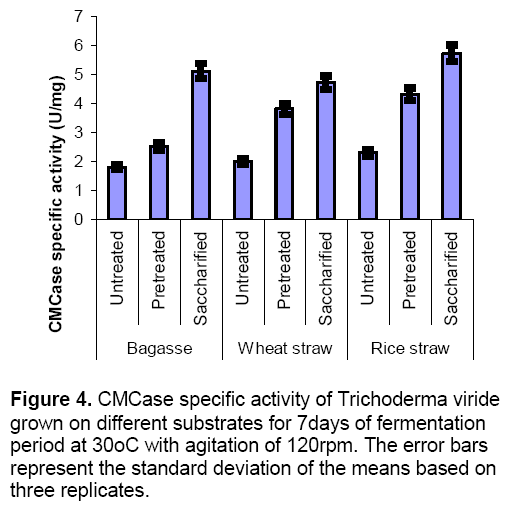
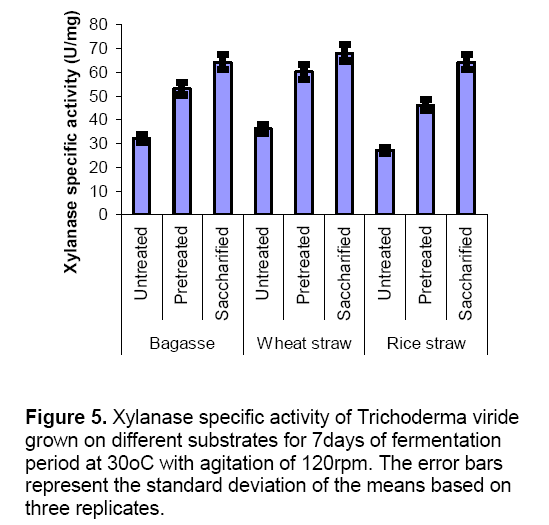
When Trichoderma viride was grown as monoculture on various substrates like wheat straw ,bagasse and rice straw under various conditions (untreated,pretreated and saccharified),it was observed that both CMCase and xylanase activities were maximum in saccharified substrates. Figure 4 revealed that CMCase specific activity was maximum in saccharified bagasse (5.1 ± 0.8 U/mg),saccharified wheat straw (4.7± 0.4 U/mg) and saccharified rice straw (5.7 ± 0.6 U/mg) as compared to untreated and pretreated substrates. Xylanase specific activity was also found maximum on saccharified substrates (Figure 5 ). Saccharified bagasse (64.2 ± 2.8 U/mg),saccharified wheat straw (68 ± 1.7 U/mg) and saccharified rice straw (64.3 ± 2.1 U/mg) yielded high xylanase production.
These findings indicated that Trichoderma viride has the ability to utilize the excess amount of glucose present in the medium.
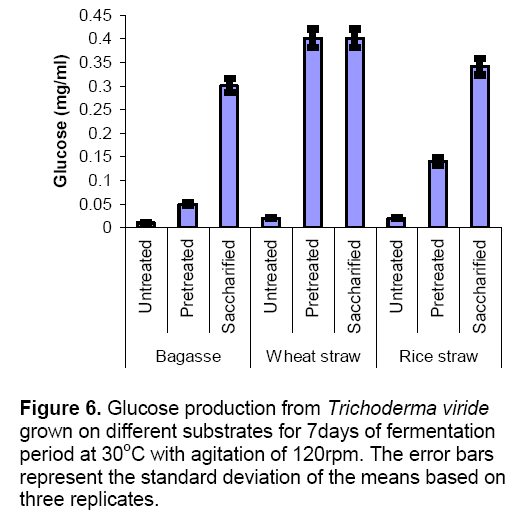
Figure 6 presented the glucose production from substrates in submerged fermentation. In bagasse and rice straw maximum glucose production was observed in saccharified substrates which indicated that the enzyme released by the fungus effectively hydrolyzed the substrates. In case of wheat straw both pretreated and saccharified substrate yielded almost equal quantity of glucose i.e. 0.4 mg/ml.
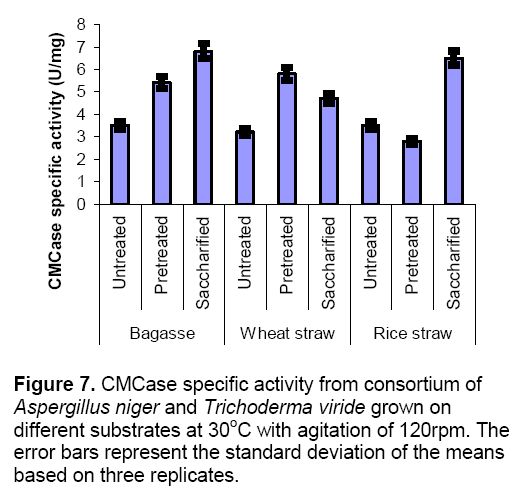
Co-cultures were also cultivated on various substrates such as bagasse,wheat straw and rice straw under different conditions (untreated,pretreated and saccharified) in submerged fermentation. When bagasse was used it was observed that highest CMCase activity was found in saccharified bagasse (6.8 ± 0.5 U/mg) as compared to untreated (3.5 ± 0.3 U/mg) and pretreated bagasse (5.4± 0.3 U/mg) respectively. These results indicated that enzyme production was increased upto 100% in saccharified bagasse as shown in Figure 7 . In case of wheat straw enzyme production was increased in pretreated substrate (5.8± 0.8 U/mg) and reduced in saccharified (4.7 ± 0.4 U/mg) and untreated substrate (3.2± 0.27 U/mg). In case of rice straw highest levels of CMCase production was observed in saccharified (6.5 ± 0.7 U/mg) but decreased in pretreated (2.8 ± 0.16 U/mg) as compared to untreated (3.5 ± 0.24 U/mg) rice straw.
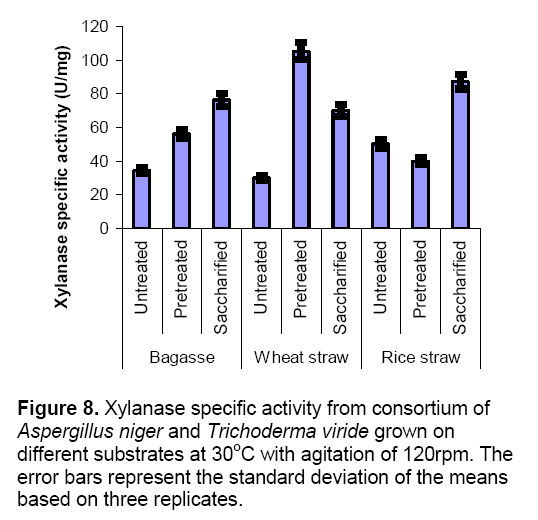
Figure 8 explains the production of xylanase from co-cultures on various substrates under different conditions in submerged fermentation. Results indicated that production of xylanase follow the same pattern as in CMCase production. In these experiment highest titers of xylanase production was 105± 2.8 U/mg observed with pretreated wheat straw. Similar findings were also reported by Haq et al [16] using co-culture of A.niger and T.viride for CMCase and xylanase production.
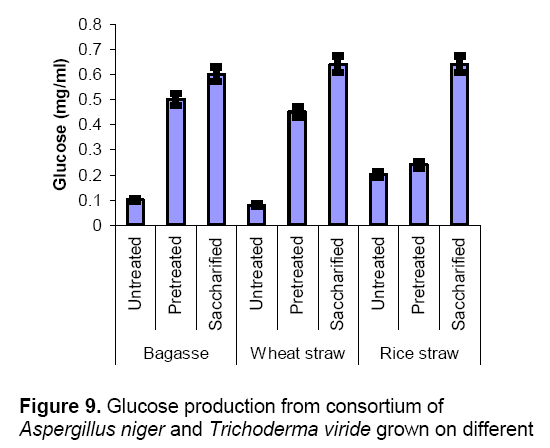
They also reported that co-cultures produce high levels of enzyme production as compared to mono cultures. Glucose production was also found better due to the synergistic action of these two fungi (Figure 9). For efficient sacchrification of substrate a complete cellulose system is very essential. Trichoderma species have deficient in ß–glucosidae which is cover come by co-culturing with Aspergillus sp [17]. Both species acts synergistically for each other for better growth,enzyme production and saccharification. Mixed cultures of lignocellulolytic or cellulolytic microorganisms increases the protein content and efficient in degrading lignocellulosic substrates by producing high activities of enzyme as compared to pure cultures [18,19]. Pure cultures or mixed cultures of celluolytic origin are used for improved conversions of cellulosic biomass to a variety of chemicals and fuels [20,21].
substrates at 30°C with agitation of 120rpm. The error bars represent the standard deviation of the means based on three replicates.
4 Conclusions
Results of this study indicated that pretreatment has a potential influence on enzyme production and this process is very helpful particularly in bioethanol production from plant biomasses. Co-culturing of fungus proved to be beneficial for increased enzyme production in submerge fermentation.
Acknowledgements
Authors thank Ministry of Science and Technology (MoST),Islamabad Pakistan for financial support under the project “Production of Bioenergy from Plant Biomass.”
References
- Solomon,B.O.,Amigun,B.,Betiku,F.,Ojumu,T.V.,Layokun,S.K.,(1999) Optimization of Cellulase Production by Aspergillus flavus Linn Isolate NSPR 101 Grown on Bagasse. J Nig Soc Chem Eng,16: 61-68.
- Taherzadeh,M.J.,Karimi,K.,(2008) Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int J Mol Sci,9: 1621-1651.
- Mosier,N.,Wyman,C.,Dale,B.,Elander,R.,Lee,Y.Y.,Holtzapple,M.,Ladisch,M.,(2005) Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresource Technol,96: 673-686.
- Tang,L.G.,Hon,D.N.S.,Pan,S.H.,Zhu,Y.Q.,Wang,Z.,Wang,Z.Z.,(1996) Evaluation of microcrystalline cellulose changes in ultrastructural characteristics during preliminary acid hydrolysis. J Appl Polym Sci,59: 483-488.
- Fan,L.T.,Gharpuray,M.M.,Lee,Y.H.,(1987) Cellulose Hydrolysis. Berlin,Germany: Springer-Verlag 3: 1-68.
- Sternberg,D.,(1976) Production of cellulase by Trichoderma. Biotechnol Bioeng Symp,35–53.
- Duff,S.J.B.,Murray,W.D.,(1996) Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresour Technol,55: 1–33.
- Sun,Y.,Cheng,J.,(2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Biores Technol,83:1-11.
- Pandey,A.,Soccol,C.R.,Mitchell,D.,(2000) New developments in solid state fermentation: I – bioprocesses and products. Process Biochem,35:1153–1169.
- Irfan,M.,Gulsher,M.,Abbas,S.,Syed,Q.,Nadeem M.,Baig,S.,(2011) Effect of various pretreatment conditions on enzymatic saccharification. Songklanakarin J Sci Technol,33: 397-404.
- Irfan,M.,Nadeem,M.,Syed,Q.,Baig,S.,(2010) Submerged cultivation of Aspergillus niger on pretreated sugarcane bagasse. World J Agric Sci,6:466-472.
- Miller,G.L. (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem,31:426–428.
- Lowry,O.H.,Rosenberg,W.J.,Farr,A.L.,Randell,R.J.,(1951) Quantization of protein using Folin Ciocalteu reagent. J Biol Chem,193:265-75.
- Tabassum,R.,Rajoka,M.I.,Malik,A.,(1990) Production of cellulases and hemicellulases by an anaerobic mixed culture from lignocellulosic biomass. World J Microbiol Biotechnol,6:39-45.
- Lakshmikant,K.,Mathur,S.N.,(1990) Cellulolytic activities of Chaetomium globosum on different cellulosic substrates. World J Microbiol Biotechnol,6: 23-26.
- Haq,I.,Javed,M.M.,Khan,T.S.,(2006) An innovative approach for hyperproduction of cellulolytic and hemicellulolytic enzymes by consortium of Aspergillus niger MSK-7 and Trichoderma viride MSK-10. Afr J Biotechnol,5: 609-614.
- Deshpande,S.K.,Bhotmange,M.G.,Chakrabarti,T.,Shastri,P.N.,(2008) Production of cellulose and xylanase by Trichoderma reesei (QM 9414 mutant),Aspergillus niger and mixed culture by solid state fermentation of water hyacinth. Ind J Chem Technol,15:449-456.
- Arora,D.S. (1995) Biodelignification of wheat straw by different fungal associations. Biodegradation,6: 57-60.
- Puniya,A.K.,Singh,K.,(1995) Biochemical changes during the solid substrate fermentation of wheat straw. Indian J Microbiol,35: 211-215.
- Lezinou,V.,Christakopoulos,P.,Li,L.,Kekos,D.,Macris,J.B.,(1995). Study of a single and mixed culture for the direct bioconversion of Sorghum carbohydrates to ethanol. Appl Microbiol Biotechnol,43: 412-415.
- Tanaka,H.,Kurosawa,H.,Murakami,H. (1986) Ethanol production from starch by a co-immobilized mixed culture system of Aspergillus awamori and Zymomonas mobilis. Biotechnol Bioengg,28: 1761-1768.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences