Molecular Detection of HCMV and Investigation of Its Relationship with Quality of Sperm Parameters in Male Infertility
Khatereh Baghdadi, FarzanehTafvizi, Nasim Hayati Roodbari
1 MSc Student, Department of Biology, Arak Branch, Islamic Azad University, Arak, Iran
2 Assistant Professor, Department of Biology, Parand Branch, Islamic Azad University, Parand, Iran
3 Assistant Professor, Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
- Corresponding Author:
- FarzanehTafvizi
Assistant Professor
Department of Biology, Parand Branch
Islamic Azad University, Parand, Iran
Tel: +989125709532
E-mail: farzanehtafvizi54@gmail.com; farzaneh.tafvizi@yahoo.com
Received date: December 28, 2015; Accepted date: February 01, 2016; Published date: February 07, 2016
Citation: Baghdadi K, Tafvizi F, Roodbari NH, Molecular Detection of HCMV and Investigation of Its Relationship with Quality of Sperm Parameters in Male Infertility. Electronic J Biol, 12:1
Abstract
Background Infertility is the inability of a couple to conceive (irrespective of causes) after one year of consecutive sexual intercourse using no birth control methods. Men are responsible for infertility in 50% of infertile spouses. Numerous factors contribute to male infertility including genital infections that may appear following microbial, fungal, and viral infections. The present study aims to identify HCMV DNA in the semen of infertile men admitted to Infertility clinic as an infertility risk factor in couples.
Material and Finding In this study, 50 semen samples of fertile men (as the control group) and 50 semen samples of infertile men were collected from Infertility Center of Qom Jihad Daneshgahi, Qom, Iran. The semen samples were analyzed according to World Health Organization’s standard methods and cytomegalovirus was detected using nested polymerase chain reaction technique. The analyzed spermogram of 50 infertile samples showed that 36%, 68%, and 72% of the samples had problems, respectively in terms of count, motility, and morphology, and 52% of the infertile samples had problems in all three parameters. Human cytomegalovirus DNA was detected in three and two samples of infertile and fertile men respectively. The results were analyzed using SPSS (version 14.1) software with a significance level of P<0.05.
Conclusions According to statistical analysis no significant correlation was obtained between the cytomegalovirus infection and male infertility. Although the results achieved may vary with changes in population size.
Keywords
Male infertility; Human cytomegalovirus; Semen parameters; Nested PCR.
1. Introduction
Infertility is one of the most important problems of 25% of the couples in the world. In about 50% of the cases, it is caused only by the men and in the rest; the reason is by either the women or both of them [1]. Major causes of infertility involving men include genital damage, infections of semen, testis, genital tract and accessory glands, varicocele; genital tract obstruction and endocrine and metabolic disorders [2].Genital system infections are considered a major cause for male infertility. Seminal vesicle or prostate gland infections in men affect the sperms in specific ways. Infected cells reduce sperm motility. Some infections obstruct the sperms in the male genital system and thus stop sperm transfer. Viral (sexually transmitted) infections of the genital system make for an underlying cause of male infertility. One such viral cause is human cytomegalovirus (HCMV), which is a human herpes virus belonging to the betaherpesvirinae subfamily [3].
In fact, 15% to 20% of infertility cases are affected by contaminated semen. Even asymptomatic men can be at their latent period of sperm contamination. This contamination is associated with the poor quality of sperm parameters [4]. HCMV can contaminate most parts of the sperm. A recent study on HCMVcontaminated organo-typical cultures confirmed the lytic effects of the presence of viral antigens and viral particles in the spermatogonia, spermatocytes and spermatids. In other words, this virus begins to replicate during all the stages of sperm development [4]. Various studies have recently been conducted on cytomegalovirus infection and its effect on the functional parameters of sperm, including motility, morphology, sperm count and reduced male fertility; however, the relationship between cytomegalovirus infection and male infertility remains to be discovered. The present study aims to identify HCMV DNA in the semen of infertile men admitted to the Infertility clinic, Qom Branch, as an infertility risk factor in couples. Results may prove helpful in fertility clinics for the selection of patients for IVF surgery and even for sperm donation.
2. Method
2.1 Samples
Semen samples were taken from the 100 men (50 infertile men and 50 fertile men as a control group) admitted to Infertility Center of Qom Jihad Daneshgahi, Qom, Iran, after obtaining their written consent. Infertile patients were considered those subjects with altered sperm parameters; at least 2 years of unprotected sexual intercourse without conception, and normal female partner (tubal, uterine, cervical abnormalities, and ovarian disorders) were excluded. Exclusion criteria were history of cryptorchidism, testicular trauma, or post-mumps orchitis. Varicocele and seminal infections were excluded, respectively.
Semen samples were obtained by masturbation after 3 days of sexual abstinence. Spermogram was performed on all samples according to the World Health Organization (WHO) criteria. Parameters assessed included volume, pH, liquefaction time, motility, morphology and sperm count. Samples collected were frozen at -80°C for DNA extraction.
2.2 Genomic DNA extraction
Total cellular DNA was extracted from samples according to Weyrich protocol [5]. Briefly, the seminal plasma was washed off with ethanol. The remaining spermatozoa were incubated overnight in lysis buffer containing Dithiothreitol (DDT) and proteinase K. After lysis, samples were centrifuged to separate the cell debris in the pellet. The supernatant was carefully removed and the DNA was precipitated using ethanol. Washed DNA pellet was dried by leaving the tubes in a 37°C for 40 minutes. DNA sample was dissolved in 50 μl TE Buffer . Genomic DNA purity was assessed with a NanoDrop™ ND-2000 spectrophotometry and calculated by ratio of the DNA optical density (OD 260) and protein optical density (OD 280).
2.3 Verification of DNA extraction
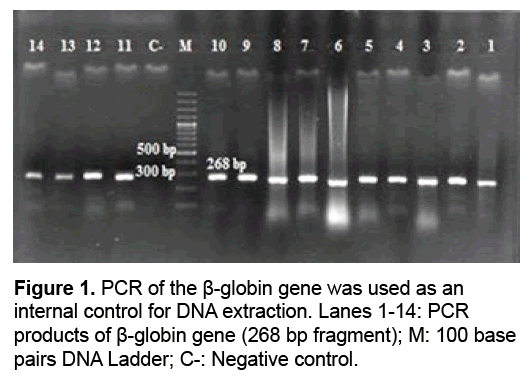
All samples were examined for DNA integrity using amplification of the β-globin. Sequences of primers were: PC04: 5' CAA CTT CAT CCA CGT TCA CC 3', GH20: 5' GAA GAG CCA AGG ACA GGT AC 3'. PCR were carried out in a final volume of 25 μl containing 12.5 μl of Amplicon master mix, 0.5 μl of forward and reverse primers and 1 μl of DNA template. Amplification was carried out in Thermal Cycler (Biorad). After an initial denaturation step at 95°C for 3 min, 45 cycles were programmed as follows: denaturation step at 95°C for 30 sec annealing step at 53°C for 40 sec, primer extension at 72°C for 40 sec and final extension step at 72°C for 5 min. PCR products were determined by visualization of amplicons on 2% agarose gels stained with gel red.
2.4 Nested PCR amplification
The first-round PCR amplification was performed in a 25 μL reaction volume containing 12.5 μl of Amplicon master mix, 0.5 μl of forward and reverse primers, 10 μl of each genomic DNA sample. Sequences of oligonucleotides primers were: 5'TCCAACACCCACAGTACCCGT-3' and 5'CGGAAACGATGGTGTAGTTCG-3' [6]. PCR program was performed as follows: pre-denaturation at 95°C for 5 minutes, 1 cycle; denaturation at 94°C for 30 seconds, annealing at 55°C to for 30 seconds, extention at 72°C for 30 seconds, 20 cycles; postextention at 72°C for 5 minutes, 1 cycle.
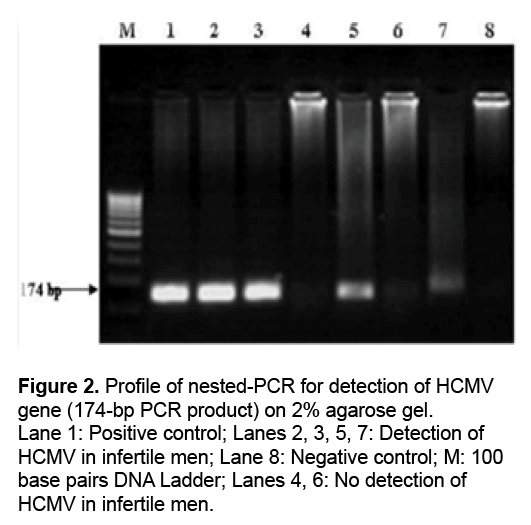
The second-round PCR amplification was carried out by using 12.5 μl of Amplicon master mix, 0.5 μl of forward and reverse primers and 5 μl of the first round PCR products as templates in 25 μL reactions. The sequences of the inside primer pair was:5'-GCCCGCCGCGGCAGCACCTGGCT-3' and 5'-GTAAACCACATCACCCGTGGA-3' [6]. PCR cycling protocol was used: 95°C for 5 minutes, 1 cycle; 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds, 30 cycles, 72°C for 5 minutes, 1 cycle.
At the end of amplification, 5 μL of the PCR products was analyzed on 2% agarose gel. The resultant product was expected to be a 174-bp fragment.
2.5 Statistical analysis
Statistical analyses were performed using the SPSS (version 19) software package. Frequency tables were analyzed using the Chi-square test, with Fischer’s exact test. P<0.05 was accepted as statistically significant.
3. Results
The spermiogram of the 100 samples indicated a normal analysis for 50 (as the controls) and an abnormal analysis for the remaining 50 (as the cases) in some of their sperm parameters. The three factors of sperm motility, count and morphology are considered major factors in assessing sperm quality and infertility. 68%, 46% and 72% of the cases were reported to be lower than the standard in terms of motility, count and morphology, in respective order, and were therefore taken to be infertile. Of the 50 infertile samples, 52% were impaired in terms of all the three factors, i.e., motility, count and morphology. To examine the samples, the quality of the extracted DNA was first assessed on 1% Agarose gel. Genomic DNA purity was assessed with a Nano Drop™ ND- 2000 spectrophotometry and calculated by ratio of the DNA optical density (OD 260) and protein optical density (OD 280), indicating that the DNA extracted from the samples is suitable for PCR amplification. To confirm the DNA extraction, ß-globin amplification was used as a control, and all samples were amplified with ß-globin-specific primers, and the produced 268 bp was confirmed on agarose gel (Figure 1). Of the 100 DNA samples extracted, including 50 fertile and 50 infertile cases, a total of 5 came out HCMV positive, 3 of which pertained to the infertile group and 2 to the fertile group. The 117 bp produced from the amplification of HCMV DNA is shown on 2% Agarose gel (Figure 2). The results were analyzed in SPSS-14.1 software given P<0.05 as the level of significance. No significant relationship was found between male infertility and cytomegalovirus infection (P=0.64).
4. Discussion
According to different studies, the prevalence of HCMV DNA in the genitalia of fertile and infertile men is reported to be between 8% and 65% [7-9]. The present study revealed that HCMV infection has no effect on infertility and the reduced quality of sperm parameters. In other words, no significant difference was observed between those with and without HCMV in the case group and the control group in terms of their sperm parameters in particular, sperm motility, count and morphology. The results of the present study are inconsistent with results obtained in previous studies on the role of HCMV infection in male infertility and yet consistent with other published reports confirming HCMV to have no effect on male infertility. A few examples of these reports are provided.
In a study on infertility, Kapranos et al. identified human cytomegalovirus in 8 (7.1%) out of 113 semen samples; however, they found no significant relationship between cytomegalovirus and male infertility [10]. Yang et al. showed that, despite the high prevalence of HCMV in infertile Taiwanese people, no effect was observed for it on the quality of sperm parameters [11]. Similarly, in study on the effect of cytomegalovirus on male and female infertility, Eggert-Kruse et al. identified human cytomegalovirus in semen samples using the Nested-PCR technique and reported the prevalence of the viral DNA in semen to be 6.5%. The viral DNA was identified in 11 out of 170 samples. The researchers emphasized that viral infection has no effect on the functional activities of sperm, including sperm motility and morphology and also anti-sperm antibodies, and therefore has no significant effect on infertility either [7].
Pallier et al. demonstrated no change in sperm motility as a result of sperm incubation with HCMV [12]. Habibi et al. diagnosed 154 men with infertility and 46 without it, in their study on 200 patients. HCMV infection was found in 25 men out of the entire population of participants. No significant differences were found in the studied variables between those with and without HCMV infection in either the case group or the control group. They argued that, despite the higher prevalence of HCMV in infertile patients, the difference was not statistically significant [13]. In another study, Yu-Shih et al. showed that, despite the high seroprevalence of HCMV infection in infertile couples, the prevalence of the virus had no effect on the quality of sperm parameters [14]. Findings of the present study also indicate that the virus has no effect on the quality of sperm parameters, including motility and morphology.
There are studies, however, that emphasize the effect of HCMV infection on male infertility. Wu et al. demonstrated that HCMV infection is greater in infertile men with pathological cells in their semen. HCMV and HSV-II infected spermatogenic cells may produce pathological lesions and affect spermatogenesis. The problem with this study was its small sample size [15]. In a study conducted by Bezold et al. sexually transmitted pathogens DNA were identified in the semen of a large percentage of asymptomatic infertile men, which was associated with reduced semen quality. The effects of the different infections have not been separately reported. The main limitation of their study was the small sample size, which prevented the accurate identification of the cause. As a result, their study showed no major role for HCMV infection in male infertility [16,17].
5. Conclusion
In the present study, the nested-PCR test was used to eliminate false results, as a result of which the prevalence of this virus was reported to be low in the study subjects. No relationship was found between reduced quality of sperm parameters and infertility. The disparity of results reported by different studies might be due to the differences in testing techniques applied and the study populations. The nested-PCR technique is a strong molecular technique for identifying viral DNA, as using two pairs of specific primers in the two rounds of PCR facilitates the detection of low levels of virus in the semen. It is recommended to conduct similar studies on larger populations. As this virus can be sexually transmitted, the infected man is able to transmit the infection to his sexual partner and subsequently to the developed fetus. One of the treatment methods used in fertility clinics is sperm donation, and infected sperms donated can transfer infection to the fertilized ovule, thus resulting in abortion. It is therefore recommended to perform initial screenings for the virus, and if infection is detected, to use special rinsing techniques to reduce the subsequent risks associated with the virus.
6. Conflict of Interest
The authors have no conflicts of interest.
References
- Sanger F, Nicklen S, Coulson AR. (1977). DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA.74: 5463-5467.
- Ahmad MK, Mahdi AA, Shukla KK, et al. (2008). Effect of Mucunapruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil Steril. 90: 627-3.
- La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE. (2012). Diabetes mellitus and sperm parameters. J Androl.33: 145-53.
- Revello MG, Gerna G. (2002). Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev.15: 680-715.
- Garollaa A, Pizzola D, Bertoldoa A, et al. (2013). Sperm viral infection and male infertility: focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J Reprod Immunol.100: 20-29.
- Weyrich A. (2012). Preparation of Genomic DNA from Mammalian Sperm. Current protocols in Molecular Biology . Berlin, Germany. 17: 1-4.
- Kühn JE, Wendland T, Eggers HJ, et al. (1995).Quantitation of human cytomegalovirus genomes in the brain of AIDS patients. J Med Virol.47: 70-82.
- Eggert-Kruse W, Reuland M, Johannsen W, Strowitzki T, Schlehofer JR. (2009). Cytomegalovirus (CMV) infection—related to male and/or female infertility factors? Fertil Steril.91: 67–82.
- Neofytou E, Sourvinos G, Asmarianaki M, Spandidos DA, Makrigian-nakis A. (2009). Prevalence of human herpes virus types 1–7 in these men of men attending an infertility clinic and correlation with semen parameters. Fertil Steril.91: 2487-2494.
- Bresson JL, Clavequin MC, Mazeron MC, et al. (2003). Risk of cytomegalovirus transmission by cryopreserved semen: a study of 635 semen samples from 231 donors. Hum Reprod.18: 1881-1886.
- Kapranos N, Petrakou E, Anastasiadou C, Kotronia. (2003). Detection of herpes simplex virus, cytomegalovirus, and Epstein-Barr virus in the semen of men attending an infertility clinic. Fertil Steril.79: 1566-1570.
- Yang YS, Ho HN, Chen HF, et al. (1995). Cytomegalovirus infection and viral shedding in the genital tract of infertile couples. J Med Virol.45: 179–82.
- Pallier C, Tebourbi L, Chopineau-Proust S, et al. (2002). Herpesvirus, cytomegalovirus, human sperm and assisted fertilization. Hum Reprod.17: 1281-1287.
- Habibi M, Bahrami A, Morteza A,et al.(2014). Study of cytomegalovirus infection in idiopathic infertility men referred to Shariati hospital, Tehran, Iran. Iran J Reprod Med.12: 151-154.
- Yu-Shih Y, Hong-Nerng H, Hsin-Fu C, et al. (1995). Cytomegalovirus Infection and Viral Shedding in the Genital Tract of Infertile Couples. J Med virol.45: 179-182.
- Wu KH, Zhou QK, Huang JH, et al. (2007). Infection of cytomegalovirus and herpes simplex virus and morphology of the infected spermatogenic cells in infertile men. Zhonghua Nan Ke Xue13: 1075-1079.
- Bezold G, Politch JA, Kiviat NB, et al. (2007). Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertile Steril. 87: 1087-1097.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences