Micrococcus Luteus Strain BAA2, A Novel Isolate Produces Carotenoid Pigment
Pawar Y Surekha, Dhanya P, Sarath Josh MK, Pradeep S, Sailas Benjamin
Enzyme Technology Laboratory, Biotechnology Division, Department of Botany, School of Biological Sciences, University of Calicut, Malappuram- 673 635, Kerala, India.
- Corresponding Author:
- Sailas Benjamin
Enzyme Technology Laboratory
Biotechnology Division, Department of Botany
School of Biological Sciences
University of Calicut, Malappuram- 673 635, Kerala, India
Tel: +91 04942407406
Fax: +91 4942400269
E-mail: benjamin@uoc.ac.in
Received date: January 27, 2016; Accepted date: February 06, 2016; Published date: February 12, 2016
Citation: Surekha PY, Dhanya P, Sarath Josh MK, et al., Micrococcus luteus Strain BAA2, A Novel Isolate Produces Carotenoid Pigment. Electronic J Biol, 12:1
Abstract
The present study describes the culture characteristics, isolation and partial purification of a yellow pigment produced by a novel bacterium, Micrococcus luteus strain BAA2. The growth conditions of M. luteus strain BAA2 were optimized. It showed the optimum growth (OD: 0.55 at λ660) at 28oC (pH 7, 24 h), while the maximum pigment (OD: 0.50 at λ466) production was at 36 h (pH 7). The yellow pigment secreted in the medium was extracted in methanol, and purified using silica column chromatography, and the pigment was characterized using thin layer chromatography (2 spots: Rf 0.38 and 0.43), UV-visible and IR spectroscopic techniques; and both spectroscopic profiles showed the characteristic peaks of carotenoid pigment.
Keywords
Micrococcus luteus strain BAA2; Yellow pigment; Carotenoid.
1. Introduction
Demand for natural pigments is increasing day-byday, because of its environmental safety as well as beneficial effects on human health. It is essential to explore various natural sources of food grade colorants and their potentials [1]. Owing to the stability of the pigments produced and easier availability of cultivation technology (production strategies), microbial colorants draw increased attention [2]. Production of natural food colorants for industrial use by microbial fermentation has several advantages; such as cheaper production, easier extraction, higher yields, availability of raw materials and lack of seasonal variations [3]. Microbial pigment production has two fundamental approaches: first is to find out new strains of efficient microbes producing pigments and the other approach is to obtain enhanced and consistent yields either through optimizing the process parameters for the better yield or through strain improvement [4]. Due to biodegradability and higher compatibility with the environment, bacterial pigments offer promising avenues for various applications in industries like food, pharmaceuticals, cosmetics, textiles, etc. [5].
Carotenoids are the most important pigment group comprising of yellow to orange-red variants, which are ubiquitous in nature with proven anti-carcinogenic and immune-modulation properties [6]. Among algae, Dunaliella salina and Dunaliella bardawil are well known producers of carotenoids [7]. Haematococcus pluvialis, a fresh water alga produces astaxanthin, an important and valuable keto-carotenoid [8]. Haloferax alexandrinus is one of the most promising microorganisms used for the commercial production of canthaxanthin, a di-ketocarotenoid [9]. Riboflavin, a yellow water soluble vitamin is produced from ascomycetes (e.g. Ashbya gossypii), filamentous fungi (e.g., Candida famata), etc. [10]. Hence, work on the bacterial pigments should be intensified, especially in cheap and suitable growth media, which can reduce the production cost and increase its applicability in industry and medicine [11].
Micrococci are Gram-positive, non-sporulating and non-motile bacteria with spherical cells, and often found in tetrads. The genus Micrococcus has several species, all described as strict aerobes and M. luteus, the type species of the genus Micrococcus, is an obligate aerobe. Netzer et al. [12] characterized the major carotenoids synthesized by the M. luteus strain NCTC 2665 as sarcinaxanthin. Pigment produced by bacteria could be extracted in suitable solvents, and be characterized using various analytical techniques such as thin layer chromatography (TLC); gel permeation chromatography; High Performance Liquid Chromatography (HPLC); UV-Vis, Fourier Transform Infra-Red (FT-IR) and Nuclear Magnetic Resonance (NMR) spectroscopies [5].
Based on this background, the present work is aimed at: (1) identification of the pigment producing bacterium based on culture, morphological and molecular characteristics; (2) cultivation of the selected bacterium in a suitable medium, and extraction of the pigment in suitable solvents; and (3) purification and characterization of the pigment produced using various analytical techniques.
2. Materials and Method
2.1 Growth conditions
The yellow pigmented bacterium Micrococcus luteus strain BAA2 (GenBank Accession No. KF550912) obtained from the culture collection of the Enzyme Technology Laboratory, Department of Botany, University of Calicut was used for this study.
2.2 Culture characterizations
Standard procedures were employed for the Gramstaining [13]. The photomicrographs were taken using image analyzer (Nikon Eclipse E 400, Towa optical, Japan) fitted with Nikon digital camera (DXM 1200F, Japan), and also by phase contrast microscope (Leica M80, Germany). The bacterial isolate was further characterized by the PCR amplification of 16S rRNA gene employing 8F and 1492R primers using BDT v3.1 Cycle sequencing kit on ABI 3730xl genetic analyzer.
2.3 Growth profile and pH effect
The growth of the bacterium was observed by measuring the maximum absorbance (λ max) at 660 nm at every 3 h interval using UV-vis spectrophotometer (Shimadzu, Japan). To determine the optimum pH for pigment production, 10 ml nutrient broth (NB) with different pH (i.e., 6, 7 or 8) was incubated in a temperature-controlled shaker (Orbitech, India) at 28ºC. Growth of the bacterium, change in pH, biomass and production of the pigment (at 466 nm) were noted at every 6 h interval.
2.4 Pigment extraction
The pigment was extracted according to the method of Jagannadham et al. [14], with some modifications. Briefly, 1 ml of the culture broth was taken in a microcentrifuge tube and centrifuged at 9,440 × g for 15 min at 4ºC. The pellet was washed with distilled water and again subjected to centrifugation as above. Methanol (0.5 ml) was added to the pellet and then mixed well until the methanol layer turned yellow. The entire suspension was centrifuged at 9440 × g for 15 min at 4ºC, and the methanol layer containing the crude pigment was recovered. The yellow pigmented cell pellet was extracted once again with methanol, and the methanol layer was recovered as described above. All methanol fractions were pooled, and used for further analyses.
2.5 Purification by column chromatography
Pigment produced by M. luteus BAA2 was purified by column chromatography using silica gel (60- 120 mesh size), and eluted initially with n-hexane (flow rate 1 ml/min). The polarity of the solvent was increased subsequently by adding ethyl acetate (5-100%), and the yellow colored fractions were collected from the column.
2.6 Characterization of the pigment
Thin layer chromatography (TLC): The procedure for TLC (silica gel GF234) was as described by Basker et al. [15] with slight modifications. A suitable solvent system containing chloroform:methanol:water in the ratio 65:25:4 was designed by trial and error method. The retention factor (Rf) was calculated subsequently.
UV-Vis spectrophotometry: Absorption spectra of both the crude and purified pigments were taken [14] using a UV-vis biospectrophotometer (Elico double beam BL 200 bio-spectrophotometer).
FT-IR spectroscopy: The purified pigment was characterized by FT-IR spectroscopy, as described by Song et al. [16]. The methanolic extract of the pigment was concentrated, pelleted with potassium bromide (KBr) and analyzed using Jasco FTIR 400 Series (Jasco, Japan). The relative intensity of transmitted light was measured against the wavelength of absorption in the region 400-4000 cm-1.
3. Results and Discussion
3.1 Culture characterizations
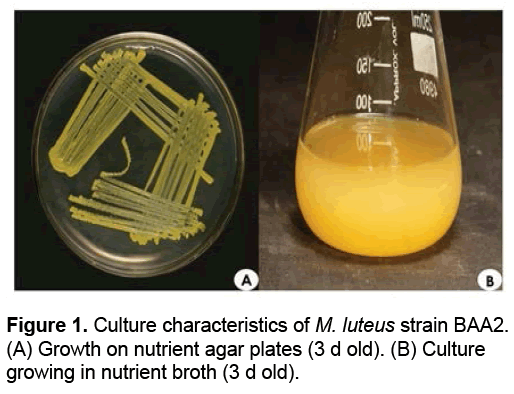
M. luteus strain BAA2 on nutrient agar plates (after 3 d of incubation at 37ºC) showed yellowish colonies. It grew well in the complex nutrient medium, both on agar plates and in broth (Figures 1A and 1B). Upon Gram-staining, the bacterium appeared as cocci; the colonies were seen arranged in tetrads and also in irregular clumps of tetrads. Thus, the bacterium M. luteus strain BAA2 was identified as Gram-positive non-sporulating coccus.
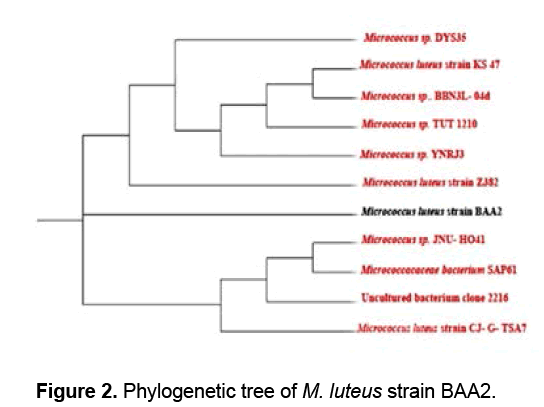
The genomic DNA was isolated from M. luteus strain BAA2 and subjected to PCR amplification. A consensus sequence of 1321 bp of 16S rRNA gene was generated to carry out BLAST with the non-redundant database of NCBI GenBank. Based on the maximum identity score, first ten sequences were selected and aligned using multiple alignment software programmes Clustal W, and the phylogenetic tree was constructed using tree view. M. luteus strain BAA2 showed 100% similarity with all the ten accessions analyzed. From the phylogenetic tree (Figure 2), it is evident that M. luteus strain BAA2 is closely related to M. luteus strain Z382 (GenBank accession number: KC2119951). The genus, Micrococcus now harbors only five species: M. luteus, M. lylae, M. antarticus, M. endophyticus and M. flavus [17,18].
3.2 Growth profile
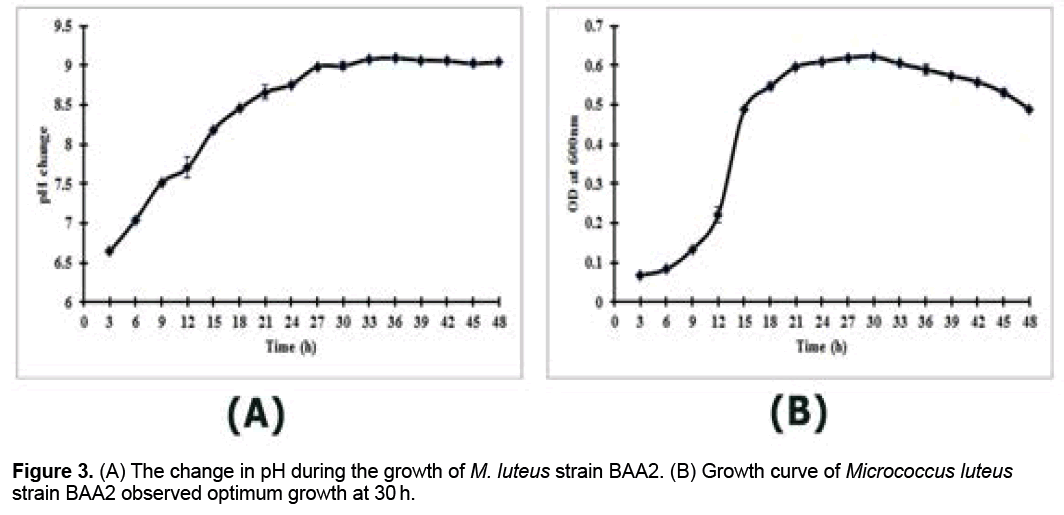
The change in pH was observed at every 3 h interval during the growth of M. luteus strain BAA2 (Figure 3A). The pH of the bacterial culture was increased at every 3 h interval till 27 h, subsequently the culture became stable (Figure 3A). From the growth profile, M. luteus strain BAA2 attained the maximum growth at 30 h of incubation (Figure 3B).
3.3 Effect of pH
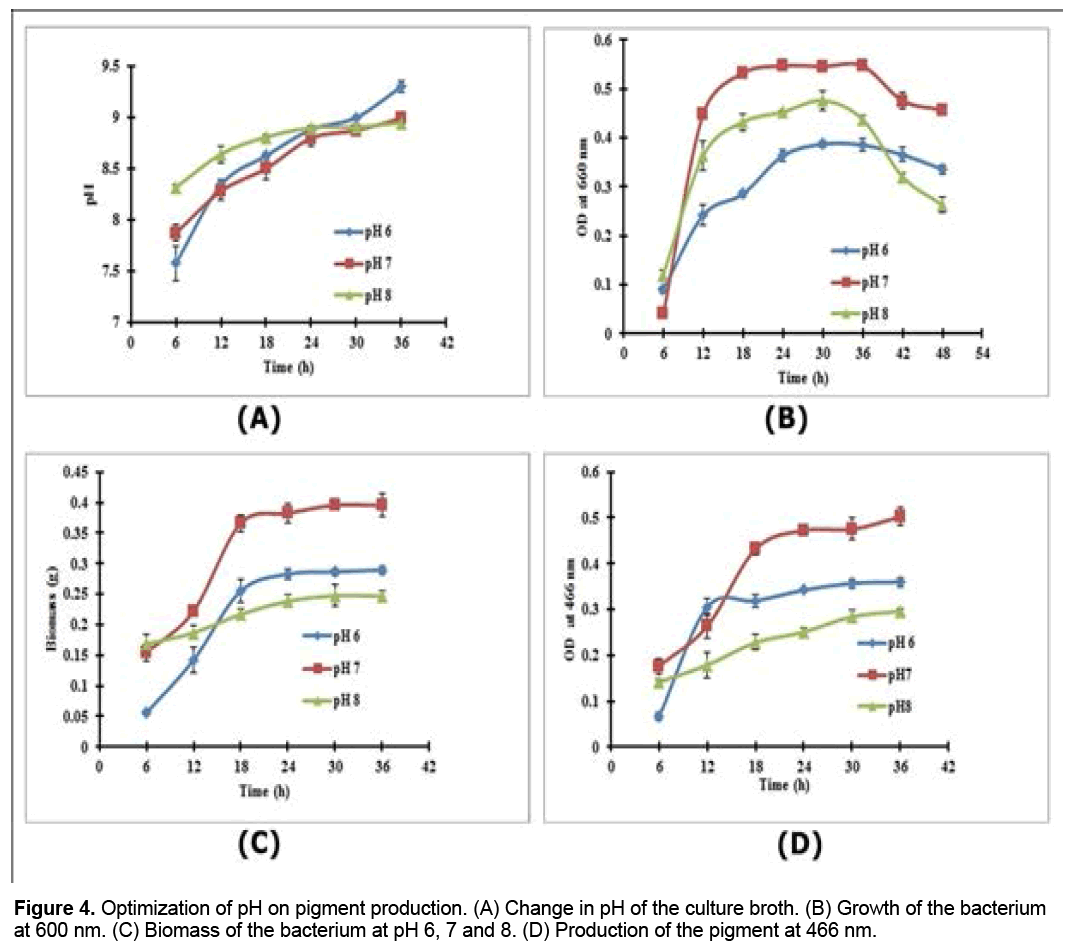
The pH change during the optimization of pH was noted (Figures 4A-4D); the maximum growth (OD, 0.55 at 660nm) was found at pH 7 in 24 h of incubation (Figure 4B), and the maximum pigment production was also observed at pH 7 (OD, 0.502 at 466 nm), but in 36 h of incubation (Figure 4D).
3.4 Pigment extraction
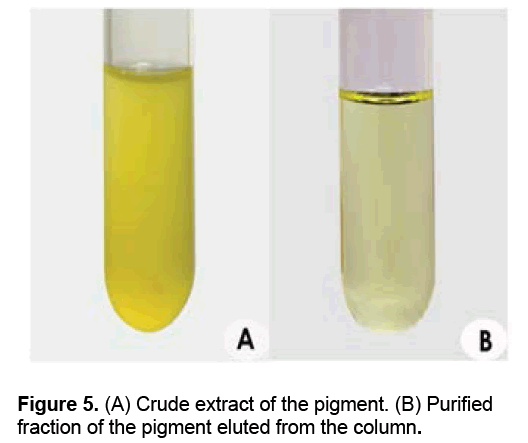
The yellow pigment produced by M. luteus strain BAA2 was extracted using different solvents after centrifugation at 9,440 × g. It was noted that the pigment was insoluble in distilled water, and showed the maximum solubility in other solvents such as acetone, methanol, chloroform and hexane. From the results, it was also inferred that methanol is an ideal solvent for extracting this water insoluble pigment.
3.5 Purification by column chromatography
The solvent system used for column chromatography of the crude pigment (Figure 5A) comprised of hexane and ethyl acetate (50 ml of n-hexane was used as initial elution solvent and colorless fractions were eluted out). The polarity of the solvent was increased by adding ethyl acetate. The major fractions of yellow pigments (Figure 5B) were separated collected when the second elution solvent (ethyl acetate: n-hexane; 15:35; v/v), which was concentrated and used for characterization studies (Figures 5A-5B).
3.6 Characterization of the pigment
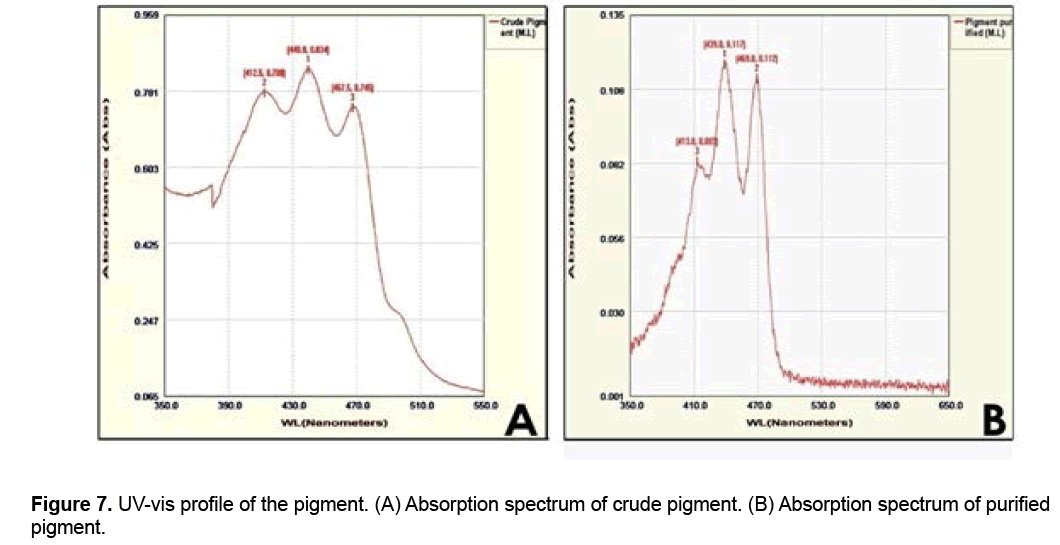
TLC was used for analyzing, identifying or separating mixtures of the crude pigment. Two spots (Figure 6) with 0.43 and 0.38 Rf values were obtained after developing the silica gel plates with chloroform:methanol:water (65:25:4, v/v) system. The chemical nature of pigments has been determined only for two mesophilic species of the genus Micrococcus so far. The pigment produced by M. luteus was a dihydroxy C50 carotenoid [19-21]; while α or β carotene derivative with canthaxanthin as the main pigment was produced by M. roseus [22,23]. Pigments purified from the strain of M. arborescens by TLC using petroleum ether and ethyl acetate (90:10 v/v) as solvent system showed four spots with different Rf values: yellow (0.15), pink (0.20), light yellow (0.47) and dark yellow (0.77) [24]. UV-vis spectroscopy helped to determine the λmax of the pigment present in both crude and purified fractions (from column chromatography). In the absorption spectrum of crude pigment (scanning range λ350-550), 3 peaks were seen i.e., one major peak and two minor peaks; and both the minor peaks showed absorbance at λ412.5 (0.780) and λ467.5 (0.745). Among the 3 peaks obtained, the middle peak showed the maximum absorbance (0.834), i.e., at λ440 (Figure 7A). The absorption spectrum of purified pigment by column chromatography also showed 3 peaks, and the middle peak showed the maximum absorbance (0.117) at λ439 (Figure 7B). The absorption spectrum of the pigment saproxanthin produced by the bacterial strain 040KA-13-27 belongs to the family Flavobacteriaceae also showed 3 peaks [25] (Figures 7A-7B). UV-Vis absorption spectra of carotenoid pigments are of immense importance, since they aid a great deal in determining the structure of carotenoids [26]. The UV-Vis absorption spectra of 5 pigments isolated from the psychotrophic M. roseus strain by HPLC appeared to be identical and exhibited a fine structure, with three absorption maxima, i.e., at λ466,493 and 523; and one of them was characteristic of carotenoids [14].
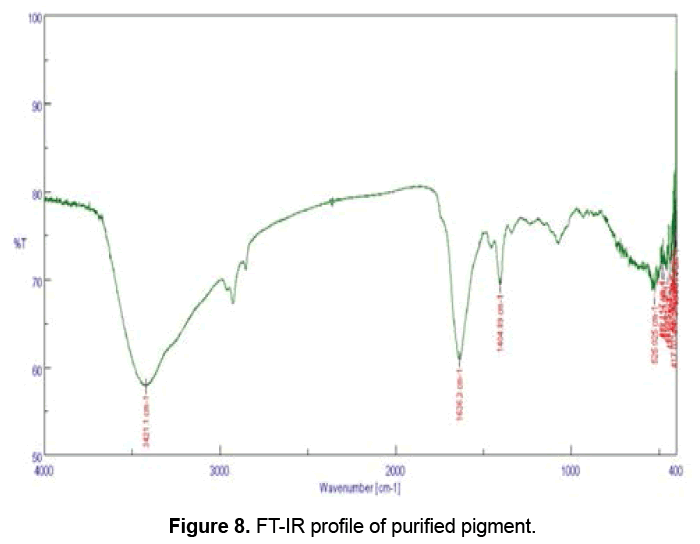
FT-IR absorption of the yellow pigment showed strong and broad peaks (Figure 8) at 3421.1 cm-1. The spectrum obtained after analysis also showed medium peaks at 1636.3 and at 1404 cm-1. The peaks at 3421.1, 1636.3 and 1404.89 cm-1 correspond to different functional groups (Table 1). FT-IR helps to determine the functional groups in the sample, and different functional groups absorb characteristic frequencies of IR radiations differently [16]. Ahmad et al. [11] isolated and characterized a crude violet pigment, which showed characteristic frequencies at a broad range (3700 to 3000 cm-1), corresponding to –OH stretching in the violet pigment. Prodigiosin, a red pigment isolated and characterized from Serratia marcescens showed characteristic vibrations at 3445.45, 2926.23, 1718, 1650.96 and 1559.85cm-1 [11].
The appropriate use of the fermentation physiology together with the metabolic engineering could allow mass production of valuable pigments from bacteria efficiently. Optimization of fermentation processes is an important strategy required to achieve the higher production of pigments [2]. Bacteria belong to the genus Micrococcus may be yellow, yellowish green or orange (M. luteus); dark yellow (M. varians); pink or red (M. roseus and M. radiodurans); dark rose-red (M. agilis); and cream or white (M. lylae, M. kristinae, M. nishinomiyensis, M. sedentarius and M. halobius) [27,28].
4. Conclusion
In comparison to the known characteristics of the pigments produced by various species of Micrococcus and the characteristics of the pigment produced by M. luteus strain BAA2, it seems that the water insoluble and extracellular yellow colored pigment secreted by M. luteus strain BAA2 seems to be a carotenoid. It was produced inexpensively in nutrient broth upon incubating for 3 d in a shaker at 28ºC and 150 rpm, and showed the maximum growth, biomass and pigment production at pH 7. Moreover, the structural elucidation of the molecule needs to be carried out further by NMR. Efficacy of the pigment needs to be confined by antioxidant, cell line, and bioinformatics aided computational docking studies on various cellular targets.
Acknowledgement
The authors are thankful to KSCSTE for a research grant. The authors declare that there exists no conflict of interests.
References
- Nagpal NN, Munjal N, Chatterjee S. (2011). Microbial pigments with Health Benefits-A Mini Review. Trends Bioscience. 4: 157-160.
- Parekh S, Vinci V, Strobel R. (2000). Improvement of microbial strains and fermentation processes. Applied Microbiology and Biotechnology. 54: 287-301.
- Malik K, Tokkas J, Goyal S. (2012). Microbial Pigments: A review. International Journal of Microbial Resource Technology. 1: 361-365.
- Nielsen J, Olsson L. (2002). An expanded role for microbial physiology in metabolic engineering and functional genomics: Moving towards systems biology1. FEMS Yeast Research. 2: 175-181.
- Venil CK, Zakaria ZA, Ahmad WA. (2013). Bacterial pigments and their applications. Process Biochemistry. 48: 1065-1079.
- Browning DF, WhitworthDE, Hodgson DA.(2003).Light?induced carotenogenesis in Myxococcus xanthus : functional characterization of the ECF sigma factor CarQ and antisigma factor CarR. Molecular Microbiology. 48: 237-251.
- Dufossé L, Galaup P, Yaron A, et al. (2005). Microorganisms and microalgae as sources of pigments for food use: a scientific oddity or an industrial reality? Trends in Food Science & Technology. 16:389-406.
- Grung M, D'Souza FM, Borowitzka M, Liaaen-Jensen S. (1992). Algal carotenoids 51. Secondary Carotenoids 2. Haematococcus pluvialis aplanospores as a source of (3S, 3′ S)-astaxanthin esters. Journal of Applied Phycology. 4: 165-171.
- Nelis H, De Leenheer A. (1989).Profiling and quantitation of bacterial carotenoids by liquid chromatography and photodiode array detection. Applied and Environmental Microbiology. 55: 3065-3071.
- Stahmann KP, Revuelta J, Seulberger H. (2000).Three biotechnical processes using Ashbya gossypii , Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Applied Microbiology and Biotechnology. 53:509-516.
- Ahmad WA, Ahmad WYW, Zakaria ZA, YusofNZ. (2012). Application of bacterial pigments as colorant. Springer. 25-44.
- Netzer R, Stafsnes MH, Andreassen T, et al. (2010). Biosynthetic pathway for γ-cyclic sarcinaxanthin in Micrococcus luteus : Heterologous expression and evidence for diverse and multiple catalytic functions of C50 carotenoid cyclases. Journal of Bacteriology. 192: 5688-5699.
- Cappuccino J, Sherman N. (1999). Microbiology-A laboratory manual. Sydney, Australia: Addision Wesley Longman.
- Jagannadham M, Rao VJ, Shivaji S. (1991). The major carotenoid pigment of a psychrotrophicMicrococcus roseus strain: purification, structure, and interaction with synthetic membranes. Journal of Bacteriology. 173: 7911-7917.
- Baskar V, Madhanraj P, Kanimozhi K,Panneerselvam A. (2010).Characterization of carotenoids from selected strains of Streptomyces sp. Annals of Biological Research. 1: 194-200.
- Song MJ, Bae J, Lee DS, et al. (2006). Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. Journal of Bioscience and Bioengineering. 101: 157-161.
- Liu H, Xu Y, Ma Y, Zhou P. (2000). Characterization of Micrococcus antarcticus sp. nov., a psychrophilic bacterium from Antarctica. International Journal of Systematic and Evolutionary Microbiology. 50: 715-719.
- Liu XY, Wang BJ, Jiang CY, Liu SJ. (2007).Micrococcus flavus sp . nov., isolated from activated sludge in a bioreactor. International Journal of Systematic and Evolutionary Microbiology. 57: 66-69.
- ThirkellD, Hunter M. (1969a).Carotenoid-glycoprotein of Sarcina flava membrane. Journal of General Microbiology. 58: 289-292.
- Thirkell D, Hunter M. (1969b). The polar carotenoid fraction from Sarcinaflava . Journal of General Microbiology. 58: 293-299.
- Thirkell D, Strang R. (1967). Analysis and Comparison of the Carotenoids of Sarcinaflava and S. lutea . Journal of General Microbiology. 49: 53-57.
- Cooney J, Marks H, Smith AM.(1966).Isolation and identification of canthaxanthin from Micrococcus roseus. Journal of Bacteriology. 92: 342-345.
- Schwartzel E, Cooney J. (1970). Isolation and identification of echinenone from Micrococcus roseus . Journal of Bacteriology. 104: 272-274.
- Godinho A, Bhosle S. (2008). Carotenes produced by alkaliphilic orange-pigmented strain of Microbacteriumarborescens -AGSB isolated from coastal sand dunes. Indian Journal of Marine Sciences. 37: 307.
- Shindo K, Kikuta K, Suzuki A, et al. (2007). Rare carotenoids, (3R)-saproxanthin and (3R, 2′ S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Applied Microbiology andBiotechnology. 74: 1350-1357.
- Goodwin TW. (1976). Chemistry and biochemistry of plant pigments. New York: Academic Press. 225-261.
- VentosaA, Marquez MC, Kocur M, Tindall BJ. (1993). Comparative study of “Micrococcus sp .” strains CCM 168 and CCM 1405 and members of the genus Salinicoccus. International Journal of Systematic Bacteriology. 43: 245-248.
- Skerman VBD, Mcgowan V, Sneath PHA.(1980).Approved lists of bacterial names. International Journal of Systematic Bacteriology. 30:255-420.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences