Chromate Reduction by Vogococcus sp. Isolated from Cr (VI) Contaminated Industrial Effluent
Kinnari Mistry, Chirayu Desai, Krishna Patel
Ashok and Rita Patel Institute of Integrated Study & Research in Biotechnology and Allied Sciences (ARIBAS), New Vallabh Vidyanagar,388 121 Gujarat, India.
Abstract
Bioremediation is the most promising and cost effective technology widely used now a days to clean up both soils and wastewaters containing organic or inorganic contaminants. Discharge of chromium containing wastes has led to destruction of many agricultural lands and water bodies. Utilization of chromium (Cr) reducing microbes and their products has enhanced the efficiency of the process of detoxification of Cr (VI) to Cr (III). This research article focuses mainly on the current technologies prevalent for remediation like natural attenuation, anaerobic packed bed bioreactors (using live cells, Cr (VI) reductase or their byproducts). A chromium resistant bacterial strain KKF was isolated from chromium contaminated soil. On the basis of different morphological and biochemical characteristics the strain KKF was identified as Vogococcus fluvialis. Hexavalent chromium resistance of the strain showed that it could tolerate very high concentration of K2CrO4 in nutrient agar medium. A self made anaerobic packed bed bioreactor has shown good efficiency to reduce chromate from industrial effluent. The bacterial isolate KKF can be exploited for bioremediation of chromate containing wastes, since it seems to have potential to reduce the toxic hexavalent form of chromium to its non-toxic form.
Keywords
Bioremediation; Vogococcus fluvialis; Hexavalent chromium reduction; anaerobic packed bed bioreactor; Vigna radiate.
1. Introduction
Heavy metals released by a number of industrial processes are major pollutants in marine, ground, industrial and even treated wastewaters [1]. Lead is widely used in many industrial applications such as storage battery manufacturing, printing, pigments, fuels, photographic materials and explosive manufacturing [2]. Heavy metals can be extremely toxic as they damage nerves, liver, kidney and bones, and also block functional groups of vital enzymes. Two stable oxidation states of Chromium persist in the environment, Cr (III) and Cr (VI), which have contrasting toxicities, mobilities and bioavailabilities. Whereas Cr (III) is essential in human nutrition (especially in glucose metabolism), most of the hexavalent compounds are toxic, several can even cause lung cancer. Chromium and its compounds are widely used in electroplating, leather tanning, cement, dyeing, metal processing, wood preservatives, paint and pigments, textile, steel fabrication and canning industries. These industries produce large quantities of toxic wastewater effluents [3]. The maximum concentration limit for Cr (VI) for discharge into inland surface waters is 0.1 mg/l and in potable water is 0.05 mg/l. Procedures for the removal of toxic metal species from contaminated environments have been developed and most of them are based on ion-exchange technologies and/or precipitation of the cation in an inert form. Unfortunately, these methods are expensive and require the use of contaminating products for desorption of metals for cleaning up of the inorganic matrix. Physico-chemical methods presently in use have several disadvantages such as unpredictable metal ion removal, high reagent requirements and formation of sludge and its disposal, in addition to high installation and operational costs [4].
Several microorganisms have the exceptional ability to adapt and colonize the noxious metal polluted environments by developing mechanisms to evade metal toxicity like metal efflux channels, metal resistance plasmids, adsorption uptake, DNA methylation and metal biotransformation either directly by specific enzymes or indirectly by cellular metabolites.
Biotransformation of Cr (VI) to Cr (III) using bacteria is the most pragmatic approach with a well-established feasibility in bioremediation. Reduction of Cr (VI) has been demonstrated in various bacterial species including Bacillus sp. [5-7] Pseudomonas sp. [8,9], Escherichia coli [10], Desulfovibrio sp. [11], Microbacterium sp. [12], Shewanella sp. [13], and Arthrobacter sp. [14].
The objective of this study was to isolate and characterize Cr (VI) reducing strains from contaminated soil, to evaluate their potential for the biotransformation of Cr (VI) to Cr (III).
2. Materials and Methods
2.1 Isolation of chromium reducing Bacteria
The effluent samples were collected under aseptic condition in sterilized bottles from electroplating industries. G.I.D.C. Ankleshwar dist. Bharuch, Gujarat. The samples used to isolate metal resistance bacterial strain and for further analysis within 1-2 hours of collection. Suspensions were made in 50ml autoclaved distilled water. Nutrient agar plates were prepared which were supplemented with 0.5mm K2CrO4. Different dilutions of samples were plated and incubated at 37 °C. Colonies obtained were picked and purified by many rounds of restreaking on Nutrient agar plates amended with 0.5mm K2CrO4. From this preliminary screening colonies showing resistance to chromium were selected for further studies. Slants were prepared from these isolated colonies and stored at 4°C. Chromium tolerance was checked by transferring morphologically different colonies on Nutrient agar plate amended with 0.5 to 25 mM of hexavalent chromium. One bacterial isolate (KKF), which could grow on plate containing 25mM chromium, was identified by biolog system. All further studies were done using KKF.
2.2 Evaluation of chromium resistance and effect of pH and temperature on growth kinetics of Chromium (VI) resistant bacterial isolate (KKF)
Chromium resistance of this strain was determined in the nutrient broth amended with 0, 0.5, 1, 1.5, and 2.0 mM K2CrO4. To determine the pH and temperature range of the isolate, nutrient broth was adjusted to different pH (5, 6, 7, 8 and 9) and temperature (24, 28, 32, 37 and 42°C).
2.3 Identification of isolated strain Morphological Characteristics Study
Morphological and colonical characteristics studies of chromium resistant bacterial isolates were carried out according to the method described by Norris and Ribbons [15]. KKF was characterized morphologically by Gram’s staining and colonical characteristics were determined on nutrient agar plate. The tests carried out were pertaining to form, arrangement, colour, size, shape, margin, elevation, texture, opacity, pigmentation, motility, and Gram’s reaction.
Biochemical Identification
Biochemical characteristics of the bacterial strain were studied using GP2 plate by biolog system (Biolog.inc.USA)
2.4 Chromate reduction Experiments using Chromium resistance bacterial Isolate (KKF)
Chromate reduction by resting cell assay, ermeablilized cell assay and cell free extract
Culture suspension of KKF was grown for overnight in 50ml Nutrient broth (pH-7) and harvested by centrifuge at 4000rpm for 10min at 4ºC. Cell pellets obtained on centrifugation were washed twice with 1ml 100mM potassium phosphate buffer (pH-7) and resuspended in same buffer. These cell suspensions spiked with 50, 100, 200, 300μm K2CrO4 solution adjusting final system volume to 1ml, vortexed for 1-2 min and incubated all tubes at 30ºC for 6hr. The tubes were centrifuged and remaining Cr (VI) is estimated from supernatant by 1,5-diphenyl carbazide (DPC) method [16]. Heat killed cells served as controls. Chromate reduction by permeabilized cell assay and cell free extract was performed as previously published protocol [16, 17].
Chromate reduction in industrial effluents
To check the efficiency of strain KKF to reduce hexavalent chromium present in the effluents, sample from an electroplating industry was collected in sterilized bottles. Chromium was estimated in effluent by inductive coupled plasma optical emission spectrophotometer (ICP-OES Perkin Elmer Optima 3300RL).Two different dilutions of effluent sample were used: sample 1 and sample 2 that contained 3 and 0.3mg ml-1 of Cr (VI), respectively. Culture was harvested after 24 h and the amount of chromium reduced was measured as described above method.
2.5 Treatment of industrial effluent using anaerobic packed bed reactor
Preparation of anaerobic packed bed bioreactor using immobilized cells of KKF
For biomass preparation, Strain KKF was grown in nutrient broth. After 24 h, cells were harvested by centrifugation at 8000 rpm for 15min and then pellets were dissolved in Tris-HCl buffer (pH-7). For each immobilized preparation, 2gm of biomass was entrapped in 100ml slurry. The preparations were obtained using Calcium alginate [18], where 4.5% sodium alginate was dissolved in Tris-HCl (pH-7) with constant stirring. At room temperature, 2gm of biomass was added in 100ml slurry under stirring condition for even dispersal. The slurry solution was dispersed drop wise into 4.5%. CaCl2. Instantaneous spherical gel beads formation occurred at the drop-solution interface as the alginate was cross-linked by Ca+2. The gel beads (3-4mm diameter) were allowed to cure for 2 hr at 4°C and were washed thoroughly with distilled water. These gel beads of KKF were packed in 500 ml polycarbonate column having internal diameter 9 centimeter this reactor was used for the treatment of effluent and remaining chromium was estimated by inductive coupled plasma- optical emission spectrophotometer (ICP-OES Perkin Elmer Optima 3300RL).
Effect of treated and untreated effluent on the seed germination of the Vigna radiata
The effect treated and untreated effluent on the seed germination of Vigna radiata plant was studied in laboratory under natural condition. After 3 days of sowing the seeds results for seed germination experiments were recorded. A similar type of experiment was settled for the control in parallel. Photographs were taken for each experiment.
3. Results and Discussion
3.1 Identification of isolated strain
The Chromium reducing bacterial strain KKF screened from the above mentioned site could tolerate up to 25mM chromate. Microscopic characterization showed the isolate to be non-spore forming non motile, gram positive cocci. Different substrate utilization by the strain KKF, showed a substrate utilization profile similar to related Vogococcus fluvialis.
3.2 Evaluation of chromium resistance,effect of pH and temperature on growth kinetics of chromate resistant bacterial isolate (KKF)
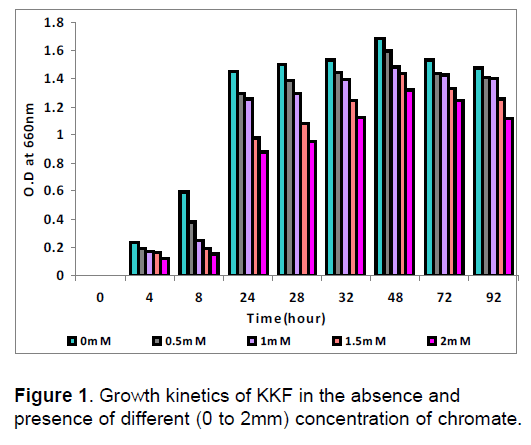
The growth curves of KKF at different concentrations of chromate revealed that the rate of growth decreased with the increase in chromate. As shown in Figure 1 lag phase was observed up to 8 hour in 0mM, 0.5mM, 1mM, 1.5mM and 2mM concentration followed by the log phase up to 24 hour of incubation period. The stationary phase was observed after 28 hours up to 48 hours, then growth declines at 92 hour of incubation period. KKF strain showed maximum growth at pH 7 and temperature 37ºC. KKF strains showed very high-level resistance against potassium chromate both in nutrient broth and on nutrient agar, which becomes the reason for the selection of this strain for future study. The chromate resistance level of the strain was more on nutrient agar as compared to the nutrient broth in the presence of the chromate. This might be due to the fact that in aqueous conditions the cells are more exposed to metal toxicity as compared to solid surface. According to Ganguli and Tripathi [9] the normal growth time of Pseudomonas aeroginosa A2Chr was 42 min but with the addition of 0.1 mg ml-1 of chromate increases germination time to 57 min.
3.3 Chromate reduction experiments using chromium resistance bacterial strain (KKF)
Chromate reduction by resting cell assay, permeabilized cell assay and cell free extract
Table 1 show that resting cells of the bacterium were expedient in reducing 50,100,200 and 300μM chromate concentrations in 6 hours at 37°C. The strains KKF showed maximum reduction in 50μM concentration followed by 100μM, 200μM and 300μM. Similar results were observed for permeabilized cells of bacterium. Resting and permeabilized cell assays provided the better evidence of the presence of an enzymatic chromate reduction mechanism in KKF as observed in previous findings by Megharaj et al. (2003)[14]. In majority of cases chromate reduction activity has been found to be associated with intracellular soluble fraction of the cells [19, 20].

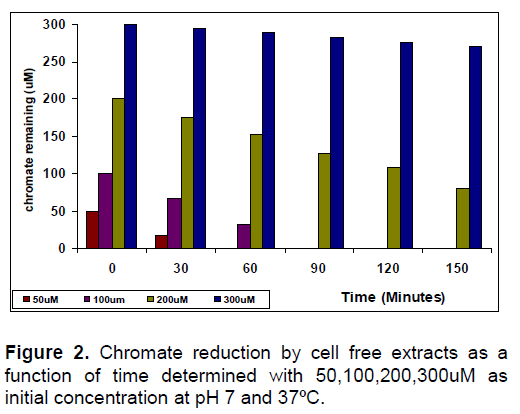
The localization of chromate reductase activity was made by performing the assays using the ultrasonicated sub-cellular fractions. Cytosolic fractions [20, 21] of the chromate reducing bacteria The chromate reduction by the cell free extracts as a function of time is shown in Figure 2; wherein, the cell-free extracts could reduce 100% of 50μM,100% of 100 μM ,60% of 200 μM chromate and 10% of 300 μM chromate in 150 min, suggesting an effective enzymatic mechanism of chromate reduction in the cytosolic fractions of the bacterium.These results confirm the presence of soluble enzymatic mechanism in the cytoplasmic fraction (crude cell-free extracts) of the strains. Heat-killed control of cell-free extracts of strains failed to reduce chromate.
Chromate reduction in industrial effluents
The strain KKF could reduce 90% of 3mg ml-1 and 92% of 0.3mg ml-1 of chromate in 6 hour. Table 2 shows immobilized cells of the KKF in packed reactor reduced chromate 91.96% and 91.26% in undiluted effluent and diluted effluent respectively in 6 hours at 37°C.

3.4 Effect of treated and untreated effluent on the growth of Vigna radiata
The seeds of Vigna radiata showed maximum growth in control but same seed showed poor growth with the undiluted and diluted industrial effluent. The significant effect was observed in seed germination of plant of Vigna radiata after treatment of same undiluted and diluted industrial effluent which suggests high hexavalent chromium concentration may affects growth of seeds of Vigna radiata. Figure 3 indicates seed germination was severely affected by the application of chromium salt. The effects of chromium salts were more severe as compared to control. Many workers also reported the adverse effect of chromium salts on germination [22-24]. Treated effluent significantly enhanced the germination when compared to non treated effluent.
4. Conclusions
A gram-positive cocci strain isolated in this study has shown a high efficiency in detoxifying dichromate by reducing it. These cells have a high potential to reduce chromate to its non toxic trivalent form and could tolerate a maximum of 25 mM chromate. Industrial effluent was treated with immobilized chromium resistant bacterial isolate strain KKF in packed reactor. This resulted in the reduction of 91.96% and 91.26 % in undiluted effluent and diluted effluent respectively in 6 hours at 37°C of chromate. It reflects the good efficiency of our designed packed bed reactor containing reducing capacity of KKF strain. The process of reduction is enzymatic and further understanding of the mechanism is in progress. Conventional technologies to clean up heavy metals ions from the contaminated waste have been utilized but these technologies are not cost effective and alternating to these more expensive reagents and system are the bioremediation method which are inexpensive and safe.
Acknowledgements
Authors are grateful to Charutar Vidya Mandal (CVM) Vallabh Vidyanagar, Gujarat for providing platform for this research work. We are also thankful to academic director Professor H.G Vyas and research director Dr.Pradip Patel of Ashok and Rita Patel Institute of Integrated Study & Research in Biotechnology and Allied Sciences (ARIBAS), for providing the facilities and their valuable suggestions during our research work. We owe reverence to Professor Datta Madamwar and Dr. Haresh Kahariya BRD School of biosciences, Sardar Patel University, for allowing us to utilize the facilities in their laboratories for a part of this research work.
References
- Martins B.L., C.C.V. Cruz A.S. Luna., et al. (2006) Sorption and desorption of Pb +2 ions by dead Sargassum sp. biomass. Biochemical Engineering Journal, 27(3): 310-314.
- Jalali R., H. Ghafourian, Y. Asef., et al. (2002) Remove and recovery of lead using non-living biomass of marine algae. Journal of hazardous Material, 92(3): 253-262.
- Raji C., T.S. Anirudhan. (1997) Chromium (VI) adsorption by saw dust: kinetics and equilibrium. Indian Journal of Chemical Technology, 4: 228-236.
- Faria P. C. C., J. J. M. Orfao., et al. (2004) Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Research, 38: 2043-2052.
- Campos J., Martinez-Pacheco M., Cervantes C. (1995) Hexavalent-chromium reduction by a chromate-resistant Bacillus sp. strain. Anton. Leeuw, 68: 203-208.
- Camargo F.A.O., Okeke B.C., et al. (2004) Hexavalent chromium reduction by immobilized cells and the cell-free extract of Bacillus sp. ES 29. J. Bioremed, 8(1–2): 23-30.
- Elangovan R., Abhipsa S., Rohit B., et al. (2006) Reduction of Cr (VI) by a Bacillus sp. Biotechnol. Lett, 28: 247-252.
- Mclean J., Beveridge T. J. (2001) Chromate reduction by a Pseudomonas isolated from a site contaminated with chromated copper arsenate. Appl. Environ. Microbiol, 67: 1076-1084.
- Ganguli A., Tripathi A. K. (2002) Bioremediation of toxic chromium from electroplating effluent by chromate-reducing Pseudomonas aeruginosa A2Chr in two bioreactors. Appl. Microbiol. Biotechnol, 58: 416-420.
- Bae W.C., Lee H.K., Choe Y.C. (2005) Purification and characterization of NADPH-dependent Cr (VI) reductase from Escherichia coli ATCC 33456. J. Microbiol, 43: 21-27.
- Mabbett A.N., Macaskie L.E. (2001) A novel isolate of Desulfovibrio sp. with enhanced ability to reduce Cr (VI). Biotechnol. Lett, 23: 683-687.
- Pattanapipitpaisal P., Brown N.L., Macaskie L.E. (2001) Chromate reduction and 16S rRNA identification of bacteria isolated from Cr (VI) contaminated site. Appl. Microbiol. Biotechn, 57: 257-261.
- Myers C.R., Carstens B.P., Antholine W.E. (2000) Chromium (VI) reductase activity is associated with the cytoplasmic membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Appl. Microbiol, 88: 98-106.
- Megharaj M., Avudainayagam S., Naidu R. (2003) Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr. Microbiol, 47: 51-54.
- Norris J.R., Ribbons D.W. (2004) Methods in microbiology, Academic Press. London.
- APHA (American Public Health Association), American water works associations (AWWA), Water environment (WFE). (1995) Standard methods for examination of water and waste water, Washington, D.C.
- Chirayu D; Kunal J; Datta M. (2008) Hexavalent Chromate reductase activity in cytosolic fractions of Pseudomoas sp. G1DM21 isolated from Cr(VI)
- contaminated industrial landfill. Process. Biochemistry, 43: 713-721.
- Asuncion lopez, Nuria Lazaro and Ana. M., Marques. (1997) The interphase technique a simple method of cell immobilization in gel beads. Journal of microbiological methods, 3: 231-234.
- Pal A., Dutta S., Paul A.K. (2005) Reduction of hexavalent chromium by cell free extract of Bacillus sphaericus AND 303 isolated from serpentine soil. Curr. Microbio., 51: 327-330.
- Elangovan R., Abhipsa S., Rohit B., Ligy P., Chandraraj K., (2006) Reduction of Cr (VI) by a Bacillus sp. Biotechnol. Letter, 28: 247-252.
- Bae W.C., Lee H.K., Choe Y.C., Jahng D.K., Lee S.H., Kim S.J et al. (2005) Purification and characterization of NADPH-dependent Cr(VI) reductase from Escherichia coli ATCC 33456. J. Microbiol, 43: 21-27.
- Hsu F., Chou C. H. (1992). Inhibitory effect of heavy metals on seeds germination and seedling growth of Miscanthus species. BotBul. Acad. Sch, 33(4): 335-342.
- Hasnain S., Sabri A. N. (1997) Growth stimulation of Triticum aestivum seedlings under Cr-Stressees by Non-Rhizospheric Pseudomonas strains. Environ. Pollut, 97: 265-273.
- Rout G. R., Samantary S., Das P. (2000) Effect of chromium and nicke on germination and growth in tolerant and non-tolerant population of Echinochloa colona, (L) Link. Chemosphere, 40: 855-859.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences