Tracking the Regenerative Growth in Earthworm: A Cellular and Molecular Perspective
Ray Subarna*,Carvalho Sweta, Hambarde Madhuri
DOI10.36648/1860-3122.21.17.197-202
Ray Subarna*, Carvalho Sweta, Hambarde Madhuri
Department of Zoology, St Xavier’s College, Autonomous, Mumbai, India
- *Corresponding Author:
- Ray Subarna
Department of Zoology
St Xavier’s College
Autonomous, Mumbai, India
E-mail: subarnaray5@gmail.com
Received: February 10, 2021; Accepted: April 19, 2021; Published: April 26, 2021
Citation: Subarna R, Sweta C, Madhuri H. Tracking the Regenerative Growth in Earthworm: a Cellular and Molecular Perspective. Electronic J Biol. Vol.17: 196-201.
Abstract
Regeneration in earthworm is studied vastly and proved to be an effective model to study regeneration. The process of regeneration is a stepwise and gradual process. The mechanism of regeneration was not clear earlier but it was deciphered later. Regenerative growth occurs firstly by wound healing followed by blastema formation. Blastema cells are majorly derived from the existing tissue specifically longitudinal muscle cells near the amputation site by dedifferentiation. Differentiation and pattern formation are the major processes in early development and organogenesis and these involve activation of various molecular signaling pathways. Neural factors are also activated along with other transcription factors for neural development. Segmentation and pigmentation occur at the last stage of development. Survival rate and body weight after amputation also has a correlation with regenerative growth. Genetic regulation and signaling controls the development and growth via various controlling genes such as distal less, notch. These genes are responsible for blastema formation, organogenesis and patterning. Pluripotent factors such as nano, regulate the dedifferentiation to form pluripotent stem cell for blastogenesis. Further studies are needed to study molecular mechanism for regulation in earthworm regeneration.
Keywords
Earthworm; Regeneration; Blastema; Gene regulation; Development
Introduction
Earthworms are classified under the phylum Annelida and they show a remarkable phenomenon of regeneration. A large variation in the regeneration is observed across the phylum Annelida. Leeches are categorized under the class Hirudinea and do not show the characteristic of regeneration [1].
Therefore, diversity in the regenerative mechanism exists in the phylum Annelida. Earthworms are often chosen and most preferred due to its easy handling, availability and segmental body, organized in three regions: clitellar and postclitellar [2]. The presence of segment provides a parameter to measure the regenerative growth. Regeneration is a gradual process and involves the formation of blastema at first. The formation of blastema is the characteristic of epimorphicre generation. The process of hemimorphic regeneration involves dedifferentiation and cell proliferation and provides a useful model to investigate the mechanism of normal development as well as differentiation [3]. Different tissue such as my fibers, satellite cells, and nerve cells contribute to blastema formation after amputation and proliferate near the wounded site [4,5].Dedifferentiation, a switch in cell type from fully differentiated to pluripotent status, is initiated by the rearrangement of extracellular matrix and integrins [6]. Earthworm regeneration is proposed to follow the epimorphotic type of regeneration where dedifferentiation leads to the formation of blastema cells and subsequent redifferentiation without any contribution from totipotent stem cells. Formation of blastema is crucial for the regeneration and subsequently restoration of the lost parts by differentiation as reported by various studies of early wound healing [7,8]. The course of blastema development and differentiation is well documented in a study by Park and described in detail [9].Studies reported that correlation of the survival rate and regeneration capacity of immature and adult indicating that higher the number of annuls retained in the amputated earthworm, the greater was the chances of survival and regeneration [10]. Have five identified pluripotent factor genes. The results suggested that the cells undergo reprograming during earthworm regeneration and dedifferentiation to form blastula.
Histological Analysis
Eisenia foetida has gained more importance for its rapid regenerative power, its survival rates are correlated with its regenerative capacity in both the immature and adult. A study relating to this correlation was performed by Xiao and suggested that the larger number of annules retained, greater is its rate of survival and regeneration. The process which follows amputation is wound healing at the amputation site. Tissue interaction occurs after wound healing where tissues such as nerve cord, epidermis and gut endoderm interact [11]. NE oblast cells are a particular type of cell found near the traumatized section as they are responsible to give rise to the regenerative blastema and form most of the missing parts [12]. Another study suggests that gut endoderm has a crucial role in the regeneration of the endoderm [13]. The origin of blastema in earthworm Eisenia andrei is still a debatable topic but histological evidences of tissue-level dynamics in support of dedifferentiation soon after amputation followed by redifferentiation in course of time was restricted to 1 and 3 day(s) after amputation [14].
Histological studies reveal the detailed trajectory of regeneration and the origin of blastema. From 1-day post amputation, neoblast cells appeared from the de-differentiating longitudinal muscle layer of the body wall facing the coelomic side. This observation was supported by the findings of Park et al [15].
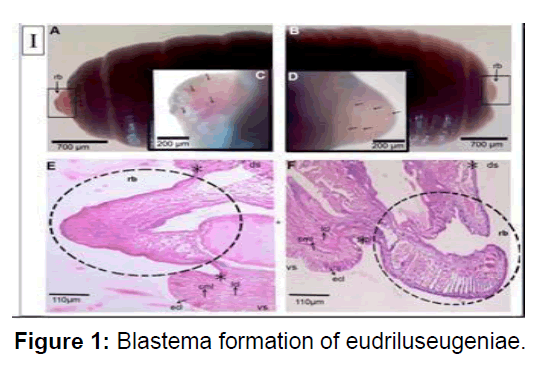
Longitudinal muscle layer was rebuilt from the dedifferentiated cell. Dedifferentiated also originate from the chloragogue tissue and intestinal tissue especially the basal cells of the typhlosole region. In contrast to the de-differentiated longitudinal muscle cells, the dedifferentiated chloragouge cells formed the blastema mass on the visceral side of the coelom. The noblest and chloragogue cells were found to accumulate in the coelomic space 5 days post amputation and occupies most of the space by 11 days post amputation. This blastema seems to act as a main nutrition reservoir for the regenerating tissue as most of the segmental structures viz. epithelium, circular and longitudinal muscle layers, wall of intestine have reformed by 11dpa. Amputees with vital organs at its anterior segment which represent pygidium. Longitudinal muscle-cells dedifferentiate possibly by losing intra cellular adhesion molecules and divide resulting into the separation of nucleated part and contractile part.Cell-tracking studies conducted on earthworm Lubricous, also confirm that old gut tissue contribute to new gut tissue [13]. debatable topic of the origin of blastema is not resolved but studies show that during regeneration in foetida the origin of neoblast cells are tissue restricted. The neoblast cells originates through proliferation of the de- differentiated muscle cells and the chloragogue cells in respective tissue layers. Furthermore, these neoblast cells were found to redifferentiate into newly formed tissues in the regenerating region of the amputated earthworm. There must be intricate cellular mechanism that controls the process of regeneration. Future investigations on model organisms may identify key molecules required to initiate and modulate the restorative events. Reports are available which suggest that nerve cord control play some cardinal role during annelid regeneration. In absence of the nerve cord at the severed region of amputated Eisenia, endodermal growth occurs but, ectodermal and mesodermal growths are inhibited (Figure 1).
A & B) Magnified (1) stereo zoom microscopical images of newly formed blastema in the worms amputated at the anterior and posterior parts, respectively. Arrow heads indicate the blastemas. C & D) Magnified (2×) stereo zoom microscopical images of unpigmented blastemal tissues showing a network of blood vessels in the worms amputated at anterior and posterior parts, respectively. Arrow heads mark the blood vessels of blastemas. E & F) Histological images (4×) of 3rd day blastema of the worms amputated at anterior and posterior parts, respectively. The sections were stained with hematoxylin and eosin. rb denotes regeneration blastema. Dotted circles indicate the blastemal region. The arrows indicate the ecl- epithelial cell layer, cml- circular muscle layer, lcl- longitudinal cell layer, ds- dorsal side, vs. ventral side, *- amputation site.
The gene expression varies across the region of regeneration i.e. genes are differentially expressed at different regions and at different time points. Direct comparison of the regenerating tissue with posterior end establishes the extent to which regeneration is completed by 30 days post amputation. In the tissue immediately anterior to the site of injury, less than hundred genes were significantly differentially expressed at any point during regeneration. In contrast, in the regenerating tissue, thousands of transcripts from a total of 71,341 transcripts were differentially expressed. The number of differentially expressed genes steadily reduced as regeneration proceeds, at later time points. Therefore, a number of genes are expressed at the early stages of regeneration by DNA replication, transcription and translation while in later stages, extra-cellular matrix remodelling is undertaken. Comparison between the regenerating tissue and adjacent tissue shows that majority of the genes are down regulated due to a reduced heterogeneity amongst the undifferentiated, rapidly proliferating cells in the regenerating tissue. The regenerating tissue, initially, is a homogenous mass of undifferentiated cells, wherein many genes appear down regulated because the corresponding cell type has not been formed. The giant extracellular hemoglobin of annelids has been studied extensively, for its exceptional oxygen carrier properties. Interestingly, the expression of the gene for giant extracellular haemoglobin of fetida is induced in the regenerating tissue at 15dpa and 20dpa.
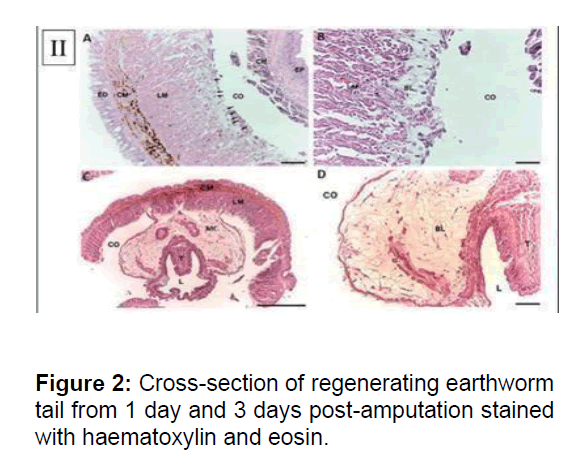
Neural regeneration involves a differential expression of neural genes. A strong induction of Nerve Growth Factor and the neurofilament gene, NF70 indicated ongoing neurogenesis. Earthworm anatomy shows a ventral nerve chord that connects the dorsal ganglia present in the anterior segments to the rest of the body. Cross section of the regenerated tissue offers a model for nerve regeneration. The annotation of the fetida transcriptome also comprises TrkB receptors, known to bind nerve growth factors. TRK1, encoding TrkB, is amongst the highly induced genes in the regenerating tissue. have previously studied the expression of orthologs of pituitary adenylate cyclase- activating polypeptide using radioimmunoassay and immunohistochemistry in fetida. Since these factors are neurotrophin in vertebrates, it was thought that their accumulation in the regenerating tissue of fetida was critical for its innervation. However, the sequences of these factors were not known and the authors relied on immunoreactivity to antibodies against orthologous human proteins (Figure 2).
(A) The dedifferentiating mesenchymal cells (arrows) were observed at the coelomic layer of the Longitudinal Muscle (LM) of body wall. Dedifferentiating cells had distinct single nucleus. No dedifferentiating cells appeared in Epidermis (ED), Circular Muscle (CM), Coelom (CO), Chloragogue (CH) tissue and epithelium of intestine. (X200). (B) Blastema cells showed typical characteristic of mesenchymal cells: large size, distinct nucleus, loosely packed and irregular shape. (X400). (C) Crosssection of regenerating earthworm tail from three days post-amputation stained with haematoxylin and eosin. Proliferating Mesenchymal Cells (MC) migrated towards coelom to form blastema. T: typhlosolar region and L: lumen of intestine. (X40). (D) The blastema cells progress and increase in cell population, migrating from the adjacent longitudinal muscle layer to the chloragogue tissue. (X200). Scale bars 100mm (B), 250mm (A, C and D)
Segmentation is another characteristic which is not immediately seen during blastema development but appears only after 3 weeks post amputation. Before that, a stark difference in the colour differentiates between the regenerated and the normal segments. At
26 days, segmentation of the regenerated tail was observed followed by rapid growth and pigmentation In addition, between 30 and 50 days post amputation, the intestine reopened and the worm were able to feed and excrete digested material. After 30 days post- amputation, the redifferentiation of blastemal cells was well advanced.Study suggests that normal development, respecification of body pattern preceded redifferentiation of blastema cells into each organ [9].segmentation or body pattern of an embryo is generally determined at a very early stage. Earthworm regeneration in general, therefore, provides a useful model to elucidate the molecular basis of dedifferentiation as well as body pattern formation of the annelid.
Relation of Survival Rate with Regeneration
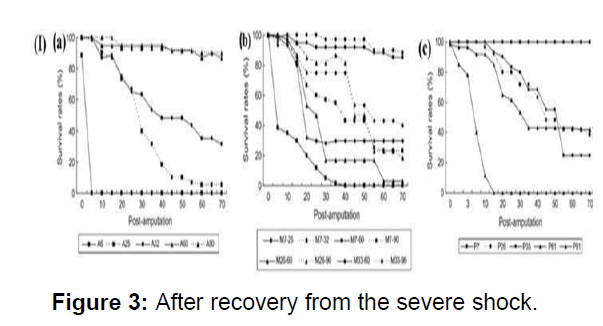
Earthworms are often encountered by various predators and external soil characters. It is therefore regeneration is important for the survival of the earthworm. The survival rate-time curves of earthworms for all treatments could be classified into three types. High survival rates with only a few changes in the process of regeneration, occurred when the numbers of segments remaining were more than half of the total number of segments, and they were amputated anterior Survival of earthworms with a number of segments of between one quarter to one half of the total numbered segments remaining after amputation, decreased gradually during the process of regeneration. Earthworms which had less than a quarter of total segments remaining had the lowest survival rate of all. Therefore, the survival rates of earthworm in response to amputation treatments depend on the number of segments remaining, and are much less related to the amputation position. The body weight is also considered as a parameter for the survival and subsequently regeneration (Figure 3).
(I)(a) Survival rates of anterior segments of immature earthworms after amputation from different segments (b) Survival rates of medial segments of immature earthworms (c) Survival rates of posterior segments of immature earthworms after amputation from different segments.
Genetic Control on Regeneration
Regeneration involved the redevelopment of various critical organs such as digestive organs, reproductive, nervous organs etc. This redevelopment of organs occurs via cell to cell communication and biosynthesis activity during the early phases of regeneration to induce dedifferentiation, regulate proliferation of pluripotent cells and specify the fate of these cells to reconstruct the missing organs. Transcriptase analysis showed that about 622 Expressed Sequence Tags(EST) were involved in regeneration which represents about 338 genes. By performing, the expression of three genes was recorded the highest in the regenerating segments during the initial phase of regeneration. Notch is an essential gene encoding a signaling receptor that is required throughout development to regulate the spatial patterning, timing, and fate of cells in both vertebrates and invertebrates. EGF like proteins which includes fibropellin, Notch receptors, and their ligands, such begins its expression initially post amputation and returns to a controlled level 12 hours post amputation. In the anterior regeneration of the earthworm, very early activation of a Notch-like receptor might be a regeneration-inducing pathway that precedes the regeneration process. DNA- binding proteins are important transcription regulators that can induce a developmental process in which undifferentiated cells become specific cell types. Distal less gene is one home box gene which plays a specific role in regeneration in various organisms such as in hydra, amphibian limb development etc. During anterior regeneration of the earthworm, the expression of Distal-less appeared to be up-regulated within the first few hours, and reached a distinct peak at 6 hours after amputation, followed by gradual decrease to the intact level which suggests it’s involvement in the regenerative processes during early anterior regeneration. Moreover, by various studies earthworm regeneration can be categorized into both i.e. the epimorphosis and morphallactic pattern of regeneration and forms the molecular basis of dedifferentiation as well as body pattern formation in annelids.
Sox2 gene is one such important gene which has a crucial role in cellular reprogramming in head, middle and tail regeneration.Sox2 encodes for a transcription factor which is well known for its role in maintaining pluripotent stem cell populations, as well as differentiation during early development.Sox2 facilitates conversion of adult differentiated cell types into pluripotent stem cells. Sox2 protein regulates the activity of other genes by attaching (binding) to specific regions of DNA. The expression of Sox2 was seen higher in the head and tail than in the midpiece at a phenotypic level. Similarly, at a protein level, Sox2 protein expression level is similar in the head and tail, while expression in the midpiece was lower. These results suggest that the expression of Sox2 gene promotes the blastogenesis and the regeneration. Retinoic acid interferes with the expression of Sox2 gene and regeneration of the anterior and posterior axis of foetida. This study can be treated as the confirmation for the role of Sox2 in regeneration. Extracellular matrix expression is also influenced by the various signaling factors very similar to Sox2 gene expression. The role of ECM is evident in the wound healing after the amputation process. The leucocytes migrate to the injured site and fuse with dedifferentiated cells responsible for blastema formation. In addition, collagen is one of the three components of ECM, and growth and differentiation factors regulate the expression of collagen genes for tissue reconstruction, damage repair and other genes are reported to be expressed during earthworm regeneration and the SOM cluster presents the same expression pattern as the Sox2 gene .Further, the SOM cluster can fuse genes with similar functions of the same or of adjacent classes. Thus, similar to the Sox2 gene, the collagen gene plays a decisive role in the formation of blastema during earthworm regeneration.
The process of regeneration in earthworms occurs via dedifferentiation to form pluripotent stem cells and involves the stem cell pluripotent factors such as octamer-binding transcription required for the maintenance of the early embryopluripotency,self- renewal and are central to the transcriptional regulatory hierarchy that governs pluripotency .myc gene family is an oncogenic transcription factor and plays an important role in maintaining the cell cycle and cell apoptosis in somatic cells and cancer cells.
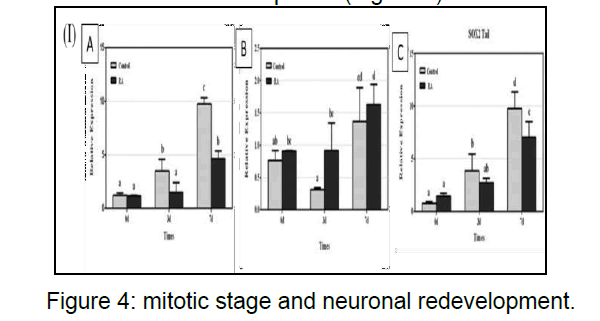
RNA-binding protein can alter developmental timing and heterochrony similar expression pattern and act synergistically during regeneration in earthworm translation or stability of mRNA during differentiation expressed during the initial phases of reprogramming during regeneration and its expression is down regulated after 3 days as the blasogenesis takes place within this timeframe. The cells are at mitotic stage and neuronal redevelopment (Figure 4).
(I)(A) Sox2 mRNA expression effects of head regeneration in the control and retinoic acid groups on days 0, 3, and 7. Sox2 mRNA expression was determined by real-time PCR and expressed as the mean ± SEM relative to basal conditions.(B)Sox2 mRNA expression effects on the sidepiece during regeneration in the control and retinoic acid groups on days 0, 3, and 7. Sox2 mRNA expression was determined by real time PCR and expressed as the mean ± SEM relative to basal conditions.(C) Sox2 mRNA expression effects during tail.
Conclusion
Earthworms have proved to be an excellent model to study regeneration. The mechanism of regeneration was not known yet but studies have found the pattern of regeneration which follows the epimorphosis and morphallactic pattern of regenerative growth. Wound healing is the first step after amputation followed by blastema formation. The blastema is formed by dedifferentiation of the chloragougecells. Histological studies reveal the detailed trajectory of regenerative growth. The contribution of longitudinal muscle cells is the most in blastema development. Starvation is also compensated by the action of bacterial activity during the initial few days of 5 days after amputation. The digestive tract is well developed and segmentation is observed. The initial hours after amputation are crucial for its survival and as a result there is high probability of bacterial infection in the natural habitat. Considering this action in a natural habitat, immune response for the infection becomes significant for the survival and growth and should be studied. Therefore, the gene expression profile could be studied by performing amputation at different sites. The genetic expression of two proximities i.e. prostomium and anus follows a different molecular signaling path and can be studied in detail about the expression levels along the entire length of the organism. Studies suggest that the cells undergo reprogramming during regeneration in earthworm. More studiesare needed to understand themolecularmechanism during regeneration in earthworm, which may be useful for us to understand how these pluripotency factors may work in the human network.
References
- Mazouri Karker S, Sandoval J, Regard S, Braillard O, Guessous I, et al. (2000) Telemedicine at the heart of the COVID-19 crisis. Rev Med Suisse 16: 1699-1702.
- Manaouil C (2020) Expertises in times of health crisis. Rev Legal Medicine 11: 49-53.
- Chen N, Zhou M, Dong X, Qu J, Gong F, et al. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395: 507- 513.
- MacLaren G, Fisher D, Brodie D (2020) Preparing for the Most Critically ill Patients with COVID-19: The potential role of extracorporeal membrane oxygenation. JAMA 323: 1245-6.
- Hekimian G, Frere C, Collet JP (2020) Covid-19 and circulatory assistance [Covid-19 and mechanical circulatory support]. Ann Cardiol Angeiol 69: 360-4
- Sobhi M, Jalel Z, Yasmine B, Faker G, Melek B, et al. La télémédecine et des réseaux sociaux dans la gestion des ECMO à l’ère de COVID-19: l’expérience Tunisienne Telemedicine and social Media in the management of ECMOs in the era of COVID-19: the Tunisian experience. Ann Cardiol Angeiol.
- Kortright KE, Chan BK, Koff JF, Turner PE (2019) Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 24: 219-232.
- Ratner V (2019) Interstitial cystitis/bladder pain syndrome research: the answer may be just around the corner. F1000Research 8: 972.
- Bastardot F, Vollenweider P, Marques Vidal P (2015) Social networks: new communication and training tools for physicians? Rev Med Switzerland 11: 2050-54.
- Wilkinson SE, Basto MY, Perovic G,Lawrentschuk N, Murphy DG (2015) The social media revolution is changing the conferenceexperience: Analytics and trends from eight international meetings. BJU Int 115: 839-846.
- Greysen SR, Chretien KC, Kind T, Young A, Gross CP (2012) Physician violations of online professionalism and disciplinary actions: A national survey of state medical boards. JAMA 307: 1141- 1142.
- Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM (1997) Interstitial Cystitis: Unexplainedassociations with other chronic disease and painsyndromes. Urology 5: 52-57.
- Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, et al. (2012) Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 186: 540- 4.
- Clemens JQ, Link CL, Eggers PW, Kusek JW, Nyberg LM, et al. (2007) Prevalence of painful bladder symptoms and effect on quality of life in black, Hispanic and white men and women. J Urol 177: 1390-4.
- Konkle KS, Berry SH, Elliott MN, Hilton L, Suttorp MJ, et al. (2012) Comparison of an interstitial cystitis/bladder pain syndrome clinical cohort with symptomatic community women from the RAND Interstitial Cystitis Epidemiology study. J Urol 187: 508-12.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences