Toxicological Evaluation of Aqueous and Acetone Extracts of Combretum molleTwigs in Wistar Rats
David Miaffo, Sylvie Léa Wansi, Marius Mbiantcha, Sylviane Laure Kamani Poualeu, Oulianovie Kamgue Guessom, Legentil Moïse Nchouwet, Albert Kamanyi
1Department of Life and Earth Sciences, Higher Teachers’ Training College, University of Maroua, Far North Region-Cameroon;
2Department of Animal Biology, Faculty of Sciences, University of Dschang, West Region-Cameroon.
- Corresponding Author:
- Tel: +237-670 30 08 07
E-mail: david_miaffo@yahoo.fr
Abstract
Combretum molle (Combretaceae) is used in folkloric medicine against pain, diabetes mellitus and microbial infections. In this study, toxicological profile of aqueous and acetone extracts of C. mo lle twigs was investigated. In acute toxicity test, female rats were divided into two groups and treated orally with distilled water (10 mL/kg) and single dose of aqueous extract (2000 mg/kg). Animals were observed during 14 days, during which toxic signs, mortality and body weight were evaluated. For sub - chronic toxicity, rats received respectively 0, 125, 250 and 500 mg/kg/day of each extract during 28 days. Body and organs weights, food and water intake, selected b iochemical, histological hematological a nd parameters were evaluated. Results showed that the acute toxicity study did not produce any grossly toxic signs, mortality and body weight changes. In the sub - chronic study, higher doses of both extracts caused significant loss in body weight, food and water intake and significant increase in transaminases levels. In increase in relative organs weight and a periportal inflammation and mesengial hyperplasia in the liver and kidneys were also observed in extract treated rats. An increase in platelet count and a decrease in proteinemia, creatininemia, MCH and MCHC levels were noticed with the aqueous extract while enlarged alveoli sacs were observed at 125 mg/kg of acetone extract. In conclusion, the aqueous extract did not exhibit acute toxicity then it app ears to be relatively non - toxic one. Prolonged administration of extracts caused hepatotoxicity and nephrotoxicity. Lower dose of acetone extract caused pulmonary toxicity
Keywords
Combretum molle twigs, toxicity, aqueous and acetone extracts, Wistar rat.
1. Introduction
The use of folk medicine for disease management is increasing globally, owing to their easy access, low cost, least risk and low side effects profile. About 80% of the world population today, relies on herbal medicines to meet their health needs [1]. However, most of the plants are used indiscriminately without adequate information on toxicity risks. Therefore, it has become imperative to assess the safety of plants used for medicinal purposes for possible toxicity [2].
Combretum molle is one of the herbal plants belonging to the family Combretaceae which includes 20 genera and about 600 species. It is a tree with large, straighter pole than most species of Combretum, distinguished by its rough bark and dense crown [3]. C. molle is distributed especially in savannah vegetation that cuts across from Senegal to West Cameroon, but generally exists in tropical and subtropical Africa regions [4]. Almost every part of these plants roots, leaves, seeds, twigs, and stem barks is used in African traditional medicine for the treatment of various ailments and diseases. In fact, decoctions of the roots of C. molle seem to have a variety of uses against hookworm, stomach pains, snake bite, leprosy, fever, dysentery, general body swellings, and abortion as well as for swelling of the abdomen, sterility and constipation [5]. Some traditional health practitioners of South Africa have also claimed that decoctions, infusions and other extracts of C. molle are effective remedies for the management and/or control of an array of human ailments, including arthritic and other inflammatory conditions [6]. C. molle like other Combretum species has also been reported to possess antifungal, antimicrobial, antiparasitic, and antioxidant activity, as well as anti-HIV type 1 reverse transcriptase [7,8,9]. Studies on its antidiabetic properties have produced promising results [10,11].
The previous toxicological studies showed that the aqueous leaf extract of C. molle is moderately toxic when given intraperitoneally [12]. Moreover, the histopathological findings on tissue sections revealed evidences of toxicity especially in experimental animals treated with stem bark methanol extract at 2000 mg/kg [13]. Despite the widespread use of the twigs of this plant in Cameroon herbal medicine, no study on its toxic effects has been reported. This study is based on the acute and sub-chronic toxicity of aqueous and acetone extracts of C. molle twigs in rats, with the aim to obtain informations on the safety of this plant and provide guidance for selecting a safe dose in its use in traditional medicine.
2. Materials and Methods
2.1 Collection and authentication of the plant material
Fresh twigs of C. molle were collected on December 2012 in Moutourwa, Maroua city of Far north region, Cameroon and authenticated in the National Herbarium of Yaoundé, Cameroon, where voucher specimen was deposited. The plant material was then washed thoroughly with water, shade-dried and milled to coarse powder using a mechanical grinder.
2.2 Preparation of plant extracts
Two hundred grams (200 g) of the aqueous extract powder mixed with 500 mL of distilled water were boiled for 15 min and then cooled for 15 min. Afterwards, the mixture was filtered using Whatman filter paper No.1 and the filtrate obtained was dried at a temperature of 45 ºC to produce 19.69 g of the crude aqueous extract, with a yield of 9.64 %.
Two hundred grams (200 g) of acetone extract powder were soaked in 1000 mL of acetone for 72 hours in cold condition. The whole mixture was successively filtered as previously. The filtrate obtained was evaporated at 80°C using rotary evaporator to obtain 14.12 g of the crude acetone extract, representing a yield of 7.48 %.
2.3 Animals
Nulliparous and non-pregnant healthy albinos female rats aged 8-10 weeks and weighing between 120-140 g were used for acute toxicity test and 8-10 weeks old Wistar rats of either sex weighing 120-140 g were used for sub-chronic toxicity test. The rats were obtained in the animal house of the Department of Animal Biology, Faculty of Science, University of Dschang, Cameroon. They were housed in polypropylene cages and maintained in ambient temperature of 24 ± 1°C, relative humidity of 55-65% and normal light/dark cycle. The rats were fed with standard laboratory feed and allowed free access to clean drinking water. Prior to the study, they were acclimatized to laboratory conditions for seven consecutive days. Animal treatment and handling were done according to the standard ethical guidelines [14].
2.4 Acute toxicity test
The acute toxicity study was conducted as per the Organization for Economic Co-operation and Development guidelines (OECD) 425 [15]. Ten female rats were divided in two groups of 5 animals each and fasted for 6 h with free access to water only. Animals of first and second groups respectively received distilled water (10 mL/kg) and a single oral dose (2000 mg/kg) of aqueous extract. After administration, rats were observed after 30 min, 4 h, 24 h and 48 h for signs and symptoms such as changes in skin and fur, eyes, behavior pattern, tremors, salivation, lethargy, sleep, respiratory pattern, convulsion, diarrhea, coma and death. The rats were weighed daily during 14 days following treatment. At the end of this period, animals were sacrificed under diazepam/ketamine anesthesia, i.p. The liver, kidneys, heart, pancreas, lungs, and spleen were immediately removed and weighted. The toxicological effect was assessed on the basis of mortality, which was expressed as LD50 and calculated using the limit test dose, up and down procedure of OECD [15].
2.5 Sub-chronic toxicity study
A total of 48 rats were randomly grouped into 8 groups, which include 3 males and 3 females in each group. Animals in groups I and II served as controls and received distilled water (10 mL/kg) and DMSO 5 % (10 mL/kg) respectively. Animals in groups III, IV and V were treated for 28 days with 125, 250 and 500 mg/kg body weight of aqueous extract, respectively. Animals in groups VI, VII and VIII were treated for 28 days with 125, 250 and 500 mg/kg body weight of acetone extract, respectively.
2.6 Determination of body weight, food and water consumption
The body weight of each rat was recorded on a weekly basis. The amount of food and water consumed was also measured weekly. Briefly, the weight of weekly feed supply and amount remaining by the following week were recorded and the difference was taken as the weekly feed intake. Similar procedure was adopted for the determination of the volume of water consumed.
2.7 Collection of vital organs, urine and blood samples
Twenty four hour urine sample were collected at the end of 28 days treatment and used for creatinine analysis. Animals were then weighted before being anaesthetized in diazepam/ketamine i.p. The abdominal cavity was quickly cleared of fur to expose the abdominal artery. The artery after being slightly displaced was sharply cut with sterile surgical blade. Blood was collected from each animal by catheterization method. Part of the blood sample was put into heparinized tubes and analyzed immediately for the hematological analysis. The remaining blood was put in non-heparinized test tubes, left for 15 minutes at 37°C for serum separation, centrifuged at 3000 rpm for 20 minutes, and then sera were carefully aspirated with Pasteur pipette and kept in eppendorf tubes at -20°C for biochemical analysis.
The rats were further dissected and the liver, kidneys, heart, lungs, and spleen were immediately removed, washed, blotted with clean tissue paper and weighed to determine the relative organ weights. Portions of the liver samples were also crushed in the mortar, and then homogenized with phosphate buffer (85 mL/15 g of organ). The resulting crude homogenates were put in centrifuge tubes and centrifuged at 4900 rpm for 20 minutes. The supernatant was assayed for proteins and transaminases.
2.8 Hematological and biochemical parameters
The Automated Hematologic Analyzer (Sysmex,KX-21,Japan) was used to analyze the hematological parameters such as red blood cells (RBC), hemoglobin (Hb), hematocrit (HCT), packed cell volume (PCV), Mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell width (RDW), white blood cell (WBC), platelet distribution width (PDW), mean platelet volume (MPV), platelets (PLT), lymphocytes, monocytes and granulocytes.
Total protein concentration of serum and supernatant of the liver was determined by the method of Biuret as described in Chronolab [16]. Alanine amino transferase (ALT) and aspatate amino transferase (AST) of serum and supernatant of the liver were measured using enzymatic method described in Chronolab [17,18]. Urine and serum creatinine concentrations were determined using alkaline picrate method described by Jaffe [19]. The Renal clearance (CR) was then calculated using the formula [20]:
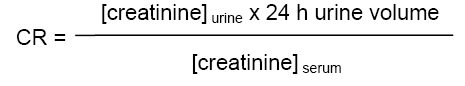
2.9 Histopathological examination
The liver, kidney and lung samples collected from the experimental animals of each group were fixed immediately in 10% buffer neutral formalin for 48 hours and washed in running water. The fixed tissues were dehydrated by serial ethanol solution and embedded with paraffin. The micrometer sections were cut by a microtome in 3-5 μm slices and stained with haematoxylin and eosin. The slides were examined under a light microscope for microscopic assessment [21].
2.10 Statistical analysis
Statistical analysis was performed using the statistical functions of the Graph pad Prism version 4.1. All the results were expressed as mean ± SEM. The significance of difference between mean values for the various treatments were tested using one-way analysis of variance (ANOVA) followed by Turkey test and two-way analysis of variance followed by Bonferroni test. Statistical significance was considered at p < 0.05; p < 0.01 and p < 0.001.
3. Results and discussion
In the acute toxicity assessment, an administration of the aqueous twigs extract of C. molle at a single dose of 2000 mg/kg did not cause mortality during 14 days of observation, which indicates that the mean lethal dose (LD50) of the extract is greater than 2000 mg/kg. None overt toxic signs was observed in rats except a mild reduced reaction of aggressivity, mobility, sensibilities to touch, to noise and to the pain which was observed during the first 30 minutes after gavage but disappeared afterward. Theses noticed behavioral changes were due to the fact that the extract may have depressant effect on the central nervous system [22]. Concurrently, no noticeable significant difference in the body weight of treated rats was shown after 14 days treatment at 2000 mg/kg as compared with control (Table 1).
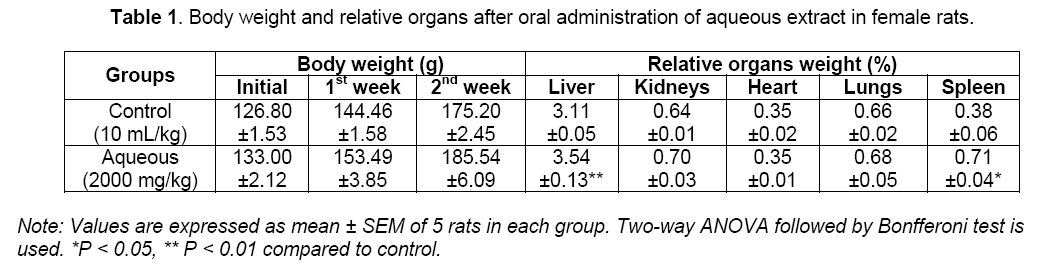
The relative weight of the liver and spleen were significantly (P < 0.01 and P < 0.05) increased in the group treated with 2000 mg/kg of the aqueous extract of C. molle, whereas that of the other organs remained unchanged (Table 1). This indicated that it did not have any adverse effect on the body weight which is used to assess the response to the therapy of drugs and adverse effects of the drugs [23].
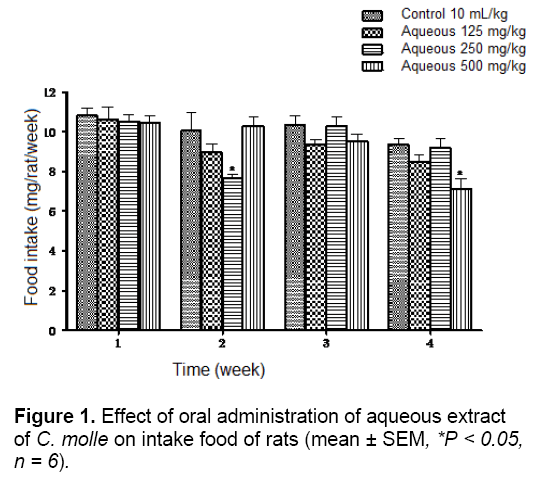
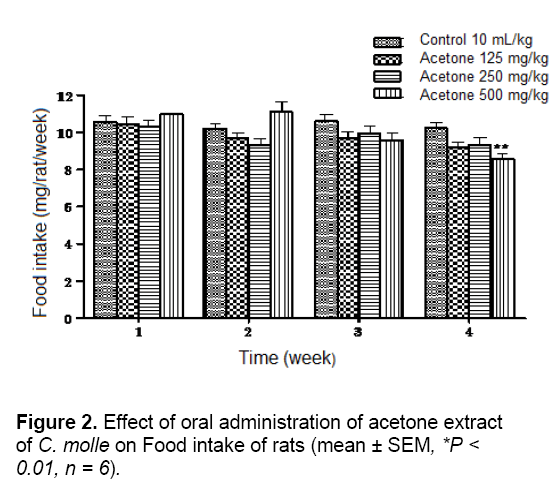
In chronic toxicity study, measurement of the weekly food intake in rats over the whole experimental period showed no significant difference between all the treated groups and theirs corresponding control groups at weeks 1 and 3 for aqueous extract and at weeks 1, 2 and 3 for acetone extract. However, rats receiving 250 and 500 mg/kg of aqueous extract and the highest dose of the acetone extract had significantly lower food intake than their corresponding control groups at week 2 and 4 in the aqueous extract groups (P < 0.05) and at week 2 in the acetone extract groups (P < 0.01), respectively (Figures 1 and 2).
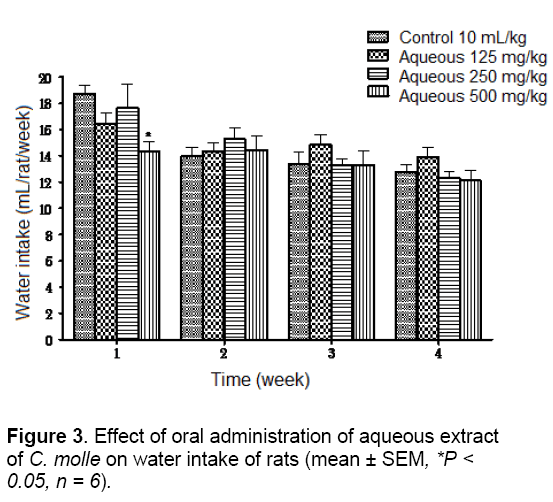
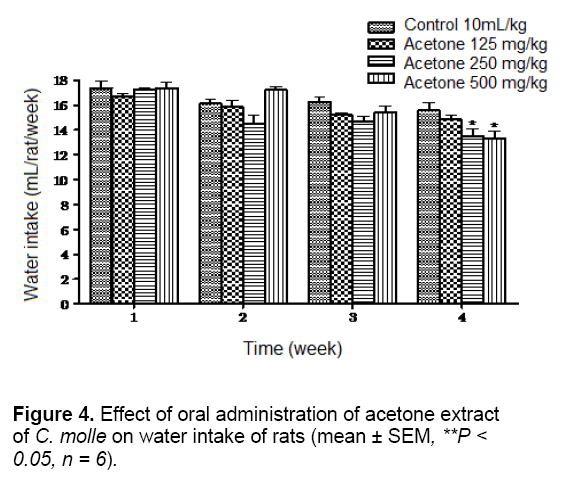
The water intake of the experimental animals was also compared with that of the control animals which had no significant change, except the animals receiving the highest doses of both extracts (500 mg/kg) which have significantly (P < 0.05) lowered average water intake than their control groups at week 1 for aqueous extract and at weeks 3 and 4 for acetone extract (Figures 3 and 4).
Water is an essential nutrient to every life since it is a most important nutrient for growth and development. Any factor influencing water intake will also affect feed consumption [24]. Therefore, it could not be stated that extracts caused probably decreased the appetite of the animals and will have consequential effects on their performances.
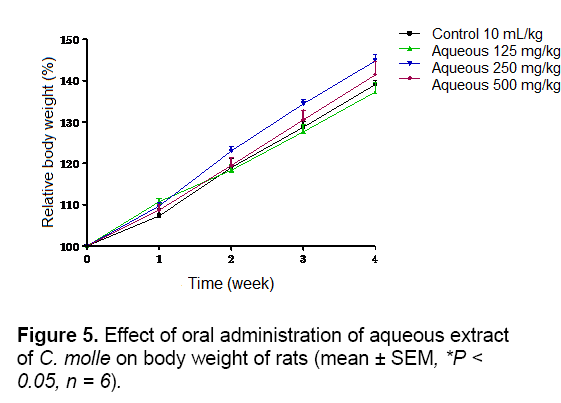
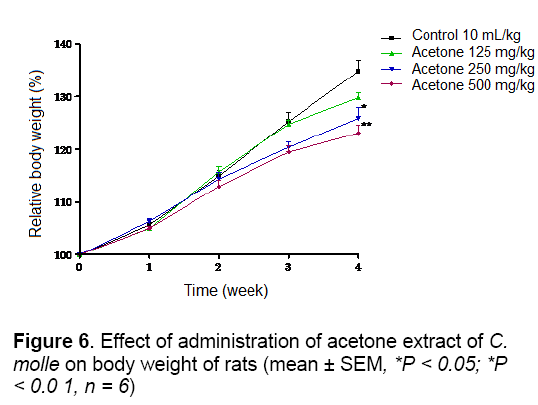
As shown in Figures 5 and 6, no significant difference in body weight gain was observed between control and aqueous treated groups during 4 weeks of observation. The same result was obtained with acetone extract group except the animals treated at doses of 250 and 500 mg/kg which showed a significant (P < 0.05 and P < 0.01) decrease of body weight at week 4, respectively According to report, reduction in body and internal organs weights are considered sensitive indices of toxicity after exposure to toxic substance [25]. In this study, the suppression of body weight gain may very well be as a result of decreased appetite and thereby lower caloric intake by the animals. Kim et al. [26] demonstrated that rat fed with Korean red ginseng containing saponins had retarded growth. Therefore, the result of poorer body weight might partially be due to the effects of saponins and tannins presents in this extract [11].
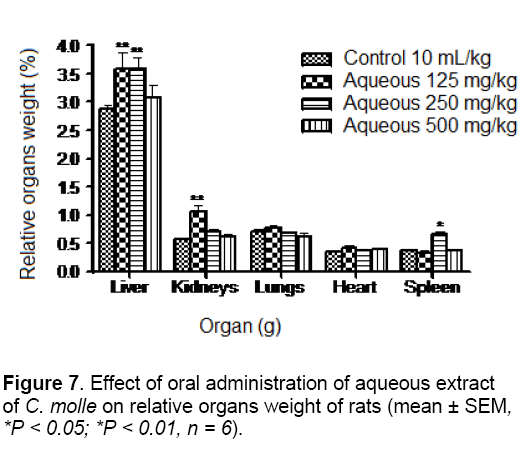
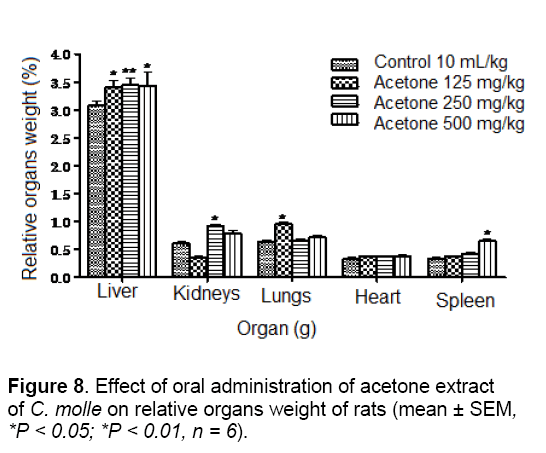
The results of organs weight are summarized in Figures 7 and 8. In fact, the relative body weight of the liver was significantly (p < 0.01) increased in the group treated with 125 and 250 mg/kg body of the aqueous extract. The weights of kidneys and spleen were also significantly elevated (p < 0.01 and p < 0.05) in the groups treated with 125 and 250 mg/kg of the aqueous extract, respectively. However, in comparison to control group, animals treated with acetone extract at doses of 125, 250 and 500 mg/kg showed a significant (P < 0.01; P < 0.05 and P < 0.01) increase in the weight of liver. Furthermore, the weights of kidneys, lungs and spleen were significantly (P < 0.05) increase in rats receiving the same extract at doses of 250, 125 and 500 mg/kg, respectively. Organ body weight ratio is an indication of atrophy, swelling and hypertrophy or inflammation [27].
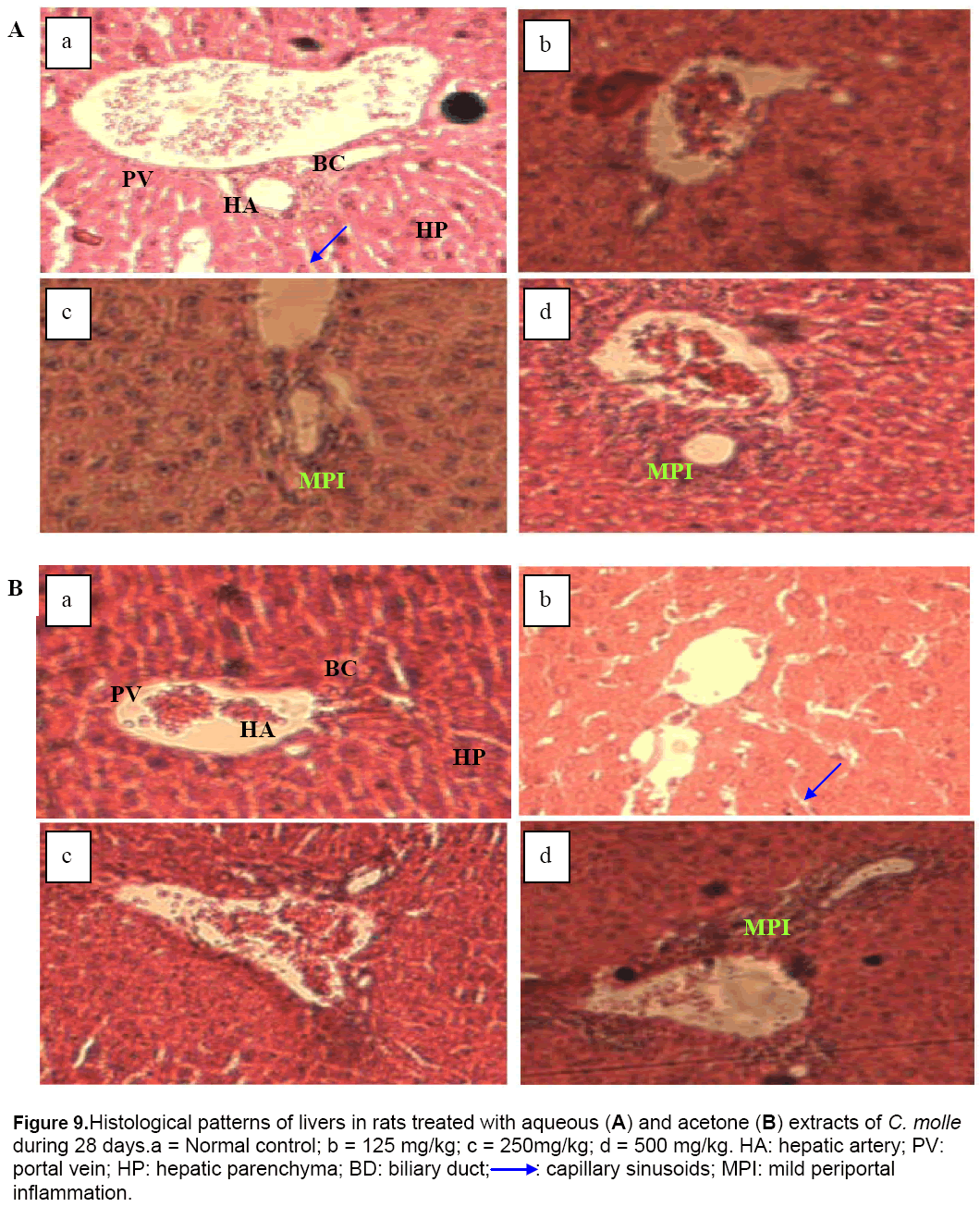
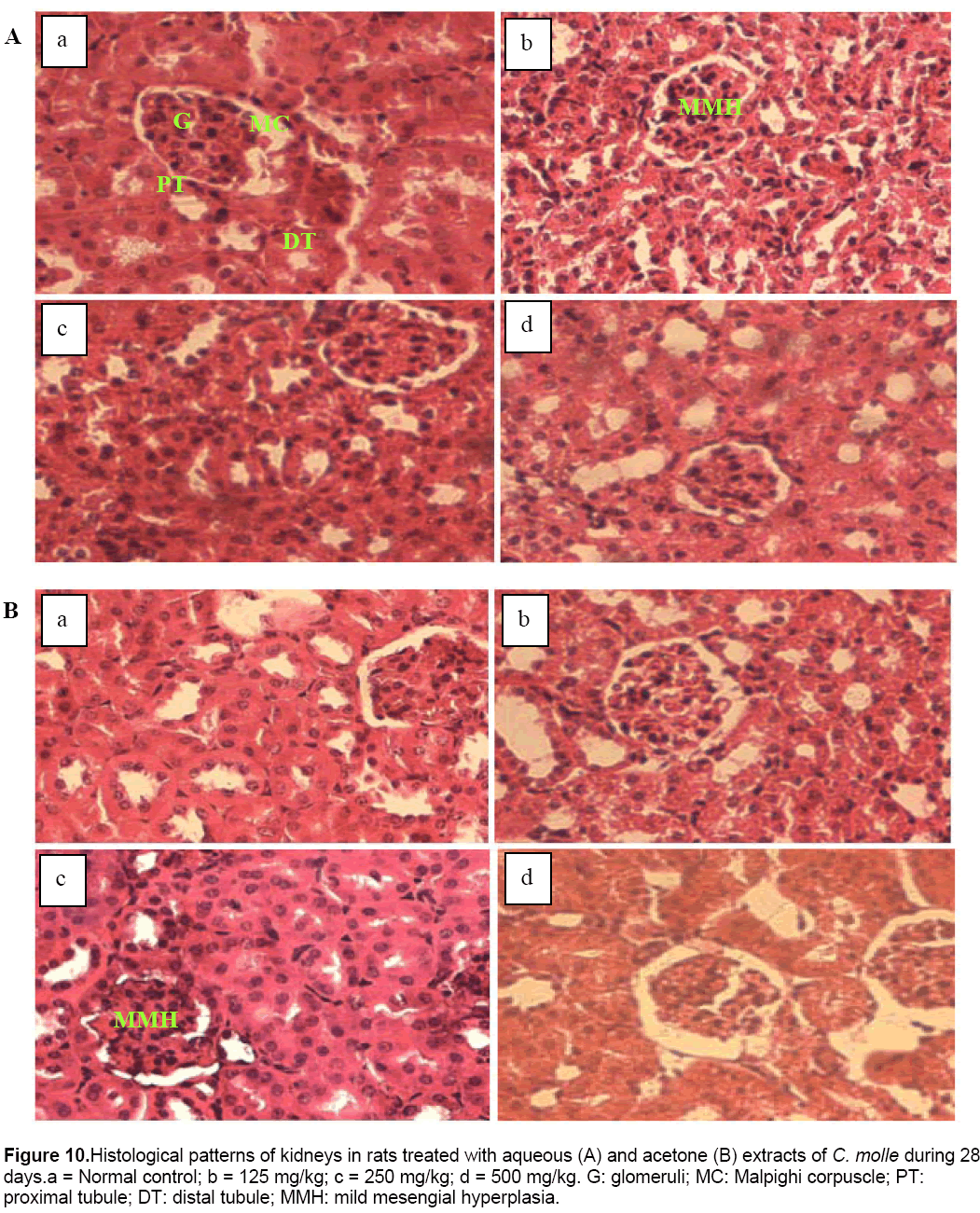
In this study, significant increase in relative liver, kidneys, lungs and spleen weights may be due to increase in the functional ability of the organ [28] and thus, may suggest hypertrophy. This finding was confirmed by the histological study in which architecture tissues showed a mild inflammatory infiltration and mesengial hyperplasia in the liver (Figure 9) and kidneys (Figure 10) of treated rats, respectively.
Figure 9: Histological patterns of livers in rats treated with aqueous (A) and acetone (B) extracts of C. molle during 28 days.a = Normal control; b = 125 mg/kg; c = 250mg/kg; d = 500 mg/kg. HA: hepatic artery; PV: portal vein; HP: hepatic parenchyma; BD: biliary duct;➝ : capillary sinusoids; MPI: mild periportal inflammation.
Figure 10: Histological patterns of kidneys in rats treated with aqueous (A) and acetone (B) extracts of C. molle during 28 days.a = Normal control; b = 125 mg/kg; c = 250 mg/kg; d = 500 mg/kg. G: glomeruli; MC: Malpighi corpuscle; PT: proximal tubule; DT: distal tubule; MMH: mild mesengial hyperplasia.
Tables 2 and 3 show the results of biochemical evaluations. Biochemical tests showed no significant differences except hepatic AST (p < 0.05 and p < 0.01) and ALT (p < 0.01) concentrations that increased significantly in rats groups treated at doses of 125, 250 and 500 mg/kg, and serum AST concentration that increased significantly (p < 0.01 and p < 0.001) in rats receiving 250 and 500 mg/kg of aqueous extract, respectively, as compared to control group (Table 2). In the same way, the rats treated with the highest dose of the acetone extract showed a significant (p < 0.05) increase in hepatic level of ALT (Table 3). The hepatotoxicity of the plant extracts is supported by marked elevation of serum transaminases (ALT and AST) which are good indicators of liver function [29]. This may be due to the fact that the liver is the first target of acute toxicity because it is the first organ exposed to everything that is absorbed in the small intestine. Furthermore, the liver metabolizes foreign substances to compounds which may be hepatotoxic [30]. In this study, appreciable increase in serum ALT and ASAT levels observed was confirmed by the histological study in which tissue architecture showed a marked mild inflammatory infiltration in the liver of animals treated with aqueous (250 and 500 mg/kg) and acetone (500 mg/kg) extracts (Figure 9).
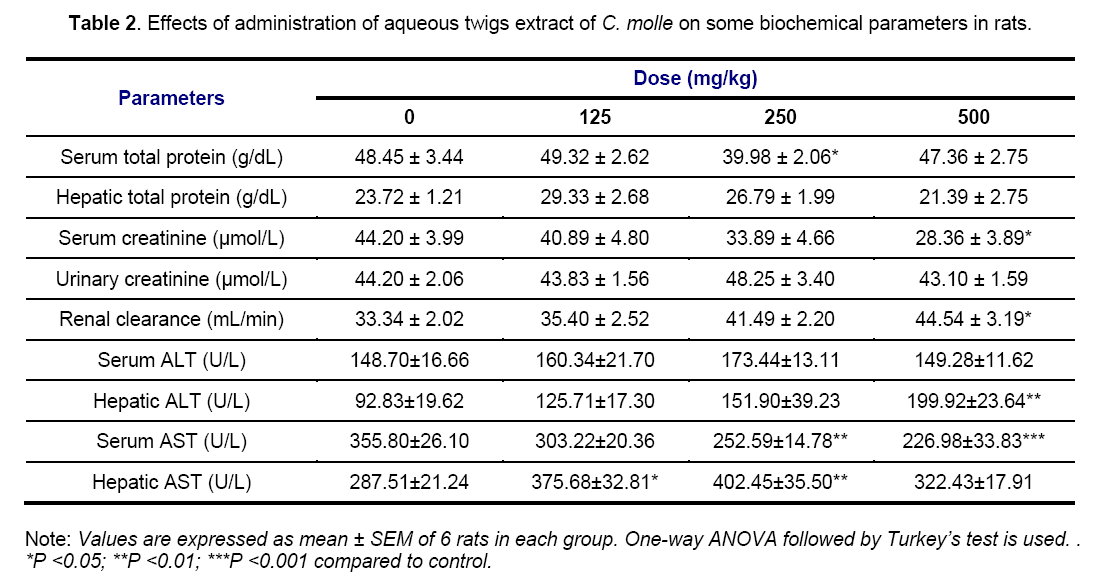
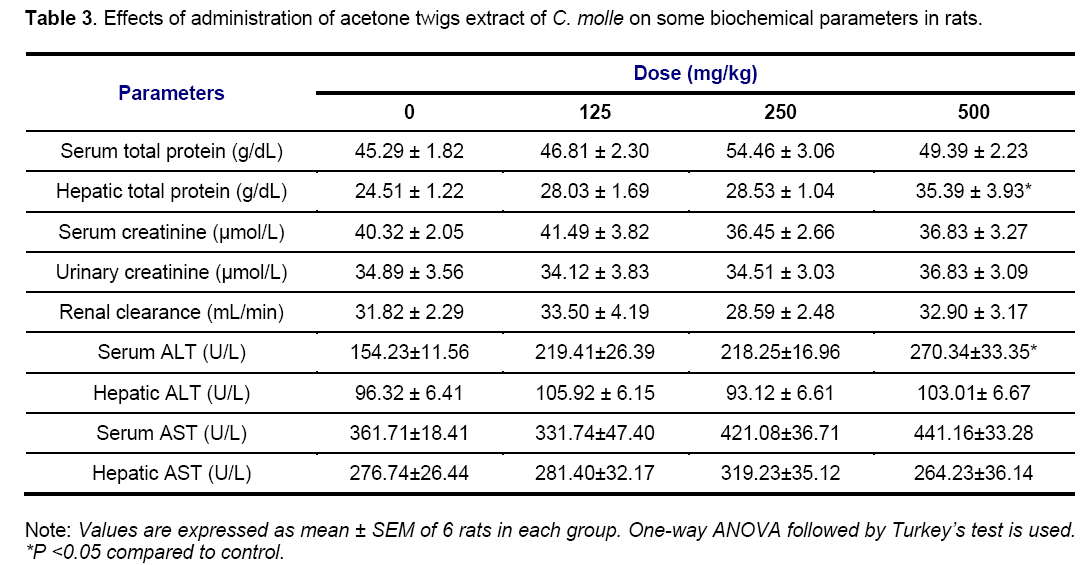
Serum concentration of total protein and creatinine was significantly (p < 0.05) decreased in animals groups treated at 250 and 500 mg/kg of aqueous extract. However, serum level of total protein of experimental rats receiving 125 and 250 mg/kg of the acetone extract is not significantly different from that obtained from the control group while rats treated with the highest dose (500 mg/kg) of the same extract showed a significant (p < 0.05) increase in hepatic level of total protein. Estimation of the total protein is one of the most widely used means of measuring hepatocellular injury. Total protein measurements can reflect nutritional status and may be used to screen for and help diagnose liver disease and many other conditions [31]. In the present study, the non-significant effect of the acetone extract on total protein in the serum could imply that the synthetic and secretory functions of the liver with respect to these proteins were not affected. Furthermore, possible causes of the decrease in serum total protein observed may be decreased protein synthesis caused by hepatic insufficiency and/or increased protein catabolism. Marzo et al [32] reported that chicken fed with tannic acid added diet exhibited a marked increase in the activities of liver which suggested the increase in protein catabolism. Thus, the tannin in C. molle extracts, at least in part, account for this finding. High total protein level may be seen with chronic inflammation or liver infections [33]. Increase in the hepatic total protein obtained in this study as well would have indicated hepatocyte damage.
Serum concentration of creatinine was significantly (p < 0.05) decreased in animals groups treated at 250 and 500 mg/kg of aqueous extract while the renal clearance of rats treated with the highest dose of the same extract was significantly (p < 0.05) increased as compared to control (Table 2). However, serum level of creatinine and renal clearance of all animals treated with the acetone extract are not significantly different from that obtained from the control group (Table 3). Serum urea, uric acid, creatinine and renal clearance are markers of damage to renal function [34]. The increase of these parameters in the blood is associated with decreased renal function and increased tissue breakdown. In this case, the normal level of serum and urinary creatinine, and renal clearance indicated that the acetone extract did not affect renal function.
Moreover, the significant decrease in serum creatinine coupled with the significant increase in renal clearance noticed in the present study, showed that the higher dose of acetone extract could partly prevent kidney damage. This finding suggests that the cause of the hyperplasia observed at doses of 125 mg/kg and 250 mg/kg of aqueous and acetone extracts respectively (Figure 10), was not due to the serum creatinine level but to the others markers of renal function not ascertained in this study.
Results of the Tables 4 and 5 did not showed any significant variations of all hematological parameters investigated except rats receiving aqueous extract which produced a significant (p < 0.001) increase in PLT count and a significant drop in MCH and MCHC levels at 125 (p < 0.05), 250 (p < 0.01) and 500 mg/kg (p < 0.05; p < 0.01) of aqueous extract, respectively. Hematological parameters Assessment can not only be used to determine the extent of deleterious effect of extracts on the blood of an animal, but it can also be used to explain blood relating functions of a plant extract or its products [35].
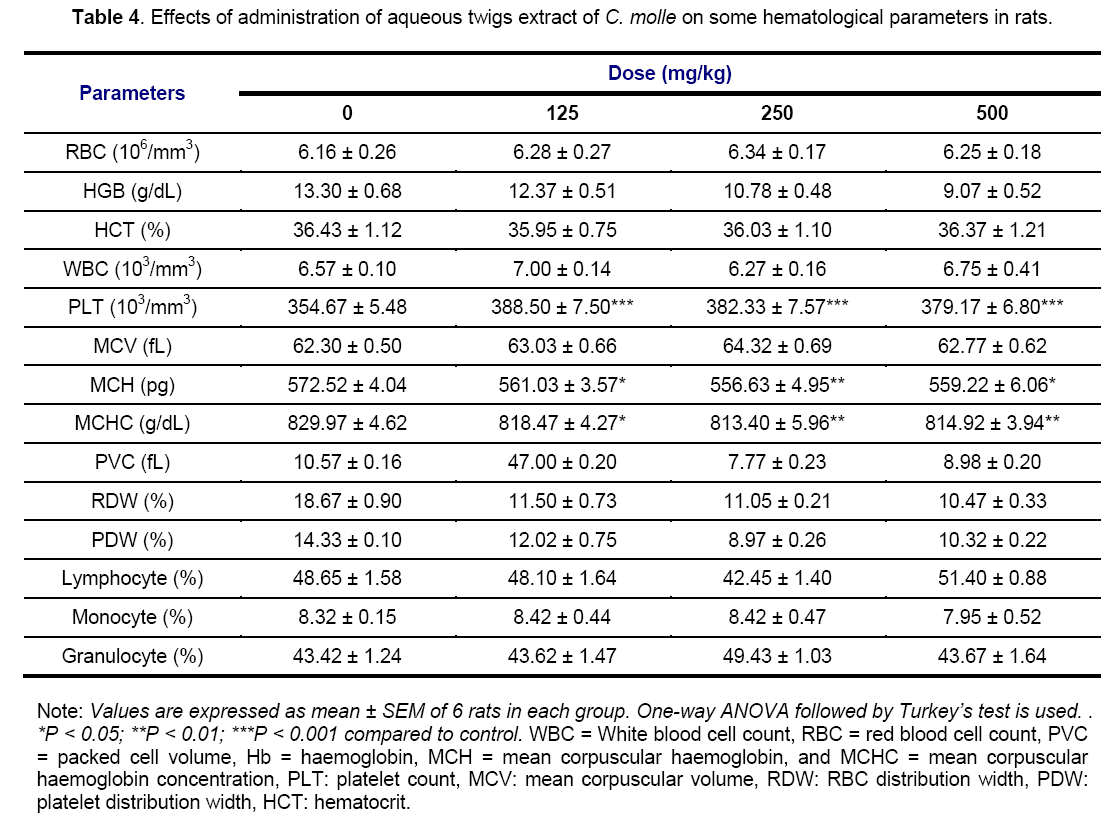
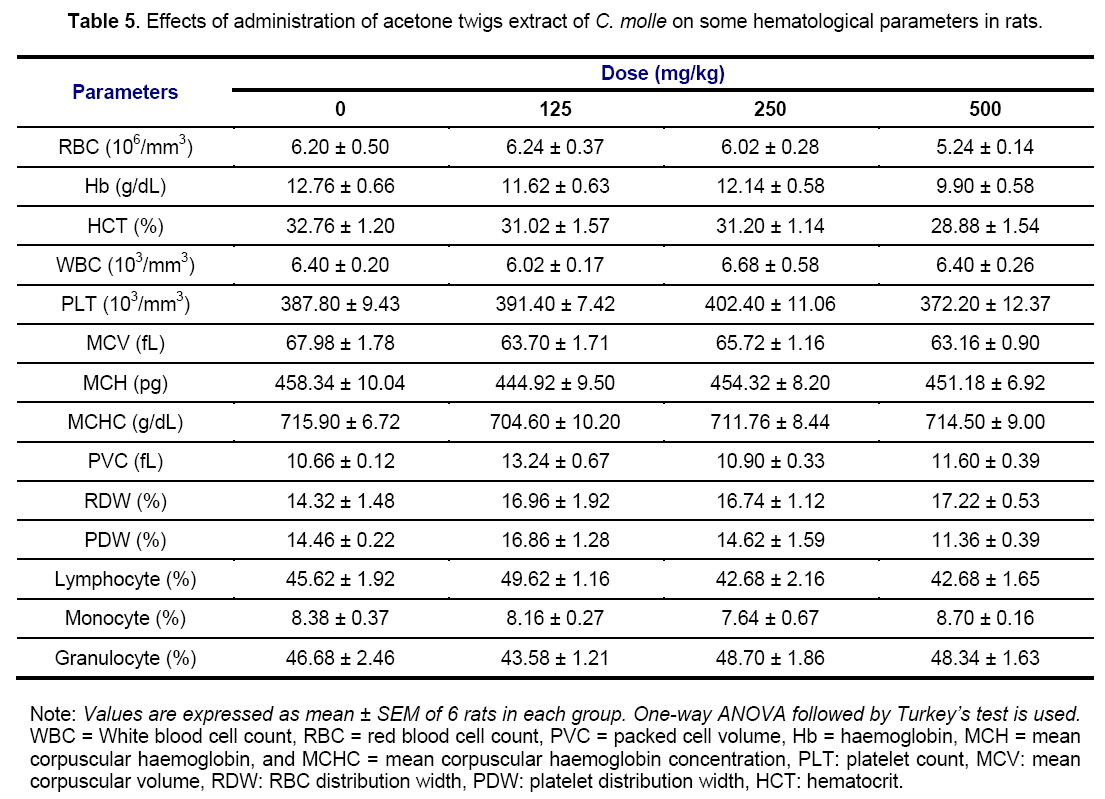
Generally, the non-significant variations of these previous elements imply that C. molle extracts would not have had toxic effects on the blood cells, hematopoiesis or leucopoiesis and bone marrow [27]. The non significant effect on the RBC, HCT, PCV and HGB implies that there was no change in the oxygen carrying capacity of the blood and amount of oxygen delivered to the tissues following the extracts administration since RBC and HGB are very important in transferring respiratory gases [36]. The calculated MCHC have a particular importance in anemia diagnosis or adverse effects on normal erythrocytes [37]. In the present study, the non-significant effects on these indices relating to RBC suggest that acetone extract does not possess any potential of inducing anemia throughout the 28 days period of administration. On the contrary, a significant decrease in MCH and MCHC level shows that the aqueous extract have the potential to stimulate erythropoietin release in the kidney, which is the humoral regulator of RBC production. WBC, lymphocytes, monocytes and granulocytes are mediators of immunity and contribute to immune-protection against inflammation [38]. The levels of these cells were the same in various both treated and normal groups, showing that drugs did not cause inflammatory changes in these groups. This result suggests that inflammatory changes observed in liver and kidneys sections (Figures 9 and 10) were not related to these hematological cells that participates in reducing blood loss, repairing of vascular injury and are also considered as an acute phase reactant to infection or inflammation [38]. Therefore, the increase in PLT count in this study suggests that the aqueous extract also contains some compounds able to cause the release of a thrombopoietin, and then to stimulate the biosynthesis of clotting factors that might help to precipitate blood coagulation or clotting, especially during severe bleeding or haemorrhage [39].
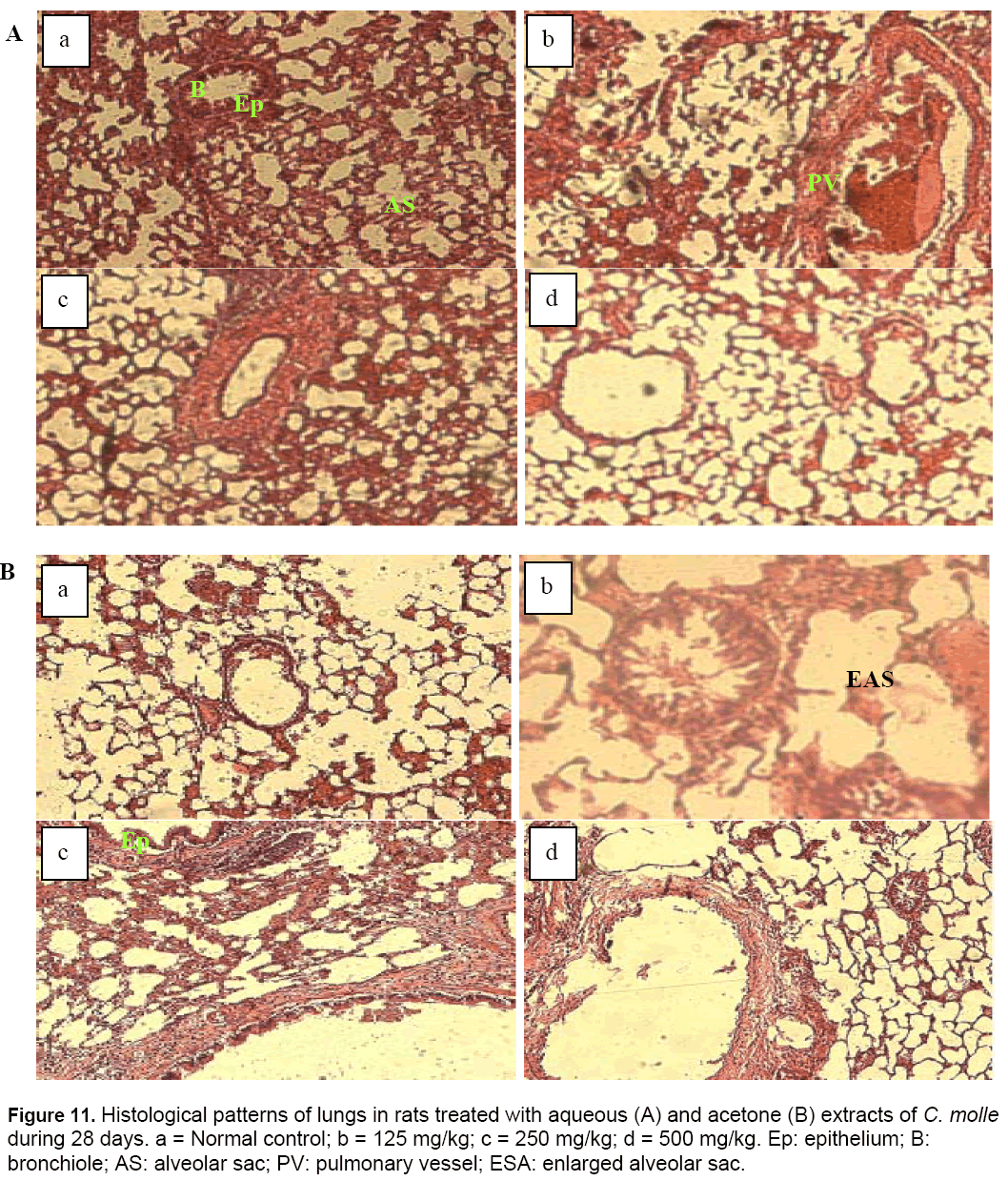
The histological section of lungs tissues of animals treated with the lower dose (125 mg/kg) of acetone extract of C. molle during the experimental period revealed an enlargement of the alveoli sacs (Figure 11). This finding could have been a result of direct toxicity or could have resulted from transportation of toxic substances from other organs like the liver and kidneys to the lungs [40].
Conclusion
The aqueous extract of C. molle twigs did not exhibit acute toxicity when given orally at concentration of 2000 mg/kg body weight. Also, the normalcy of or insignificant changes in clinical parameters and body weight reveal that the extract appears to be relatively non-toxic in the doses up to 2000 mg/kg body weight. Prolonged administration of both aqueous and acetone extracts caused hepatotocixity and nephrotoxicity but it was not immunotoxic, rather, it stimulated erythropioesis and enhanced immunity especially the cell-mediated immunity. The lower dose (125 mg/kg) of the acetone extract caused pulmonary toxicity. Therefore, it is prudent to undertake additional research in order to characterize other toxicological effects which might arise following long term use of these extracts.
References
- Polasa K., Nirmala K., Ginger. (2003). Its Role in Xenobiotic Metabolism. ICMR Bull., 33 (6): 57-63.
- Roch ABD., Lopes RM., Schwartsmann G. (2001). Natural products in anticancer therapy. Curr. Opin. Pharmacol., 1: 364-369.
- Keay RWY. (1989). Trees of Nigeria. 3rd eds. Clavevdo Press Oxford. pp.146-216.
- Burkill HM. (1985). The Useful Plants of West Tropical Africa, Families A-D. Kew: Royal Botanic Gardens; United Kingdom; 2nd edition. pp. 960.
- Fyhrquist P., Mwasumbi L., Haeggstro CA., Vuorela H., Hiltunen R., Vuorela P .(2002). Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) growing in Tanzania. J. Ethnophar-macol., 79: 169-177.
- Ojewole JAO (2008). Analgesic and anti-inflammatory effects of mollic acid glucoside, a 1a- hydroxycycloartenoid saponin extractive from Combretum molle R.Br. ex ex G. Don (Combretaceae) leaf. Phytother. Res., 22: 30-35.
- Eloff JN., Famakin J.O, Katerere D.R.P (2005). Isolation of antibacterial Stilbene from Combretum woodii (Combretaceae) leaves. Afr. J. Biotechnol., 4: 1167-1171.
- Chaabi M., Benayache S., Vonthron-Senecheau., E, Weinger B., Anton R., Lobstein A. (2006). Antiprotozoa activity of saponins from Anogeissus leiocorpus (Combretaceae). Biochem. Syst. Ecol., 36: 59-62.
- Bessong PO., Rojas LB., Obi CL., Tshisikawe MP., Igunbor EO. (2006). Further screening of Venda medicinal plants for activity against HIV type 1 reverse transcriptase and integrase. Afr. J. Biotechnol., 5: 526-528.
- Ojewole JA., Adewole SO. (2009). Hypoglycemic effect of mollic acid glucoside, a 1alpha-hydroxycycloartenoid saponin extractive from Combretum molle R. Br. ex G. Don (Combretaceae) leaf, in rodents. J. Nat. Med., 63(2): 117-23.
- Miaffo D., Poualeu SL., Kamanyi A. (2014). Antidiabetic activity of the methanol and acetone extracts of twigs of Combretum molle in dexamathasone induced-insulin resistance in rats. W. J. Pharma. Sci., 2(9): 955-965.
- Dodehe Y., Rita B., Bernard ND., Jean DN. (2012). Acute and Subacute Toxic Study of Aqueous Leaf Extract of Combretum molle. T.J.Pharma Res., 11 (2): 217-223.
- Simon MK., Ajanusis OJ., Jegede OC. (2012). Dose determination and toxicity studies of combretum molle stem bark methanol extract in rats. C. J. Pharmacol. Toxicol Res., 5(1): 30.
- Zimmerman M. (1983). Ethical guidelines for investigation of experimental pain in conscious animal. Pain., 16(2): 109-110.
- OECD. (2001). The OECD guideline for Testing of Chemicals 425. pp. 425.
- Jaffe. (1999). Creatinin: Kinetic method. Linear Chemicals Laboratories. pp.1.
- Chronolab. (2000). Biuret Method Colorimetric. Quantitive Determinations of Total of Protein. Avenida Diagonal 609, Planta 10,08028 Barcelona , Spain. pp.1.
- Chronolab. (2000). Kinetic UV Method. Quantitative determination of alanine aminotransferase ALAT (GPT). Avenida Diagonal 609, Planta 10, 08028 Barcelona, Spain. pp.1.
- Chronolab. (2000). Kinetic UV Method. Quantitative determination of aspartat aminotransferase ASAT (GOT). Avenida Diagonal 609, Planta 10, 08028 Barcelona, Spain. pp.1.
- Guyton AC., Hall JE. (2001). Textbook of medical physiology. 10th ed. India, New Delhi: Elsevier. pp. 309-10.
- Speijers GJ., Dederen LH., Keizer H. (2009). A sub-chronic (13 weeks) oral toxicity study in rats and an in vitro genotoxicity study with Korean pine nut oil (PinnoThin TG). Regul. Toxicol. Pharmacol., 55(2) : 158 - 165.
- Hassan LG., Dangoggo SM., Hassan S W., Muhammad S., Umar K J. (2010). Nutritional and Antinutritional Composition of Sclerocarya birrea fruit juice. Nigerian Journal of Basic and Applied Sciences. 18(2) : 222-228.
- Teo S., Stirling D., Thomas S., Hobermann A., Kiorpes A., Khetani VA. (2002). 90 day oral gavage toxicity study of D-methyl penidate and DL methyl penidate in Sprague-Dawly rats. Toxicology. 179: 183-196.
- Counotte G. (2003). Avicultural professional: conocer la calidad del aqua de bebida. Doetinchem: Reed Business Information. pp.20-22.
- Thanabhorn S., Jenjoy K., Thamaree S., Ingkaninan K and Panthong A. (2006). Acute and subacute toxicity study of the ethanol extract from Lonicera japonica Thunb. J. Ethnopharmacol., 107: 370-373.
- Kim JH., Hahm DH., Yang DC., Kim JH., Lee HJ., Shim I. (2005). Effect of crude saponin of Korean red ginseng on high fat diet induced obesity in the rat. Journal of Pharmacological Sciences. 97: 124–131.
- Amresh GR., Singh PN., Rao CV. (2008). Toxicological screening of traditional medicine Laghupatha (Cissampelos pareira) in experimental animals. Journal of Ethnopharmacol., 116: 454-460.
- Crook MA. (2006). Clinical Chemistry and Metabolic Medicine. 7th Edition. Hodder Arnold, London. pp.426.
- Konan NA., Bacchia EM., Lincopan N., Varelac SD and Varandac EA. (2007). Acute, Subacute toxicity and genotoxic effects of a hydroethanolic extract of the Cashew (Anarcidium occidentale L.). J. Ethnopharmacol., 110: 30-38.
- Pimainog Y., Yothinarak A., Jornrakate P. (2003). Reference ranges for hematological and clinical chemistry values in Wistar rats. Bull. Dept. Med. Sci., 45(1): 27-36.
- Marzo F., Urdaneta E., Santidrian S. (2002). Liver proteolytic activity in tannic acid-fed birds. Poultry Sci., 81: 92-94.
- Aniagu SO., Nwinyi FC., Akumka DD., Ajoku GA., Dzarma S., Izebe KS., Ditse M., Nwaneri PEC., Wambebe C., Gamaniel K. (2002). Toxicity studies in rats fed nature cure bitters. Afr. J.of Biotechnol., 4(1): 72-78.
- Padmanabhan S., Rajasekhar., Siva VK. (2003). Cardiac and renal toxicity to a herbal concoction. Indian J. Nephrol., 13: 113-115.
- Yakubu MT., Akanji MA., Oladiji AT. (2007). Haematological evaluation in male albino rats following chronic administration of aqueous extract of Fadogia agrestis stem. Pharmacol. Manag., 3: 34- 38.
- Tripathy S., Pradhan D., Anjana M. (2010). Antiinflammatory and antiarthritic potential of Ammania baccifera Linn. Int. J. Pharma Bio Sci., 1.
- Coles EH. (1986). “Veterinary Clinical Pathology”. W. B Saunders, Philadelphia, USA. pp.10-42. [37] Sanchez-Elsner T., Ramirez J R., Rodriguez- Sanz F., Varela E., Bernabew C., Botella LM. (2004). A cross talk between hypoxia and TGF-beta orchestrates erythropoietin gene regulation through SPI and smads. J. Mol. Biol., 36(1): 9-24.
- Oyedemi SO., Bradley G., Afolayan., AJ. (2010). Toxicological effects of the aqueous extract of Strychnos henningsii Gilg in Wistar rats. J. Nat., 1:1. [39] Dahlback B. (2008). Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood, 112: 1927.
- Dahlback B. (2008). Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood, 112: 1927.
- Adekomi DA., Tijani A A., Adeniyi TD., Musa AA and Usman B. (2011). Exposure to smoke extract of Datura stramonium leaf: Some of its effects on the heart, liver, lungs, kidneys and testes of male Sprague Dawley rats. Journal of Pharmacognosy and Phytotherapy. 3(1): 6-12..

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences