The Systematic Review of Proteins Digestion and New Strategies for Delivery of Small Peptides
Sina Vahdatpour, Aniseh Pourrasmi-Mamaghani, Mehdi Soleymani- Goloujeh, Naser Maheri-Sis, Hamid Mahmoodpour, Tohid Vahdatpour
Sina Vahdatpour1, Aniseh Pourrasmi-Mamaghani2, Mehdi Soleymani- Goloujeh3, Naser Maheri-Sis4, Hamid Mahmoodpour1, Tohid Vahdatpour4,*
1Faculty of Veterinary Medicine, Tabriz Branch, Islamic Azad University, Tabriz, Iran;
2Faculty of Management and Medical Informatics, Tabriz University of Medical Sciences, Tabriz, Iran;
3Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran;
4Faculty of Animal and Veterinary Sciences, Shabestar Branch, Islamic Azad University, Shabestar, Iran.
- *Corresponding Author:
- Tohid Vahdatpour
Faculty of Animal and Veterinary Sciences
Shabestar Branch, Islamic Azad University
Shabestar, Iran.
Tel: +984114777882
E-mail: vahdatpour@iaushab.ac.ir
Received date: March 16, 2016; Accepted date: May 16, 2016; Published date: May 23, 2016
Citation: Vahdatpour S, Mamaghani AP, Goloujeh MS, et al. The Systematic Review of Proteins Digestion and New Strategies for Delivery of Small Peptides. Electronic J Biol, 12:3
Abstract
The digestive system is rich in different proteolytic enzymes, acid, and other secretions, also lined with a layer of mucus, which provides extracellular and intracellular barriers to peptide absorption in all epithelial surfaces of lumen. However, small peptides may be crossing from these barriers and absorbed from different regions of the intestine. These peptides as small, mini or small peptides called bioactive peptides. Bioactive peptides have defined as peptides with quasi-hormone or drug like activity that eventually modulate physiological function through binding interactions to specific receptors on target cells. Bioactive peptides may be classified as antimicrobial, anti-diabetic, antithrombotic, antihypertensive, opioid, immune modulator, mineral binding and anti-oxidative. Most of the final protein digestive products that are absorbed are individual amino acids, with lower values absorption of small peptides. The peptide transporter 1 (PEPT1) is primarily responsible for the absorption of dietary diand tripeptides from the small intestinal. Currently, there is an interest in small peptides in pharmaceutical research and developments are being evaluated in clinical trials. The aim of this review is to summarize the existing knowledge for a better understanding of the challenges about protein digestion, small peptide absorption and oral delivery enhancers with an emphasis on small peptides.
Keywords
Barrier; Digestive; Peptide; PEPT1.
1. Introduction
Almost all proteins and many peptides compound digested in alimentary system. Therefore, pharmacologically active proteins and peptides like hormones cannot be administered orally because of inadequate oral bioavailability, and this may limit the usefulness of these compounds. Lower gastrointestinal bioavailability can be caused by weak aqueous solubility, degradation within the gastrointestinal contents, low membrane permeability or pre-systemic metabolism. Compounds can have poor membrane permeation due to largemolecular weight as is the case with proteins and other macromolecules, or insufficient lipophilicity to partition into biological membranes, as with many hydrophilic low-molecular weight compounds. There are numerous pharmacologically effective compounds currently used that must inject because of inadequate bioavailability by non-injecting routes. Absorption enhancement is the technology aimed at non-injectable delivery of poorly membranepermeable compounds. Consumption of the peptides and proteins show several advantages as compared to conventional drugs. These include high activity, high specificity, low toxicity, and minimal nonspecific and drug-drug interactions [1]. Developments of biotechnology resulted in production of peptides. Oral administration route has advantages, including: patient compliance, ease of administration and reasonably low cost of production. Low oral bioavailability of macromolecular drugs stems mainly from pre-systemic enzymatic degradation and poor penetration across the intestinal membrane. The present review summarizes the physiological barriers to oral delivery of peptides and provides novel pharmaceutical approaches to improve oral bioavailability of bioactive peptides.
2. Digestion of Protein
2.1 Digestion in the stomach
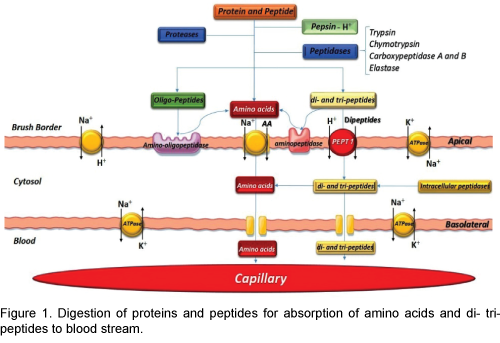
The Pepsin, an important peptic enzyme of the stomach, is most active at a pH of 2.0 to 3.0. The gastric glands secrete a large quantity of hydrochloric acid. This hydrochloric acid is secreted by the parietal (oxyntic) cells in the glands and the pH averages around 2.0 to 3.0, a highly favourable range of acidity for pepsin activity [2]. Pepsin is digesting the protein collagen. Collagen is a major constituent of the intercellular connective tissues; therefore, for the digestive enzymes to penetrate meats and digest the other meat proteins, it is first necessary that the collagen fibers be digested. Pepsin only initiates the process of protein digestion, usually providing only 10 to 20 percent of the total protein digestion to convert the protein to proteases, peptones and a few polypeptides. This splitting of proteins occurs as a result of hydrolysis at the peptide linkages between amino acids which are digested to the final stage to form single amino acids and little small peptides. More than 95 percent of the final protein digestive products that are absorbed are individual amino acids, with only 5 percent absorption of di- and tripeptides and very rare absorption of other small peptide molecules. Even these very few absorbed molecules of whole peptides and/or protein can sometimes cause serious allergic or immunologic disturbances [3].
2.2 Digestion in the intestine
Most protein digestion occurs in the initial of small intestine, in the duodenum and jejunum, by proteolytic enzymes from pancreatic secretion. In the small intestine the partial breakdown products of the protein foods attacked by major proteolytic pancreatic enzymes: trypsin, chymotrypsin, carboxypolypeptidase and proelastase. The trypsin and chymotrypsin split protein molecules into small polypeptides; carboxy polypeptidase then cleaves individual amino acids from the carboxyl ends of the polypeptides. Proelastase, in turn, is converted into elastase, which then digests elastin fibers that partially hold meats together. The small percentages of the proteins are digested all the way to their constituent amino acids by the pancreatic juices [4]. Therefore, most remain as dipeptides and tripeptides. The last digestive stage of the proteins in the intestinal lumen is achieved by the enterocytes that line the villi of the small intestine, mainly in the duodenum and jejunum. These cells have a brush border that consists of hundreds of microvilli projecting from the surface of each cell. Two types of peptidase enzymes are important, aminopolypeptidase and several dipeptidases that succeed in splitting the remaining larger polypeptides into tripeptides and dipeptides and a few into amino acids. The amino acids with the dipeptides and tripeptides are easily transported through the microvillus membrane to the interior of the enterocytes [5].
3. Absorption of Peptides
Therefore, peptides for absorption must firstly diffuse across the mucus layer before absorption across the epithelia is possible. The aqueous boundary or unstirred water layer can be act as a limiting factor for highly lipophilic peptides. Once a protein crosses the monolayer of intestinal epithelial cells, it can enter either the capillaries of the portal venous system or the lymphatic lacteal [6]. The lipophilic peptides are more likely to be absorbed by the lymphatic system [7]. The lymphatic circulation bypasses the liver and thus the attractive approach to delivery of peptides and proteins. Absorption into the lymphatic lacteals provides very slow systemic delivery over several hours as the lymph moves at a slow rate. Although, absorption into the portal venous system results in rapid delivery within minutes to systemic circulation after an initial hepatic pass.
PEPT1 mediated active absorption is responsible for the high bioavailability of orally active peptides so that the proton-coupled uptake of the more than 8000 different di- and tripeptides is performed by the high-capacity/low-affinity peptide transporter isoform PEPT1 (SLC15A1) [8]. For examples: betalactam antibiotics of the cephalosporin and penicillin classes, ACE-inhibitors, ester prodrugs of enalapril and fosinopril, bestatin, alafosfalin, amino acidconjugated antiviral drugs (valacyclovir), L-DOPA, as well as artificial di- and tripeptides such as Gly- Sar [9]. PEPT1 prefers peptides containing L amino acids over D enantiomers. Several PEPT1 inhibitors such as sulfonylurea antidiabetic drugs, nateglinide, glibenclamide, tolbutamide, chlorpropamide, sartans and ester pro-drugs of ACE inhibitors have been identified. Di-peptides such as Gly-Sar and Val- Ala have also demonstrated inhibitory potential; therefore di- and tri-peptides produced by digestion of milk proteins may also inhibit PEPT1-mediated drug absorption, causing reduced exposure of the victim drug (Figure 1).
4. Barriers to Peptide Absorption
Barriers to peptides absorption are extracellular and intracellular barriers. Potent barriers exist to the oral absorption of peptides; the development of oral replacements for injectable peptides is a high-priority research for the consumption of peptides [10]. The oral bioavailability of the most peptides is less than one percent. Inhibition of proteolytic enzymes and opening of tight junctions increase para-cellular transport of peptides from intestine [11].
4.1 Extracellular barriers
Peptide absorption from the digestive system is encountering with enzymatic and penetration barriers. Hydrolysis of peptides and proteins in the digestive system can occur in the lumen, in the brush border, intracellular fluid or in the cytosol of the enterocytes [12]. As the protein is ingested, it reaches the stomach, where it is acted on by gastric juice in a very acidic environment. Gastric juice contains a family of aspartic proteinases called pepsins. Pepsins digested the protein into a mixture of polypeptides and move down to the duodenum. As the protein enters the duodenum, the pH rises to about 6.0 to 8.0. This pH change approximately from 2.0 to 8.0 can also cause precipitation of the protein through its iso-electric point and then may not easily re-dissolve [4].
In the duodenum, the polypeptides are acted by pancreatic proteases. These proteases are classified to endopeptidases such as trypsin, chymotrypsin, and elastase or exopeptidases such as carboxy peptidase A. These enzymes degrade the polypeptides into smaller peptides. These peptides are then further degraded by the proteases in the brush border and the cytosol of the enterocytes. For peptides with four or more residues, more than 90 percent of the proteolytic activity is in the brush border membrane. For tripeptides, the activity is 10 to 60 percent; for dipeptides, it is only about 10 percent. Lysosomes and other cell organelles can also act as potential sites of peptide and protein degradation [13,14]. The brush border contains exopeptidases that act at the N-terminal end of the protein (amino peptidases). Amino peptidases in the intestine include leucine amino peptidase and amino peptidases N, A, and B. The amino peptidase activity in the Payer's patches of the jejunum and ileum is only about 20 to 30 percent of that in the neighboring patch-free areas [14]. Knowledge of the distribution of brush border membrane peptidases along the intestine helps to predict the preferential uptake of peptides and proteins from various intestinal regions [13]. Because this distribution may vary for different enzymes, sitespecific oral delivery may be dependent on the amino acid sequence of the peptide. This is because of the substrate specificity of the brush border membrane peptidases. Also, the activities of brush border membrane peptidases may be controlled by the surface pH of mucosal cells rather than the luminal pH [15]. Therefore, it should be realized that several enzymes that act on carbohydrates may also affect the drug if it is a glycoprotein.
4.2 Intracellular barriers
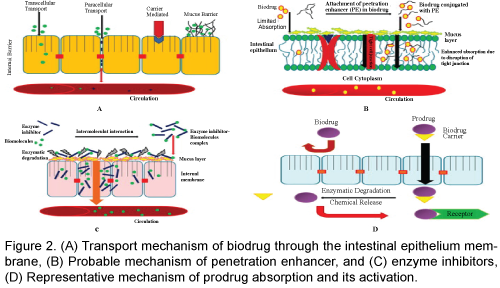
Peptides can move from epithelia by two routes. The transcellular route involves intracellular transfer from the apical to the basolateral surface of an individual epithelial cell. This transport can take place either through specific uptake mechanisms of the cell or through sequential partitioning, events from an aqueous environment to a hydrophobic environment and then back into an aqueous environment. The transcellular route is important for the uptake of lipophilic peptides by sequential partitioning events. The carbohydrates and amino acids are transported by a carrier-mediated process. Although L-amino acids are absorbed by an active transport mechanism, D-amino acids are absorbed by passive diffusion. There are four separate transport systems for amino acids, and a separate system transports dipeptides and tripeptides into the mucosal cells.
These carrier systems can even transport amino acid-type or peptide-type drugs. The active transport mechanisms for the transcellular route include Na+ coupled glucose transport and H+ coupled dipeptides transport. Studies on the kinetics of glycylproline transport in intestinal brush border vesicles have shown that dipeptides transport is saturable process and follows Michaelis-Menten kinetics [16]. Using brush border membrane vesicles (BBMVs) prepared from rabbit duodenum and jejunum and rat upper small intestine, no evidence was found in the oral absorption of TRH by a Na+ or H+ dependent carrier system in the brush border membrane. Of course, it appears that the TRH absorption in vivo may be accounted for by passive absorption of the peptide across a paracellular route and its resistance to luminal hydrolysis.
The paracellular route involves transfer between adjacent cells. The villous cells have tight intracellular junctions, which prevent paracellular transport of solutes. Movement from this route is limited by the junction complex to molecules with a radius less than about 8 Å [17]. If a peptide can pass from the paracellular route, it will not be subject to digestion by the intracellular proteases. Although the paracellular route may be preferable for this reason, structural features of peptides that may encourage their paracellular transport are not well understood. The partition coefficient between n-octanol and water may be one predictor, and drugs with a log P of less than zero (i.e., hydrophilic molecules such as most peptides) are more likely to follow a paracellular pathway. Peptides following a paracellular pathway may also be more affected by penetration enhancers such as zonula occludens toxin (ZOT), a protein from Vibrio cholera that can reversibly open tight junctions between intestinal cells [18]. Paracellular transport is constrained by the physical properties of permeate. Larger peptides (greater than three amino acids) are usually absorbed in small amounts by passive diffusion via the paracellular route. Although larger proteins are not typically absorbed in the digestive tract, protein antigens can be taken up by M cells, which are specialized intestinal epithelial cells that overlie aggregates of lymphoid tissue (Payer's patches). The tight junctions for paracellular diffusion have been reported to be generally impermeable to molecules with radii of more than 11 to 15 Å, which may represent the limit for the hydrodynamic radius for oral delivery of spherical rigid molecules. However, peptides have some conformation flexibility, and even larger molecule scans permeate the tight junctions [19].
5. The Site of Peptide Delivery
The different peptides absorbed from different regions of the intestine by several manners. This is believed to be caused by decreased proteolytic activity in the distal area. Also, the distal region has higher paracellular permeability despite a decreased absorption area [20]. The protease activity in the cytoplasm does not show regional variation, but the same is not true for the brush border or for the luminal fluid. The stomach, with its low pH and enzymatic activity, presents very harsh conditions for a protein drug. A typical approach to prevent dissolution of a dosage form in the stomach is to use enteric coating.
The intestinal segments have progressively fewer and smaller villi in the more distal sections. This leads to a progressively reduced surface area, with the colon having the lowest surface area for a particular length. The colon also has variable pH and the presence of solid fecal matter, which may interfere with drug absorption. However, the colon has relatively low enzymatic activity and is promising in this regard. Using isolated luminal enzymes and studies in intact mucosa, calcitonin was found to degrade much more in the small intestine compared to the colon [21]. The colon has a high population of bacteria; largely anaerobic species. This fact has been exploited for an ingenious approach to target peptides and proteins to the colon [22]. In a study polypeptides such as insulin or vasopressin were coated with polymers cross-linked with azo-aromatic groups to protect orally administered polypeptides from digestion in the stomach and small intestine of rats. Once the polypeptide reached the colon, the indigenous micro flora reduced the azo bonds, thus breaking the cross-links and releasing the polypeptide for absorption. The upper half of the large intestine is drained by hepatic portal veins; the lower half is drained by lymphatic. If a polypeptide is destroyed in the liver, it may be possible to adjust the thickness or composition of coating so that the drug is released in the lower colon, where it will bypass the hepatic veins. Delivery of insulin and an absorption promoter to the colon has also been attempted using a soft gelatin capsule coated with a poly acrylic polymer (Eudragit) having pH-dependent properties [23]. Delivery of insulin-like growth factor I (IGF-I) to rat and mini pig colonic mucosa under in vitro conditions has been investigated. IGF-I is a 7649- Da protein of 70 amino acids that exerts its biological actions through specific IGF-I receptors. It has been found useful to lower blood glucose levels in insulinresistant diabetic patients in clinical studies. IGF-I was absorbed intact across the rat colonic mucosa as determined by reverse-phase high-performance liquid chromatography (RP-HPLC), sodium dodecyl sulfate–poly acrylamide gel electrophoresis (SDSPAGE), and Western blotting [24]. A time-based drug release system for colon-specific delivery has been developed. This system exploits the relatively constant small intestinal transit time of dosage forms [11]. Time-based systems can be designed to release their drug after a predetermined lag time, with the lag time independent of normal physiological conditions such as pH, digestive state of the subject and anatomical position at the time of release [19,25].
The apparent permeability of insulin from rat intestine shows a site-dependent variation as measured by the averted rat gut sac technique. The permeability was significantly greater in the jejunum and the ileum than in the duodenum. In these in vitro experiments, insulin was remarkably stable. This suggests that insulin metabolism at the brush border is not significant. However, insulin was metabolized almost completely in intestinal homogenates. Thus, it appears that degradation of insulin under an in vivo situation would be caused by luminal and cytosol enzymes [1]. In situ experiments have shown that the absolute bioavailability of insulin was higher when administered in the more distal region of the rat intestine than that absorbed from a more proximal region of the intestine [26]. Insulin absorption from isobutyl cyanoacrylate Nano capsules administered to diabetic rats was dependent on the site of absorption. The hypoglycemic effect following absorption from various sites was as follows: ileum, stomach, duodenum and jejunum, and colon.
6. Strategies for Delivery of Peptides
The five main methods are most important strategies, including: chemical modification, bioadhesive delivery systems, penetration enhancers, protease inhibitors, carrier systems. The other formulation and manners referred at the end of this review which will be considered in the near future.
6.1 Chemical modification
The carrier molecules used to reversibly destabilize the native peptides. This no covalent interaction between the carrier and partially unfolded protein conformation increases solvent exposure of hydrophobic side chains, thereby increasing lipid solubility and oral absorption of the protein by a passive and transcellular route [27]. The chemical modification approach is more applicable to peptides than to proteins because of the structural complexity of proteins. A peptide can be chemically modified to improve its enzymatic stability or membrane permeation. For example, substitution of D-amino acids for L-amino acids in the primary structure may improve the enzymatic stability of the peptide. An example of chemical modification of a peptide that results in increased enzymatic stability without affecting membrane permeability is the various analogues of the naturally occurring penta-peptide methionine (Met)-enkephalin. The metabolism of these analogues by BBMVs shows large differences in degradation rates, but they all have similar effective permeability across Caco-2 cells [28].
Another clue to chemical modification comes from the fact that a lipophilic peptide, cyclosporine A, is readily absorbed from the gastrointestinal tract. Thus, efforts have been directed toward imparting lipid solubility to peptides by bonding an acyl group of a fatty acid to an amino terminus of the peptide. Using a range from tripeptides to proteins, lipid solubility was achieved for thyrotropin-releasing hormone, tetra-gastrin, insulin, and lysozyme. These new derivatives maintained their biological activity and had increased absorption from the intestine [29]. Another approach to increase lipophilicity can be cyclization, which will remove charged N- and C-terminal groups, reducing overall solvent-accessible surface area of the molecule. Also, more lipophilic synthetic amino acids such as t-butyl glycine, b-naphthyl alanine, and p-phenyl phenylalanine can be used to synthesize peptide analogues provided biological activity is not lost. The use of a conjugate system, which combines structural features of lipids with those of amino acids and peptides, is likely to provide a high degree of membrane-like character for the conjugate, which may allow its passage across membranes [30]. Chemical modification of salmon calcitonin has also been done to make its oral absorption feasible. In a study modified salmon calcitonin by a new method to prepare fatty acid-polypeptide conjugates; it can be carried out in aqueous solutions and can regenerate the original active polypeptide in tissues or blood. Using this reversible aqueous lipidization approach, the area under the curve (AUC) of modified calcitonin delivered orally was about 20 times higher than that of unmodified calcitonin [19]. PEGylated proteins may also have a potential for oral delivery. PEGylation of recombinant human granulocyte colony-stimulating factor has been reported to increase its stability and in vivo bioactivity when administered by the intraduodenal route. Its bioavailability by the internal route was 1.8 to 3.5 percent; the unmodified protein did not produce any quantifiable response [31].
Chemical modification does not always lead to improved oral absorption. Diacyl derivatives of insulin exhibited higher proteolysis than native insulin in the small intestine of rat under in vitro conditions. This was because insulin association was inhibited by diacylation, making more monomers available for proteolysis [32]. The structural requirements for intestinal absorption of peptide drugs have been reviewed [23]. Barlow and Satoh [9] conducted a series of elementary analyses to define the basic design features for a potent, specific, and absorbable peptide drug. Recognition of the peptide by its target receptor seems to need about 4 to 6 amino acid residues, and the rest of the structure may be “redundant” for bioactivity. The resulting structure is still too big for transport by paracellular transport. Also, peptide transporters for molecules larger than the three residues are unlikely to exist, so that active transport is also not feasible. For transport by simple diffusion, the lipophilicity of the peptide needs to be increased. Based on molecular modeling, it was predicted that an active absorbable peptide should have a total surface area of around 350 Å2, of which the polar surface area should be 50 Å2 or less. This could be attempted by methylation the peptide NH groups, eliminating charged termini or cyclization of the molecule so that peptide CO and NH groups are made inaccessible to solve because of intra molecular hydrogen bonding. Computer simulations to design peptides or to predict their oral absorption may be possible. A theoretical analysis to estimate the extent of peptide absorption has been developed on the basis of a mass balance approach. Using this analysis, simulations showed that the intestinal absorption of insulin is approximately 1 percent of the administered dose [33]. Because some peptide transporters are known to exist in the intestinal mucosa, knowledge of its structure will lead to rational design of peptide mimetic having affinity for this receptor. However, passive diffusion will be limited due to substrate specificity. Structural features of peptides to achieve drug delivery are not necessarily the same as those required for bioactivity. Therefore, a collaborative effort by a multidisciplinary team is required for rational design of peptide mimetic with adequate oral absorption [33].
Briefly, among common standard modifications of peptides as follows:
- D-amino acids
- Unnatural amino acids (6-Aminocaproic acid, Amino butyric acid, Citrulline, Norleucine, etc.)
- Heavy amino acids (labeled with 13C and/or 15N)
- Cyclisation
- Phosphorylation or sulfurylation (at Ser, Tyr, Thr)
- Biotinylation
- Conjugation to carrier proteins (BSA, KLH, OVA)
- Branching of peptides (MAPs – multiple antigenic peptides)
6.2 Bioadhesive delivery systems
Bioadhesive delivery systems have been widely investigated to prepare oral peptide consumption [34]. This increases the overall time for peptide absorption as the delivery system will not be dependent on the gastrointestinal transit time for removal. Peptides will not have to diffuse through luminal contents or the mucus layer to reach the mucosal epithelium. Because of intimate contact with the mucosa, a high drug concentration is presented for absorption. Also, site-specific delivery may be possible if bioadhesion can occur at a particular site in the digestive system. Bioadhesive delivery systems may be affected by the mucus turnover time in the digestive system, which varies based on the site. In the digestive system of rats, the colon and cecum were found to be the best location for mucoadhesion of poly carbophil disks. Mucoadhesive intestinal patches have also been investigated for oral delivery of conventional drug molecules [5]. Bioadhesive polymers can be used to improve the oral absorption of peptide drugs. The Bioadhesive polymers, poly carbophil, and chitosan derivatives have been used to enhance the absorption of the peptide drug 9-desglycinamide, 8-argininevasopressin (DGAVP) in the vertically perfused intestinal loop model of the rat [35]. Buccal adhesive systems offer innumerable advantages in terms of accessibility, administration and withdrawal, receptivity, low enzymatic activity, economy and high patient compliance. Adhesions of these drug delivery devices to mucosal membranes lead to an increased drug concentration gradient at the absorption site and therefore improve bioavailability of systemically delivered drugs. Investigations are continuing beyond traditional polymer networks to find other innovative drug transport systems. In the current global scenario, scientists are finding ways to develop buccal adhesive systems through various approaches to improve the bioavailability of drugs used orally by manipulation of the formulation strategies like the inclusion of pH modifiers, enzyme inhibitors, permeation enhancers, etc. The future direction of buccal adhesive drug delivery lies in vaccine formulations and delivery of peptides.
Another important aspect concerns the in vitro and ex vivo techniques which are employed for evaluation of the performance of the materials and dosage forms. Important factors affecting mucoadhesion including:
• Polymer Related Factors:
o Molecular weight
o Concentration of active polymer
o Spatial Conformation
o Chain flexibility of polymer
o Degree of Hydration
o Functional Group Contribution
• Environmental – Related Factors: 21-25
o pH
o Applied strength
o Initial contact time
o Selection of the model substrate surface
• Swelling
• Physiological variables
6.3 Penetration enhancers
Recently, the peptides are considered as penetration enhancers like skin penetrating peptides (SPPs) and/ or cell penetrating peptides (CPPs) have garnered wide attention in recent years and emerged as a simple and effective non-invasive strategy for macromolecule delivery into the skin or cells, respectively. Generally, penetration enhancers can improve oral absorption by their action on the transcellular or paracellular pathway. For effects on the transcellular pathway, surfactants and fatty acids may alter the membrane lipid organization and may thus increase oral transport. Surfactants can be incorporated into lipid bilayers, thus changing the physical properties of the cell membranes. For effects on the paracellular pathway, chelating agents can disrupt the integrity of occluding junctional complexes by chelating calcium or magnesium around tight junctions [36]. Bile salts such as sodium deoxycholate and sodium cholate can also be used in the formulation to promote the absorption of insulin from the colon mixed micellar systems have also been used to enhance the oral absorption of polypeptides [37]. Such systems are also known to form naturally in the gastrointestinal system to aid the absorption of lipids. The dietary fats are first emulsified by bile salts in the intestine and then acted on by pancreatic lipase to produce mono glycerides and free fatty acids. Lipoidal dispersions of insulin in fatty acids using sodium glycol cholateas an emulsifier and absorption promoter have been investigated. The hypoglycemic effects after oral administration to rabbits were found to be dependent on the fatty acid used. Penetration enhancers may enhance the absorption of drugs preferentially in some specific region of the gastrointestinal tract. Cyclo-dextrins have also been used to enhance the absorption of insulin from the lower jejunal/upper ileal segments of the rat by an in situ closed-loop method.
When penetration enhancers are used to enhance oral absorption, it could be realized that they have limitations that may prevent their general acceptance for usage. Also, the potential lack of specificity of penetration enhancers may have long-term toxicity implications that can only be evaluated in chronic studies. The potential lytic nature of surfactants raises safety concerns because the intestinal epithelium provides a barrier to the entry of toxins, bacteria, and viruses. Similarly, chelators that cause Ca2+ depletion do not act specifically on tight junctions but rather may induce global changes in cells, such as disruption of actin filaments or adherent junctions. Thus, it will be difficult to induce the opening of tight junctions in a rapid, reversible, and reproducible manner [37].
6.4 Protease inhibitors
Protease inhibitors may also promote oral absorption of therapeutic peptides and proteins by reducing their proteolytic breakdown in the gastrointestinal tract. Generally, inhibitory agents may be classified as (1) polypeptide protease inhibitors (e.g., aprotinin); (2) peptides and modified peptides (e.g., bacitracin, chymostatin, and amastatin); (3) amino acids and modified amino acids (e.g., a-aminoboronic acid derivatives), and (4) others (e.g., p-aminobenzamidine and camostat mesilate) [38]. An amino peptidase inhibitor, amastatin, has been reported to reduce the hydrolysis of the penta peptide, leucine (Leu)-enkephalin (YGGFL) at a high pH. At lower pH (below 5.0), the endopeptidase inhibitors, tripeptides YGG and GGF, were found to be effective. Coperfusion of YGGFL with a combination of amino- and endopeptidase inhibitors was most effective to inhibit hydrolysis in the rat jejunum. In the absence of these inhibitors, extensive hydrolysis of YGGFL was observed in the rat jejunum, primarily by brush border enzymes and secondarily by luminal peptidases [39]. In another study, an aminopeptidase inhibitor (puromycin) was able to increase the absorption of metkephamid (MKA), a stable analogue of Met-enkephalin, across the rat intestine. However, in this study, an endo-peptidase inhibitor (thiorphan) was ineffective. This is because the dominant enzyme participating in MKA metabolism during absorption is amino-peptidase [40].
Bile salts, in addition to acting as penetration enhancers, can also act as protease inhibitors to enhance oral absorption. Bile salts have been shown to inhibit brush border membrane and cytosolic proteolytic hydrolysis and would thus be useful to reduce intestinal degradation of peptide drugs [10]. A bacterial protease inhibitor from Brucella abortus called U-Omp19 has been reported as an ideal constituent for an oral vaccine formulation against infectious diseases. When U-Omp19 was coadministered orally with Toxoplasma gondii antigen (Ag), U-Omp19: i) could bypass the harsh environment of the gastrointestinal tract by inhibiting stomach and intestine proteases and consequently increased the half-life of the co-administered Ag at immune inductive sites. Finally, this bacterial protease inhibitor in an oral vaccine formulation conferred mucosal protection and reduced parasite loads after oral challenge with virulent Toxoplasma gondii [41]. In an in situ study with closed small and large intestinal loops in rats, no marked hypoglycemic response was observed when insulin alone was administered. However, a significant hypoglycemic effect was obtained following large intestinal administration of insulin with 20 mM sodium glycocholate, camostatmesilate, and bacitracin [42]. It has been suggested that if a protease inhibitor such as soybean trypsin inhibitor is used to prevent the proteolysis of insulin in the rat intestine, then its absorption is promoted by the endogenous bile acids present in the intestine [43]. In another study a decrease in insulin degradation with the co-administration of protease inhibitors to improve the oral bioavailability of insulin has been reported. A significant decrease of blood glucose levels in both lean and diabetes induced obesity rat models as well as a significant increase in plasma insulin levels 20 min and 135 min post-administration of oral insulin with the peptidase inhibitor have been shown in this study [44]. Very small doses (about 1 mg) of vasopressin in solution produced anti diuresis in rats following oral administration. The biological response was enhanced for AVP and LVP by the simultaneous administration of 1000 units of aprotinin, a protease inhibitor. The synthetic analogue DDAVP was more active than the natural hormones, but the effect of aprotinin with DDAVP was inconsistent. The relatively greater oral activity of DDAVP is caused by the unnatural D-arginine, which makes it resistant to attack by trypsin. Starchg- poly (acrylic acid) copolymers and starch/poly (acrylic acid) mixtures have been synthesized and may have potential for enabling oral peptide delivery because of their proteolytic enzyme inhibition activity and ion-binding capacity [20].
6.5 Carrier systems
Carrier systems such as nano-particles, microspheres, liposomes, or erythrocytes can also be used to improve the oral absorption of peptides and proteins. Emisphere Technologies, Incorporated (Tarrytown, NY) initiated clinical trials for oral delivery of insulin using its carrier eligen® technology. The company has also initiated oral delivery of recombinant human growth hormone in collaboration with Novartis and is currently in phase II clinical trials for oral delivery of calcitonin. These carrier molecules, in high concentrations, cause the protein to undergo a conformational change to a partially unfolded or molten globule state that has a higher oral permeability. The carrier molecules are small organic molecules with a molecular weight of about 200 to 400 Da. The protein is used in its native state rather than by a chemical modification approach, and the interaction between carrier and protein is non-covalent. Using cell mono-layers, it has been shown that the tight junctions between cells are not disrupted. The company has also used PYY 3-36 to demonstrate proof of concept for its oral delivery technology. PYY 3-36is a 34-residue gut hormone that physiologically inhibits food intake and has potential for treatment of obesity [12,45]. The use of different carrier systems to improve the absorption of insulin in anesthetized diabetic rats following intraduodenal administration from amid line incision has been evaluated. Several erythrocyte–membrane carrier systems were tested. These included erythrocyte ghosts (EGs) prepared by hemolysis of human red blood cells, erythrocyte vesicle (EVs) prepared by sonication of EG suspension, and liposome-incorporating ghosts or vesicles (LEGs and LEVs, respectively). Compared to a control group, these carriers enhanced oral absorption of insulin, with LEV the best carrier for more efficient delivery [45].
Uptake of liposomes by Payer's patches can increase the uptake of any entrapped drug. Negatively charged liposomes with at least 25 mol of phosphatidyl serine have been reported to be taken up readily by the rat Payer's patches following intra-luminal administration. Proteins such as albumin have also been used to prepare micro particles to improve the stability of drugs in the gastrointestinal tract. Thermally condensed amino acids (proteinoids) can spontaneously form microspheres when exposed to an acidic medium. Proteinoid microspheres have been used with positive results to deliver encapsulated calcitonin to rats and monkeys. In rats, the serum calcium levels decreased by 23 mg/ml 1 h after dosing encapsulated calcitonin. In contrast, rats receiving control calcitonin (no microspheres) had a decrease of only 6.5 mg/ml [1].
The advantage of using nano-particle formulations over other methods such as liposome formulations is the capability of controlled release in addition to the ability of improving drug stability, absorption and targeting [44]. The absorption and tissue distribution of 14C-labeled poly (D, L-lactide-co-glycolide) nanoparticles after oral administration to the mice has been determined in comparison to the intravenous route. The gastrointestinal transit of the nano-particles was very fast, with most of the radioactivity appearing rapidly in the colon 4 h after administration and in the feces 24 h after administration. Of the amount absorbed through the intestinal barrier (about 2%), most was found in the carcass and liver [16]. Nanocapsules may prefer initially absorbed through the Payer's patches and may be visible in M cells and intercellular spaces around lymph cells. It seems that this uptake by Payer's patches is especially important in the ileum. Absorption of nano-capsules in the jejunum may be by a paracellular pathway, possibly through the intercellular spaces formed by the desquamation of well-differentiated absorptive cells at the tip of the villi [16]. A palmitic ester prodrug of the model drug leucine-5-enkephalin was encapsulated within chitosan amphiphilic nanoparticles. Palmitic acid was used for increasing the lipophilicity of Leucine-5-enkephalin also stabilizing the peptide in the plasma and chitosan amphiphilic nano-particles were used to enhance gastrointestinal uptake. Via the oral route the nano-particle pro-drug formulation increased the brain drug levels by 67% and significantly increased leucine5-enkephalin’s anti-nociceptive activity. The nano-particles facilitated oral absorption and the pro-drug prevented plasma degradation; enabling brain delivery [46]. Free insulin did not affect glycemia when administered orally under the same experimental conditions. The intestinal absorption of insulin and calcitonin encapsulated in poly-isobutyl cyano-acrylate nano-particles has been investigated in rats, and the resulting pharmacokinetic profiles were characteristic of sustained delivery. A relatively higher plasma concentration was seen at the later time points, but was balanced by lower initial concentrations; thus, there was no significant net enhancement of absorption. This suggests that the nano-capsules slowly released the peptide into the intestinal lumen, with small amounts absorbed [47]. Hydrogel nano-spheres composed of polymetha crylicacid-grafted-poly (ethylene glycol) have also been investigated for oral protein delivery and have been reported to be capable of opening the tight junctions between epithelial cells in Caco-2 cell mono-layers [48]. Thus, our current knowledge provides some promising approaches on how to deliver peptides based drugs not only to the site of disease but also inside the target cell for enhanced therapy. Traditional methods of intracellular delivery, such as electro-portion or microinjection are invasive and applicable for in vitro experiments, but not for clinical conditions. The use of various pharmaceutical nano-carriers, such as liposomes, possessing pH-sensitivity and being able to escape from the endosomes upon the endocytic uptake, or the modification of peptide and protein drugs with cellpenetrating peptides, can allow for efficient and noninvasive intracellular delivery. Although the majority of experiments with pH-sensitive pharmaceutical nano-carriers and cell-penetrating peptide-modified drugs and drug carriers are still in pre-clinical stage, we can expect the appearance of new drugs and treatment protocols based on these methods in the very near future (Figure 2).
6.6 Other formulation
Several formulations have been reported for the gastrointestinal absorption of peptides. The oil phase contains a lipid composition similar to those of chylomicrons. A protinin, a protease inhibitor, will prevent peptide degradation; chylomicrons will improve absorption into the enterocyte. The emulsion is coated on carrier powders, which are then filled in hard gelatin capsules. The capsules are then enteric coated to prevent dissolution in the stomach. Another approach involves non-covalent linking of the peptide to phospholipids so that the complex can be absorbed into the enterocytes by endocytosis. These approaches are used to develop oral formulations for insulin, calcitonin, porcine somatotropin, erythropoietin, and an interferon [48]. Oral administration of insulin in solid form to nondiabetic and diabetic dogs has been attempted by mixing insulin with cholate and soybean trypsin inhibitor and delivering it orally as enterocoated micro tablets. Following administration of the drug, plasma insulin levels increased and plasma glucose levels decreased after a gap of about 60 to 140 min. Because delivery of insulin by the oral route leads to targeting of the entero-hepatic pathway, the authors of this study felt that this or a similar formulation may serve as an adjuvant treatment for patients with type II diabetes mellitus [34]. He et al. [49] stabilized biocompatible nano-emulsions by food proteins which could deliver fenofibric acid in vivo. In this study Food proteins (soybean protein isolate, whey protein isolate, β-lactoglobulin) were used as stabilizers for nano-emulsions to deliver hydrophobic drugs such as fenofibric acid. Food proteinstabilized nano-emulsions, with small particle size and good size distribution, exhibited good stability and bioavailability. The nano-emulsions enable the lipophilic drug to be absorbed more rapidly and better when compared with the oil solution also a much better stability was observed in protein-stabilized nanoemulsions relative to nano-emulsions stabilized with surfactants so Food proteins are viable replacements for traditional surfactants. It should be noted that the bioavailability of SPI-stabilized nano-emulsions were dramatically greater than that of nano-emulsions stabilized by β-lg and WPI [49].
7. Conclusion
Proteins and many peptides compound digested in alimentary system. Therefore, active proteins and peptides like hormones cannot be administered orally because of inadequate oral availability. Successful peptide delivery by the gastrointestinal route needs a succession of events to bypass the various penetration or enzymatic barriers at each stage. A site-specific delivery system and approaches to minimize proteolytic degradation are required. The use of penetration enhancers, carrier systems, especially new designed protease inhibitors, or chemical modification of the peptides offers promising approaches to enhance their oral delivery. Designing of absorbable small peptides that penetrate the intestinal mucosa by the paracellular pathway and absorbed to blood seems to be a possible approach. The peptide transporter1 (PEPT1) is primarily responsible for the absorption of dietary di- and tripeptides from the small intestinal lumen. Substrate type interactions by PEPT1 have been successfully exploited with pro-drugs that were designed to introduce peptide- and peptide bond like moieties on the parent molecule. It seems that in the future an important witness will be reported about the valuable effects of small peptides which using with protease inhibitors, and/or chemical modification could be easily absorbed at high levels from the gastrointestinal tract.
References
- Sarubbi D, Variano B, Haas S, et al. (2012). Oral delivery of calcitonin in rats and primates using proteinoid microspheres. Proceedings of the International Symposium on Controlled Release of Bioactive Material. Deerfield, IL: Controlled Release Society.
- Porter CJ. (1997). Drug delivery to the lymphatic system. Crit Rev Ther Drug Carrier Syst. 14: 333–393.
- Ashford M, Fell JT. (2012). Targeting drugs to the colon: delivery systems for oral administration. J Drug Targeting. 2: 241–257.
- Bai JPF. (1994). Distribution of brush-border membrane peptidases along the rat intestine. Pharm Res. 11: 897–900.
- Shen Z, Mitragotri S. (2002). Intestinal patches for oral drug delivery. Pharm Res. 19: 391–395.
- Langguth P, Bohner V, Heizmann J, et al. (1997). The challenge of proteolytic enzymes in intestinal peptide delivery. J Controlled Release. 46: 39–57.
- Porter CJ. (1997). Drug delivery to the lymphatic system. Crit Rev Ther Drug Carrier Syst. 14: 333–393.
- Spanier B. (2014). Transcriptional and functional regulation of the intestinal peptide transporter PEPT1. J Physiol. 592: 871–879.
- Barlow D, Satoh T. (2006). The design of peptide analogues for improved absorption. J Controlled Release. 29: 283–291.
- Bai JPF. (2012). Effects of bile salts on brush-border and cytosolic proteolytic activities of intestinal enterocytes. Int J Pharm. 111: 147–152.
- Gazzaniga A, Iamartino P, Maffione G, et al. (1994). Oral delayed-release system for colonic specific delivery. Int J Pharm. 108: 77–83.
- Goldberg MM. (2003). Review of the large and growing database of oral delivery of macromolecules using eligen (TM). Protein and Peptide Drug Delivery: Scientific Advances in Enabling Novel Approaches and Improved Products IBC Meeting, Philadelphia.
- Langguth P, Bohner V, Heizmann J, et al. (1997). The challenge of proteolytic enzymes in intestinal peptide delivery. J Controlled Release. 46: 39–57.
- Sasaki I, Fujita T, Murakami M, et al. (2004). Intestinal absorption of azetirelin, a new thyrotropin-releasing hormone (TRH) analogue. I. Possible factors for the low oral bioavailability in rats. Biol Pharm Bull. 17: 1256–1261.
- Lee VHL. (1994). Oral route of peptide and protein drug delivery. BioPHARM. 5: 39–50
- Le Ray AM, Vert M, Gautier JC, et al. (2014). Fate of [C-14] poly (DL-lactide-co-glycolide) nanoparticles after intravenous and oral administration to mice. Int J Pharm 106: 201–211.
- Bernkop-Schnurch A, Krajicek ME. (1998). Mucoadhesive polymers as platforms for peroral peptide delivery and absorption: Synthesis and evaluation of different chitosan–EDTA conjugates. J Controlled Release. 50: 215–223.
- Fasano A. (1998). Novel approaches for oral delivery of macromolecules. J Pharm Sci. 87: 1351–1356.
- Wilding IR, Davis SS, Pozzi F, et al. (1994). Enteric coated timed release systems for colonic targeting. Int J Pharm. 111: 99–102.
- Stoll BR, Leipold HR, Milstein S, et al. (2000). A mechanistic analysis of carrier-mediated oral delivery of protein therapeutics. J Controlled Release. 64: 217–228.
- Lu RH, Kopeckova P, Kopecek J. (1999). Degradation and aggregation of human calcitonin in vitro. Pharm Res. 16: 359–367.
- Ashford M, Fell JT. (2012). Targeting drugs to the colon: Delivery systems for oral administration. J Drug Targeting. 2: 241–257.
- Pauletti GM, Gangwar S, Knipp GT, et al. (1996). Structural requirements for intestinal absorption of peptide drugs. J Controlled Release. 41: 3–17.
- Quadros E, Landzert NM, LeRoy S, et al. (1994). Colonic absorption of insulin-like growth factor I in vitro. Pharm Res. 11: 226–230.
- Pozzi F, Furlani P, Gazzaniga A, et al. (1994). The TIME CLOCK system: A new oral dosage form for fast and complete release of drug after a predetermined lag time. J Controlled Release. 31: 99–108.
- Takaya T, Niwa K, Takada K. (1994). Pharmacological effect of recombinant human colony-stimulating factor (rhG-CSF) after administration into rat large intestine. Int J Pharm. 110: 47–53.
- Milstein SJ, Leipold H, Sarubbi D, et al. (1998). Partially unfolded proteins efficiently penetrate cell membranes—implications for oral drug delivery. J Controlled Release. 53: 259–267.
- Shah RB, Ahsan F, Khan MA. (2002). Oral delivery of proteins: Progress and prognostication. Crit Rev Ther Drug Carrier Syst. 19: 135–169.
- Snider DP, Marshall JS, Perdue MH, et al. (2010). Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J Immunol. 153: 647–657.
- Toth I. (1994). A novel chemical approach to drug delivery: Lipidic amino acid conjugates. J Drug Targeting. 2: 217–239.
- Andrysek T. (2001). The role of particle size distribution on bioavailability of cyclosporine: Novel drug delivery system. Biomed Pap. 145: 3–8.
- Asada H, Douen T, Mizokoshi Y, et al. (2004). Stability of acyl derivatives of insulin in the small intestine: Relative importance of insulin association characteristics in aqueous solution. Pharm Res 11: 1115–1120.
- Borchardt RT. (1994). Rational delivery strategies for the design of peptides with enhanced oral delivery. Drug Dev Ind Pharm. 20: 469–483.
- Plate NA, Valuev IL, Sytov GA, et al. (2002). Mucoadhesive polymers with immobilized proteinase inhibitors for oral administration of protein drugs. Biomaterials. 23: 1673–1677.
- LueBen HL, Lehr CM, Rentel CO, et al. (1994). Bioadhesive polymers for the peroral delivery of peptide drugs. J Controlled Release. 29: 329–338.
- Ameye D, Voorspoels J, Foreman P, et al. (2001). Trypsin inhibition, calcium and zinc ion binding of starch-g-poly(acrylic acid) copolymers and starch/poly(acrylic acid) mixtures for peroral peptide drug delivery. J Controlled Release. 75: 357–364.
- Hochman J, Artursson P. (1994). Mechanisms of absorption enhancement and tight junction regulation. J Controlled Release. 29: 253–267.
- Bernkop-Schnurch A. (2008). The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J Controlled Release. 52: 1–16.
- Conradi RA, Wilkinson KF, Rush BD, et al. (1993). In vitro/in vivo models for peptide oral absorption: comparison of Caco-2 cell permeability with rat intestinal absorption of renin inhibitory peptides. Pharm Res. 10: 1790–1792.
- Takada K, Nakahigashi Y, Tanaka T, et al. (2011). Pharmacological activity of tablets containing recombinant human granulocyte colony-stimulating factor (rhG-CSF) in rats. Int J Pharm. 101: 89-96.
- Ibañez AE, Coria LM, Carabajal MV, et al. (2015). Bacterial protease inhibitor protects antigens delivered in oral vaccines from digestion while triggering specific mucosal immune responses. J Controlled Release. 220: 19-28.
- Yen WC, Lee VHL. (2012). Paracellular transport of a proteolytically labile pentapeptide across the colonic and other intestinal segments of the albino rabbit—implications for peptide drug design. J Controlled Release. 28: 97-109.
- Shao ZH, Li YP, Chermak T, et al. (1994). Cyclodextrins as mucosal absorption promoters of insulin. II. Effects of b-cyclodextrin derivatives on a-chymotryptic degradation and enteral absorption of insulin in rats. Pharm Res. 11: 1174-1179.
- Yin N, Brimble MA, Harris PWR, et al. (2014). Enhancing the oral bioavailability of peptide drugs by using chemical modification and other approaches. J Med chem. 4: 763-769.
- Goldberg M, Gomez-Orellana I. (2003). Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2: 289-295.
- Lalatsa A, Lee V, Malkinson JP, et al. (2012). A prodrug nanoparticle approach for the oral delivery of a hydrophilic peptide, Leucine (5)-enkephalin, to the Brain. Mol Pharm. 9: 1665-1680.
- Lowe PJ, Temple CS. (1994). Calcitonin and insulin in isobutylcyanoacrylatenanocapsules: Protection against proteases and effect on intestinal absorption in rats. J Pharm Pharmacol. 46: 547-552.
- Torres-Lugo M, Garcia M, Record R, et al. (2002). Physicochemical behavior and cytotoxic effects of p(methacrylic acid-g-ethylene glycol) nanospheres for oral delivery of proteins. J Controlled Release. 80: 197-205.
- He W, Tan YN, Tian ZQ, et al. (2011) Food protein-stabilized nanoemulsions as potential delivery systems for poorly water-soluble drugs: Preparation, in vitro characterization and pharmacokinetics in rats. Int J Nanomed. 6: 521-533.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences