The State of Art and Possible New Applications of Nano/Meso Porous Silica Aerogel
T.Faez, M.S.Yaghmaee, S.Sarkar
Research Center for Science & Technology in Medicine,Tehran University of Medical Sciences,Tehran,Iran
Abstract
Aerogel is synthesized using sol-gel processing followed by supercritical solvent extraction, which leaves the original gel structure virtually intact. Although aerogels have been formed from metal oxides, polymers and carbon, the most interesting composition is silica. Aerogels are meso/nanostructured, open pore materials with many unusual properties including transparency, high thermal resistance, very low refractive index and sound velocity and high surface area. One limitation, however, on current processing methods centers on the polydispersity of pore sizes in the final aerogel, thus limiting some high performance applications. Here, we see an overview of the history and uptoday usage of silica aerogels.
Key words
IgE,Schistosoma,Th2 response,mast cell,basophil,eosinophil,infection.
1. Introduction
Aerogel,the lightest solid known,only three times denser than air which can protect virtually anything from the heat and cold,and could support up to 1000 times its own weight. It is known also as frozen-smoke or air-glass. Microscopically consists of nano-meter sized particles 1-10 nm diameter,which stick together and form chains. These particles have so many points of contact that a stable three-dimensional network is established in which the distance between the chains (the diameter of pore channels) is typically 10-100 nm.
Many people assume that aerogels are recent products of modern technology. In reality,the first aerogels were prepared in 1931 by Steven. S. Kistler. If the wet gel is simply allowed to dry on its own,the gel would shrink,often to a fraction of its original size,since the liquid-vapor interface of the evaporating liquid exerted strong surface tension forces that collapsed the pore structure. If a liquid is held under pressure always greater than the vapor pressure,and the temperature is raised,it will be transformed at a critical temperature into a gas without two phases having been present at any time [5]. They had been largely forgotten when,in the late 1970s,the French government was seeking a method for storing oxygen and r°Cket fuels in porous materials. This directly led to the major advances in aerogel science,namely the application of sol-gel chemistry to silica aerogel preparation. The reaction of a metal alkoxide with water a metal hydroxide is formed and then a condensation reaction °Ccurs between each two metal hydroxides. The molecular weight of the produced oxide species continuously increases and as they grow they begin to link together and making an alcogel. The drying alcogel under supercritical alcohol conditions produces aerogel.
Different products of aerogel could be: powders,monolithic,flexible blankets and clamshell preformed insulation. The disposal of silica aerogels is perfectly natural. In the environment,they quickly crush into a fine powder that is essentially identical to one of the most common substances on earth,namely,sand. Additionally silica aerogels are completely non-toxic and non-flammable.
2. Properties
2.1 Microstructure:
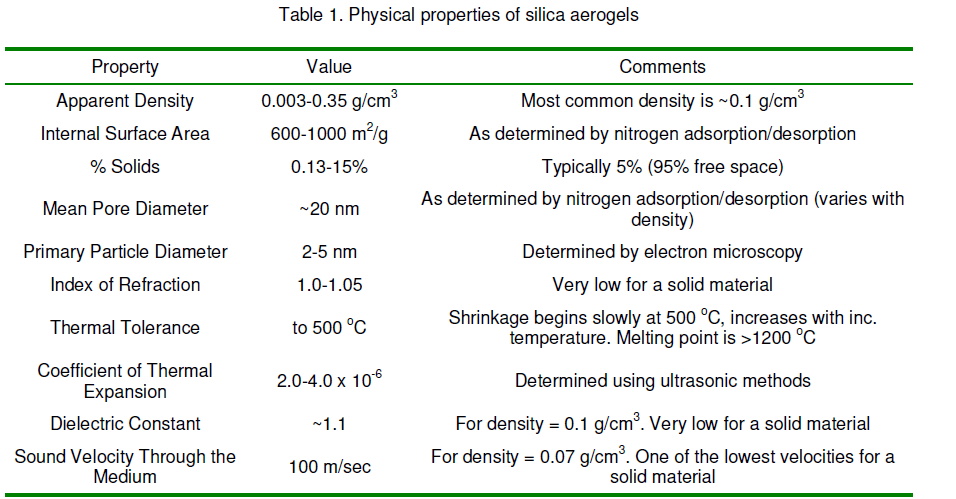
silica aerogels contain primary particles of 1-10 nm diameters. Silica particles of such a small size have an extraordinarily large surface-to-volume ration as 2*109 m-1 and a corresponding high specific surface area of 900 m2/gr. It is not surprising,therefore,that the chemistry of the interior surface of an aerogel plays a dominant role in its chemical and physical behavior. It is this property that makes aerogels attractive materials for use as a catalyst substrate,and adsorbent. Some other physical properties of silica aerogel are summarized in the Table.1. Most of the properties listed here are significantly affected by the conditions used to prepare the aerogel and any subsequent postpr°Cessing.
2.2 Thermal property:
A single one-inch thick windowpane of silica aerogel is equivalent to the insulation provided by 20 windowpanes of glass. Window heat loss accounts for up to 30 % of energy lost from home,but a welldesigned aerogel window could lower the needed heating and cooling cost by comparable figure. At higher temperature mostly above 200 °C,radiative transport becomes the dominant mode of thermal conduction,and must be dealt with. If silica aerogels are to be used at temperature above 200 °C,this mode of energy transport must be suppressed. This is accomplished by adding an additional component to the aerogel,either before or after supercritical drying. One of the most promising additives is elemental carbon,which is an effective absorber of infrared radiation and actually can also increase the mechanical strength of the aerogel. Generally these additives are with dimensions on the order of nanometers so the product can also legitimately be classified as nano-composite.
2.3 Transparency property:
Most of the pores in aerogel are too small to scatter visible light,but once in a while a few of the pore are larger. The lager pores scatter light as it passes through aerogel and this creates the hazy appearance. Aerogel produced on earth is cloudy,but scientist hope to produce a transparent variety in space that could lead to advances such as super insulating windows and extraordinary high speed computers.
3. Applications
3.1 Space technology:
It is also use for catching the stardust in space. The particles coming off the comet will probably be smaller than grains of sand,but they will be hitting the aerogel at an extremely high velocity almost 6 km/sec. The impact is so powerful that,with substance other than aerogel,the particles would either vaporize upon impact or become so distorted that scientist could not study them. By using aerogel as insulators in the Mars mission,engineers were able to cut the weight of the 23-pound Sojourner by 6 pounds (a weight saving of 20 %) and keep costs down.
3.2 Kinetic energy absorber:
aerogel at first,seem to be a poor choice for cushioning material. However,as silica aerogels are usually very low-density materials,the collapse of the solid network occurs gradually,spreading the force of impact out over a longer time. It should not even be forgotten the rebound effect which can be often do further damage to the object being protected,which is relatively low in the case of aerogels. These usages may include personal protection in motor vehicles and protection of sensitive equipment.
3.3 Microelectronics:
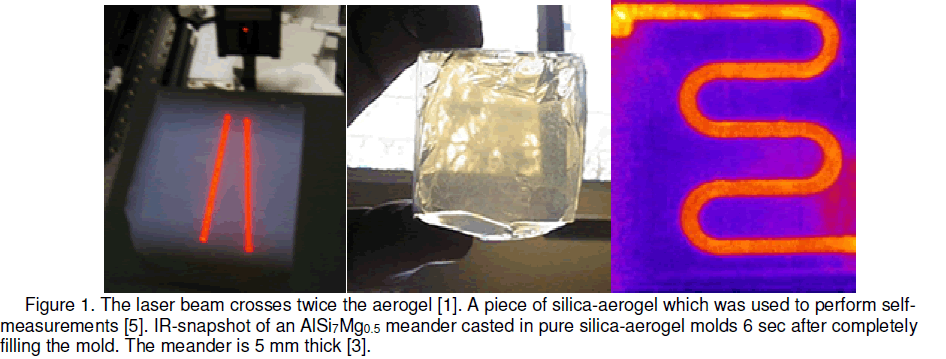
silica aerogel with having a dielectric constant as low as 2 which is more near to air than a solid material will help the researchers to double the computing speeds of computers in future. Further on the improvements in the field of microelectronics are directly correlated with the improvements in the crystal growth process. A silica crucible allows us to detect the crystallization front with a suitable camera due to its transparency. The silica-aerogel is not wetted by metallic melts and melts like Pb,Sn,Zn,Ge and GeSi and there are no chemical reactions with the melts [6]. This application may help to study the real time solid/liquid interface,growth velocity and temperature gradient of crystallization in silica aerogel mold in the future.
3.4 Transparent metals:
Usually metals are malleable and opaque and glasses are brittle and transparent,but a glass-like metal with 70 % emptiness is not only transparent but also provides metallic properties. This combination from the first side seems to be unimaginable but on March 1999 the researches at Rice University in Houston reported their ability to produce this new class of materials through silica meso-porous solid matrix [7].
The used solid material was silica spheres with 40 nm diameters that were packed and heat treated to 800 °C to get a networked structure of silica particles. The particle size of this matrix is higher than usual classified silica aerogel also they were produced by direct chemical heat treating process and not through sol-gel method,but they could still be categorized as meso-meters porous (nano/meso-cellular) foam class materials. These meso-porous silica foams were filled through different chemical steps with metals and after solidifying the metal the silica matrix were dissolved by hydrochloric acid. That is how they produced the first glass-like metal.
This exotic form of metal explore also the fantasy of engineers as one could simply imagine just as having any aluminum pieces in any device or automotive to be transparence,also a electricaldevice or panel-displays which could be checked from outside just as easy as one looks through it. There are also some fans of materials-science fictions that recall these transparency metals in Star Trek films,or even some of UFO-reports include the ability of the percipient to see through the craft's skin,as if it was made of glass. Well,at least now some of us have the knowledge to make it rather than just dream of having it. Obviously still a long way to do as the exact production method and refinement procedures should be developed further to produce the desired glass-like metals.
3.5 Drug Delivery System:
Being chemically inert and non-harmful to the human body,silica aerogels may easily find an application in the pharmaceutical industry and agriculture. Golob P. [13] described the application of fine silica aerogel powders for the storage protection of grains in the agricultural industry. Having a small particle size and very large surface area,aerogel powders can absorb the protective lipid layer of insects causing the organisms to lose body fluid and,consequently,die.
The chemical composition of silica aerogels is identical with that of fumed silicon oxide,produced by combustion of silicon chloride. Amorphous silicon oxide has been used in the pharmaceutical industry for many years. The corresponding product is called “Aerosil”. It has been shown that orally administrated Aerosil passes through the gastrointestinal tract without being resorbed in detectable quantities. It is expected that silica aerogels having the same chemical composition and amorphous structure as Aerosil would have similar clinical characteristics. Aerogels have much larger internal surface (500-1000 m2/g) than Aerosil (around 200 m2/g),which enables them to exhibit superior properties to Aerosil,in particular applications. Schwertfeger F.,et al. [14] reported the use of silica aerogels as potential carrier materials in medicine and agriculture. Here both hydrophobic and hydrophilic aerogels were loaded with pharmaceuticals by means of adsorption from corresponding liquid solutions. By choosing a suitable hydrophilic or hydrophobic aerogel,the substance with which the aerogel is loaded can be released in an accelerated or a delayed form. Loading with the target compound took place and then the resulting mixture was filtered in order to yield the loaded aerogel. The resulting powder was dried and could be used as a drug delivery system (DDS).
4. Self measurements
Previously we have attempt to measure the mechanical property of silica aerogels by a compressing test method [8]. The results showed a maximum detected load between 1300-1400 N which considering also the original contact area of loading the value of 650-700 kN/m2 can be registered for maximum compressing stress limits. Unfortunately,it is still difficult with the available methods to characterize the true porosity of aerogels. Silica aerogels possess pores of micro-,meso- and macro-pores sizes (pore size less than 2 nm,between 2-50 nm and greater than 50 nm,respectively). However,the majority of the pores fall in the meso-pore regime,with relatively few micropores. In previous work [8] for determining the true density of the aerogel the gas adsorption method with He gas was used in order to answer two questions: the ratio of the closed and open pores and the characteristic size of pores. The amounts of the closed pores were found to be negligible in the material and the apparent density was determined by weighting a silica aerogel sample having a welldefined geometry. Using the true and the apparent density data the porosity were calculated 90 %. Further on it was found that material showed an apparent density of 0.20 g/cm3,a true density of 2.02 g/cm3,a total specific pore volume of 4.5 cm3/g,a mezo- and micro-pore specific volume of 0.40 cm3/g,a micro-pore specific volume of 0.19 cm3/g,an average pore radius of 26 nm and a specific surface area (N2,77 K) of 332 m2/g.
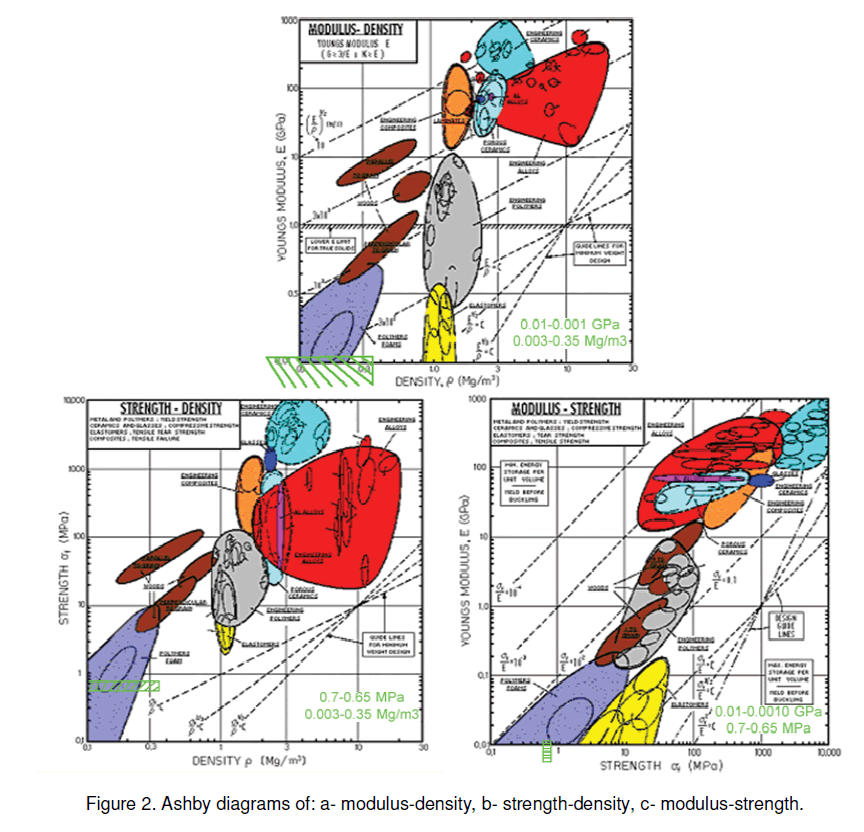
Using a compressing test and gas adsorption method [8],we tried to study more deeply the interconnection between the pore structure and the physical properties of the silica aerogel. Considering the concept of Ashby material-selection,this information will help us to figure out the position of this material more precise,in order to select a specific material for special application. The measured data and the data available in literature were put in to some Ashby diagrams in order to compare the Silica-aerogel with some known material [4].
5. Conclusion
The production of insulating and transparent materials through aerogel manufacturing in space can develop into a substantial market for residential and commercial applications. The excellent thermal properties and transparent nature of silica aerogel make it an obvious choice for super insulation windows,skylights,solar collector covers,specialty windows and the future of the computing industry as a new chip material. They are also nonflammable,nontoxic,lightweight,transparent and thermally stable to about 800°C. The production of these nano/meso-cellular groups of materials with brilliant extra properties should be further on improved in other to get uniform designed network structure and in parallel using nano-additives a new class of nano-composite materials could be created which widen the engineering applicability of silica aerogels and their related materials classes.
References
- URL: https://castore.mib.infn.it/ lhcb/aeropictures.html
- URL: https://stardust.jpl.nasa.gov/photo/aerogelbrick .jpg
- URL: https://www.kp.dlr.de/WB-RS/Erstarrung/web_eng /Feing_eng.html
- URL: https://edu.bzlogi.hu/anyagkivalasztas/ashby/fra me.htm#s0001.htm
- Yaghmaee M.S.,Kaptay G. (2000) Pr°Ceeding of micr°CAD Conference,Applied Chemistry section,Miskolc,Hungary,87-92.
- Sing K.S.W.,Everett D.H.,Haul R.A.W.,et al. (1985) Pure and Appl. Chem,57: 603-619.
- Weast R. (1963) Handbook of Chemistry and Physics,44.ed. The Chemical Rubber Pub. Co. Cleveland. pp.646.
- Lowell S.,Schields J.E. (1998) Powder Surface Area and Porosity,3. ed. Chapman and Hall,New York,pp.17.
- Rodriguez R.F.,Linares S.A. (1986) Microporous structure of activated carbons as revealed by adsorption methods. In Chemistry and Physics of Carbon (PA Thrower,ed.). Dekker,New York,NY,Vol. 21. pp. 2-146.
- URL: https://eande.lbl.gov/ECS/aerogels/saphoto.htm
- URL: https://science.nasa.gov/newhome/headlines/ms ad04feb99_1a.htm
- URL: https://www.kalwall.com/main.htm
- Golob P. (1997) Current status and future perspectives for inert dusts for the control of stored product insects,Journal of Stored Products Research 33: 69-79.
- Schwertfeger F.,et al. (2001) US Patent 6,280,744,issued 28.08.2001.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences