The Effects of Zirconium Oxide Nanoparticles on FSH, LH and Testosterone Hormones in Female Wistar Rats
Shahin Omidi, Masoud Negahdary, Heydar Aghababa
1Department of Biology, Islamic Azad University, Arsanjan Branch, Arsanjan, Iran;
2Yazd Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
- Corresponding Author:
- Tel: +989177013906
E-mail: heydar2001@yahoo.com
Abstract
Nowadays, nanoscience has attracted the attention of many researchers and different industries. Although nanostructures produced by nanoscience have many advantages, but their toxic and harmful effects are always the researchers' main concern. In the present study, the effects of Zirconium Oxide nanoparticles on FSH, LH and testosterone hormones were investigated in female rats. The main aim of the study was to find ways to prevent entry nanoparticles to human body. Zirconium oxide nanoparticles with dosage of 100, 200, 400 ppm doses were intraperitoneally injected to 40 female rats which were divided into 5 groups (each group 10 rats). Then, their blood samples were collected. The levels of LH, FSH and testosterone were measured by the Biochemical Kit and the groups were compared. The results showed no significant changes of studied hormones up to 200 ppm dose of nanoparticle; while in higher 400 ppm, the concentrations of LH, FSH and testosterone in the test groups were decreased significantly in comparison with those of the control group (P < 0.05). These changes are related to inhibitory effect of zirconium oxide nanoparticles on cells producing LH, FSH and testosterone functions. These findings suggest that zirconium oxide nanoparticles can change the levels of LH, FSH and testosterone in female rats that may have results in metabolic interferences and liver insufficiency.
Keywords
Zirconium Oxide nanoparticles; Steroid Hormones; Female rats.
1. Introduction
Nanoparticles have wide applications in biomedical fields, such as tumor therapy, gene and drug delivery, labelling cells and macromolecules [1]. Based on these applications it is very important to have focus on their possible toxic and harmful effects on the growth, proliferation and viability in cells and organisms [2]. The findings of these studies concern the researchers about harmful effects of nanoparticles. This challenge can also inform the researchers & institutes about working on production of nanoparticles; therefore they could perform required actions to changes & control manufacturing methods effectively as well as to reduce harmful effects by adopting the proper methods [3,4]. According to the previous researches can be found that entry nanoparticles can cause various embryonic disorders in the tissues and organs of developing embryos. these effects may be raised from releasing the ionic species which are still able to be oxidized, interfere in ion and electron transport chains inside the cell and mitochondria, release the reactive oxygen species [ROS] which have result in peroxidation and degradation of cell membranes by affecting their components [5], and entry the nanoparticles to the cell nucleus. Eventually, nanoparticles can inhibit the fetus’s normal growth and maturation after birth by penetrating to the very microscopic cell nucleus. As a result of in vivo effects of nanoparticles, the produced oxidative stress could be considered as a response to the cell damage [6]. Oxidative stress caused by nanoparticles might have some reasons: (a) direct ROS forming from nanoparticles due to presence of oxidizing agents and free radicals on the surface of particles [7]; (b) many studies revealed that nanoparticles arrived to the mitochondria can cause the physicals damages, which may result in oxidative stress [8]; (c) the action of inflammatory cells including macrophages and neutrophils of lung alveoli which are involved in phagocytosis of nanoparticles in a way that the phagocytotic function of these cells may lead to production of reactive oxygen and nitrogen species [9]. In addition, metal nanoparticles (e.g. ferrous, copper, chromium, zirconium, etc.) can produce ROS. Generating of Hydroxyl free radicals can have result in ROS forming which can lead to critical and inheritable DNA damages. For instance, chemical modifications in histones or other proteins which are involved to DNA structure, DNA supercoil and expose it to any other modifications [10]. Mitochondrial genome is considerably vulnerable Vs the oxidative attack [10]. Agarwal et al., suggested that the high level of ROS cause damages to mitochondrion outer and inner membranes that lead cytochrome C release from the mitochondrion and activate cascade reactions and at finally step stimulate apoptosis. Therefore, ROS has functions as a mediator in apoptosis process [11]. In vitro studies on spermatozoid cells showed that silver nanoparticles can affect the sperm cells and led to infertility [12]. Furthermore, previous researches have focused on inhibitory effects of silver nanoparticles on cells proliferation. There are increasing interests in studying the effects of nanoparticles on cancer cells with regard to their anti-proliferation effects. The particles size is important feature of nanomaterials that play a key role in special nanoparticles properties. The particles size can reflect their physicochemical properties and increase the possibility of its sorption to and interacting with biological tissues [14,15]. In fact, the size of the nanoparticles has been investigated more than other nano-scale properties [16]. Up to now, findings showed that biological activity by using of nanomaterials is increased as their size decreased [17]. Today, this science is used in multiple industries [17]. The main aim of the present study was investigating the toxicity effects of zirconium oxide nanoparticles on experimental animals as a simulation model of the human body.
2. Materials and Methods
2.1. Apparatus
The devices were used in this study included a Centrifuge machine (eppendorf), an Electrochemiluminescence device, a Transmission Electron Microscope (JEM-200CX), and a diffractometer (XRD) using CuKα radiation (TU-1901 double-beam). The absorbance properties of Zirconium oxide nanoparticles were investigated by using a TU-1901 double-beam UV-visible spectrophotometer. Biochemical tests were conducted using Electrochemiluminescence device Elecsys 2010 (manufactured by HITACHI Co.; Japan and Roche Co., Germany) which is designed in a completely automatic procedure using specific kits to perform all hormonal tests.
2.2. Materials
Ketamine was used to anesthetize rats. Laboratory kits (FSH, LH, and TSS) were purchased from Pars- Azmoon Co., Iran, which has reference laboratory confirmation. Zirconium oxide nanoparticles in the size of 100 nm and distilled water were also used. Zirconium oxide nanoparticles (100 nanometer) were Synthesized and the particles surface area were analyzed by particle Size Analyzer. Suspensions of nanoparticles were shaken for 2 minutes and were fed to the rats with different doses (100, 200, 400 mg/kg) via oral gavage [1].
2.3. Laboratory animals, diets and the required conditions to maintain
Fifty adult female Wistar rats weighing 250-350 grams at the age of 8 weeks were purchased from Shahrekord University (Shahrekrd, Iran) and were used to do experiments. The animals were grouped into 5 groups including a sham group had received normal food and water; a control group had received saline and 3 experimental groups. Ten rats were included in each group and all groups were kept in polypropylene cages with sawdust-covered floors and water container. The groups were kept under the controlled temperature conditions (22 ± 1°C), humidity (60 ± 10%), and light (12 hours brightness, 12 hours darkness), with free access to water and complete food. All rats were kept in the nest with similar conditions for 2 weeks prior to start of the experiments in order to adapt to their environment and diet. All procedures followed were in accordance with the ethical committee. Rats of treatment groups were marked by signs and were fed by oral gavage for 10 days.
2.4. Blood Samples Collection and Serum Preparation
To perform the blood biochemical tests, blood sampling was done for 10 days. The blood samples were centrifuged and the sera of samples were separated at 3000 RPM enabled 10 minuts. The concentrations of LH, FSH and Testosterone of sera specimens were measured by Electrochemiluminescence device. this device was used for hormonal tests.
2.5. Synthesis of Zirconium oxide nanoparticles
The preparation of Zirconium oxide was performed by adding an aqueous solution of ammonia (25 wt %) in the form of drops to an aqueous solution containing Zirconium precursor and a nonionic surfactant (Pluronic P123 block copolymer) at room temperature with continuous stirring. The surfactant to zirconium molar ratio was chosen as 0.03. Following the precipitation, the resulting slurry was stirred for 30 minutes and then refluxed with continuous stirring at 88 °C for 24 h. Following refluxing, the mixture was cooled at room temperature and then cooled mixture was filtered and then washed first with deionized water and then with acetone. This procedure is required for effective removal of the surfactant from the formed precipitate. The final product is dried at 100 °C for 24 h and then calcinated at different conditions.
2.6. Nanoparticles Suspension Preparation
To prepare a stock solution of Zirconium oxide nanoparticles, a suspension was prepared with 10g of nanoparticle per liter of sterilized medium. For suitable dispersing of nanoparticles the ultrasonic device (PARSONIC 7500s, Pars Nahand ENGG.Co.Iran) was used for 30 minutes. Nanoparticle suspensions were prepared to prevent of error incidence.
2.7. Statistical Analysis
Data obtained from Electrochemiluminesence device was saved and analyzed using SPSS software, version19. Analysis of variance (ANOVA) test was performed by SPSS, and then the outputs were transferred to the Microsoft Excel 2010. The results were presented as mean and standard deviation (Mean ± SD). The data were normally distributed; therefore, repeated measures ANOVA were used to compare the enzymatic results among the groups before and after experiment, as well as the ANOVA and LSD tests were applied to compare the groups in each time duration. The Level of significance was considered less than 0.05.
3. Results
3.1. Electron microscope study of Zirconium oxide nanoparticles
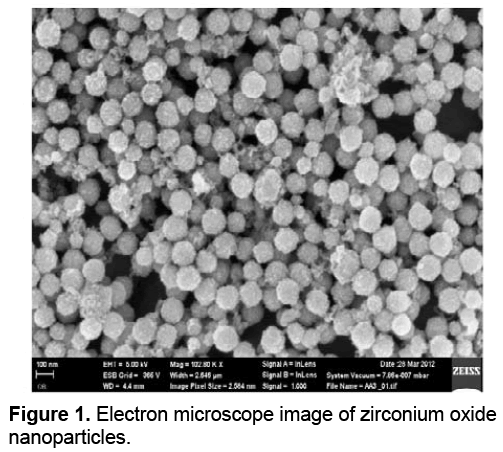
Transmission electron microscope (TEM) has larger visual zoom vs. light microscope. Although the principle of its operation is similar to the light microscope, the beam unlike the light microscope shines from top-to-bottom. The TEM has a long column such that electron beams source that is located at the top of this piston. Electron beams after passing through the sample, hit a film or a screen (which is made of fluorescent material) and cause an image to be formed. Images of electron microscope are black and white, because some beams do not cross through the sample and creates black dots. Transmission electron microscope (TEM) image of synthesized Zirconium oxide nanoparticles is shown in Figure 1.
Due to the increased surface to volume ratio in nanoparticles, by reducing the size of the smaller nanoparticles, they can play a suitable role in the immobilization processes. According to the results of the TEM studies the diameter range of 10 nm had been found for Zirconium oxide nanoparticles [18].
3.2. X-Ray diffraction of synthesized nanoparticles
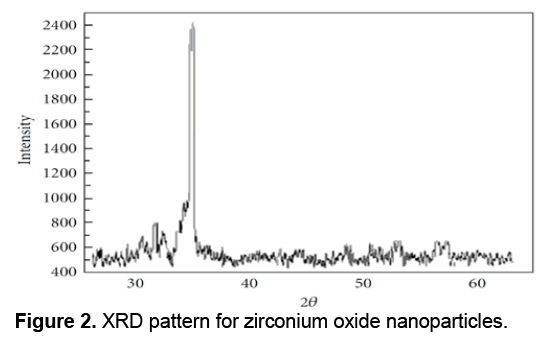
XRD or X-Ray Diffraction is a widely used technique for characterization of crystals in a way that the X-ray diffraction of samples is used to study the characteristics of the samples. XRD can be used to determine the overall quantities related agents to the structure of crystals, including lattice constant, lattice geometry, qualitative determination of unknown materials, to determine the crystal phases, crystal sizes, single crystal orientation, stress, tension, lattice errors, etc. X-Ray Diffraction is a cost effective technique due to simplicity of its physical principles. Data from the X-ray diffraction included angle of maximum diffracted beam intensity; diffracted beam intensity at each angle and the width of each maximum is dependent on a wide range of properties and quantities of crystals. Due to benefits of XRD technique and its application for providing useful information, it becomes a widely used method. For example vacuum condition is not needed in XRD resulted in reducing the costs of construction and make it preferred to the electron techniques. The XRD technique is a non-contact and non-destructive technique and requires no complicated and difficult preparation. In X-ray diffraction apparatus, X-ray shines cross through an unknown sample via a light generating tube and the diffracted beam intensity measured at different angles. Therefore, the operation of this apparatus is determining the angles by which the diffraction phenomenon occurs based on Bragg equation (2dsinθ = nλ). The XRD pattern for the Zirconium Oxide nanoparticles is shown in Figure 2.
Diffraction peaks was absorbed in the 2θ value. Scherrer Formula was used to estimate the pattern size with the prominent peak [19]; D = Kλ/ (β cos θ) where K is a constant (0.9), λ is the wavelength (λ = 1.5418 Å) (Cu Kα), β is the full width at the half-maximum of the line and θ is the diffraction angle. The size estimated using the density peak for nanoparticles was ranged from 100 to 8000 nm and an increase in sharpness of XRD peaks indicates the crystalline nature of particles. All showed peaks in Figure 2 refer to Zirconium Oxide nanoparticles that should be matched with together in any powder diffraction study.
3.3. Characteristics of the visible-UV spectrum of Zirconium Oxide nanoparticles
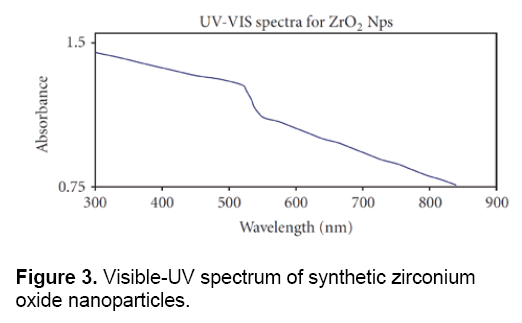
Figure 3 shows the visible-UV spectrum of Zirconium Oxide nanoparticles which were synthesized by chemical methods. In this cleared that the absorption peak is located between 900- 300 nm. The results of visible-UV spectrum, confirmed the data obtained from electron microscopy and proved the special and quantum properties of these nanoparticles.
3.4. Analysis of blood factors
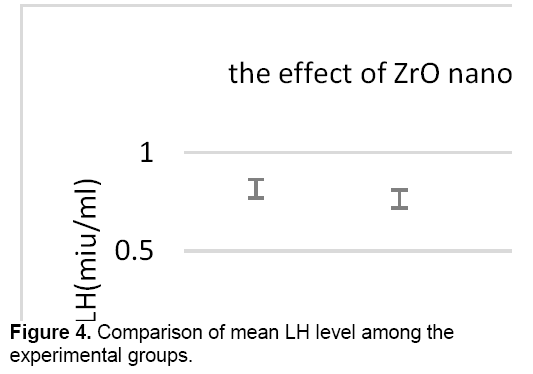
There was no significant difference in the level of LH between control groups and sham, therefore the sham group was applied to other experiments. As shown in Figure 4, the concentration of LH decreased in all test groups against control group. However, this reduction is significant only in the test group that received 400 mg/kg of zirconium oxide nanoparticles.
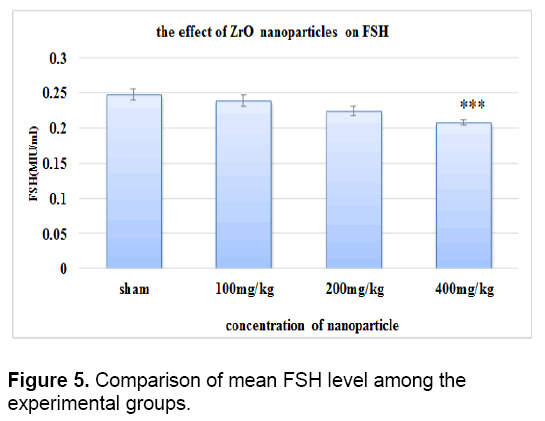
There was no significant difference in the level of FSH between control groups and sham. As it is shown in Figure 5 the concentration of FSH decreased in all test groups against control group. This reduction is significant only in the test group that received 400 mg/kg of zirconium oxide nanoparticles.
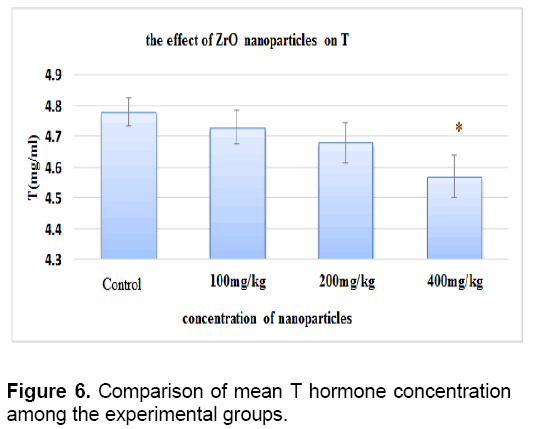
There was no significant difference between level of testosterone in control groups and sham. The concentration of testosterone decreased in all test groups against the control group. This reduction is significant only in the test group that received 400 mg/kg of nanoparticles (Figure 6).
4. Discussion
In the present research to investigate toxicity effect, the dosage 100, 200, 400 mg/kg.w.b of Zirconium oxide nanoparticles were used. The female Wistar rats included in the test groups were treated with nanoparticles by oral gavage for 10 days. To following treatment, the blood samples were collected and Testosterone, FSH, LH hormones were measured. The results of this study showed that by increasing the concentration of nanoparticles the levels of mentioned hormones were reduced and lower level of these hormones are released into the blood. Other researchers have examined the effect of Titanium Dioxide nanoparticles on sex hormones and testicular tissues and concluded that there was a significant increase of LH by increasing the dose of nanoparticles while the level of testosterone is significantly decreased and FSH levels did not change significantly, however there were not found any significant pathologic changes [20]. The result of above mentioned study about testosterone was similar to the findings of present study. Fuse et al. showed that there was a significant dependence between zinc and plasma testosterone concentrations and also sufficient amount of zinc was necessary for the normal sperm cells function [21]. Other studies showed that zinc nanoparticles disorder leads to atrophy of the seminiferous tubules and impaired spermatogenesis in rats [22]. Carlson et al. founded that nanoparticles can affect leydig cells mitochondrial activity and thus lead to reduce their secretory activity. Furthermore, the nanoparticles increase the releasing of free oxygen and this enhance the oxidation of macromolecules such as proteins [23], and eventually leading to reduce the leydig cell numbers and decrease the production of testosterone which is related with the present study. Another opinion is nanoparticles probable effects on gene expression of a protein which has a key role in transportation of cholesterol to the mitochondria membrane and increase the synthesis of steroids. Maybe nanoparticles lead to reducing the gene expression and inhibit the transportation of cholesterol to mitochondrial inner membrane. This causes to prevent the conversion of cholesterol to prognolone and reduces the level of testosterone. Karpenko studied the toxicity effects of cerium oxide nanoparticles on sex hormones and concluded that the nanoparticles have toxic effects on the sexual glands and testosterone, as the level of testosterone increases after 70 days [24]. In another study the effects of Zinc oxide nanoparticles were examined by different doses (5, 10, 20, 40 mg/kg), which Zinc oxide nanoparticles were intraperitoneally injected to 48 wistar rats. After 21 days the serum levels of LH, FSH and testosterone were measured. The results of above study, showed that Zinc Oxide nanoparticles had a significant effect on the concentration of testosterone, and a significant decrease was also found in the level of FSH at the dose equal to 40 mg/kg, however there was not found any significant changes in the level of LH. It is likely that the negative feedback from increased thyroid-stimulating hormone (TSH) decreased the levels of LH and FSH significantly [25]. The present study also reported a decrease in level of FSH.
5. Conclusion
The results of present research showed reduction in the levels of LH, FSH, TSS hormones. According to worldwide applications of nanoparticles especially in our country (Iran), we should have attention and more detailed & comprehensive studies on the toxicity effects of nanoparticles on the hormones. Finally, it is noteworthy that considering the Zirconium oxide nanoparticles have harmful effects on the hormones effects and due to the increasing use of nanotechnology, solutions must be found that preventing the nanoparticles to have toxicity effects on human body.
References
- Bing W, Weiyue F, Meng W, Tiancheng W, Yiqun G, Motao Z, Hong O, Junwen S, Fang Z, Yuliang Z, Zhifang C, Haifang W, Jing W (2008) Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice, J Nanopart Res. 10: 263-276.
- De Jong WH, Roszek B, Geertsma RE (2005) Nanotechnology in medical applications: possible risks for human health, RIVM report 265001002.
- Ingrid H, Sylvia F, Werner L, Robert H, Wilfried A, Rudolf H, Werner AK (2003) Cytotoxicity of selected magnetic fluids on human adenocarcinoma cells, J Magnetism and Magnetic Materials 261: 7-12.
- Tetley TD (2007) Health effects of nanomaterials, Biochemical Society Transactions, 35 (3): 527-531.
- Melanie Auffan, Je Rose MR, Wiesner JB (2010) Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro, Environmental Pollution 157: 1127-1133.
- Sikka Sc, Rajasekaran M, Hellstrom WJ (1995) Role of oxidative stress and antioxidants in male infertility. J Androl. 16: 464-468.
- Larrisa M, Hempel M (2012) Recent Advances in Intracellular andIn Vivo ROS Sensing: Focus on Nanoparticle and Nanotube Applications. Int. J. Mol. Sci. 13: 10660-10679.
- Singh N, Manshian B, Jenkins G, Griffiths S, Williams P, Maffeis T, Wright CH, Doak Sh. (2009) NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials. 30: 3891-3914.
- Jiaen Li, Muralikrishnan S, Ng Ch, Lanry Yung L, Bay B (2010) Nanoparticle-induced pulmonary toxicity. Experimental Biology and Medicine, 235: 1025 -1033.
- Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl R (2009) Titanium Dioxide Nanoparticles Induce DNA Damage and Genetic InstabilityIn vivo in Mice. Cancer Res. 69: 22.
- Agarwal A, Saleh RA, Bedaiwy MA (2003) Role of reacyive oxygen species in the pathophysiology of human reproductive. Fertile Steril. 79(4): 829-843.
- Burd A, Kwok CH, Huang SC, Chan HS, Gu H, Lam WK, Huang L (2007) A comparative study of the cytotoxicity of silver-based dressings in monolayer cell,tissue explant, andanimal models. Wound Repair Regan, 15: 94-104.
- Silver S (2003) Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 27:341-353
- Jianrong C, Yuqing M, Nongyue H, Xiaohua W, Sijiao L (2004) Nanotechnology and biosensors. Biotech Advances. 22: 505-518.
- Zhang XD, Wu HY, Wu D, Wang YY, Chang JH, Zhai ZB, Meng AM, Liu PX, Zhang LA, Fan FY (2010) Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine. 5: 771-781.
- Gade A, Ingle A, Whiteley C, Rai M (2010) Mycogenic metal nanoparticles: progress and applications, Biotech. Lett. 32: 593-600.
- Sinha R, Kim GJ, Nie S, Shin DM (2006) Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery, Mol. Cancer. Ther. 5(8): 1909-1917.
- Rezaei-Zarchi S, Saboury AA (2007) Use of silver nanoparticles as an electron transfer facilitator in electrochemical ligand-binding of haemoglobin, J. Appl. Electrochem., 37:1021-1026
- W. Li, G. S. Hsiao, D. Harris, R. M. Nyffenegger, J. A. Virtanen, R. M. Penner, (1996) Mechanistic study of silver nanoparticle deposition directed with the tip of a scanning tunneling microscope in an electrolytic environment, The Journal of Physical Chemistry., 100 (51): 20103-20113.
- Mohammadi Fartkhooni F, Noori A, Momayez M, Sadeghi L, Shirani K, Yousefi Babadi V (2013) The effects of nano titanium dioxide [TiO2] in spermatogenesis in wistar rat. European Journal of Experimental Biology, 3(4):145-149.
- Hammadeh ME, Alixides Filippos A, Hamad MF. (2009) Reactive oxygen species and antioxidant in seminal plasma and their impact on male fertility. Int J Fertil Steril. 3 (3): 87-110.
- Fuse H, Kazama T, Ohta S, Fujiuchi Y (1999) Relationship between zinc concentrations in seminal plasma and various sperm parameters. International Urology and Nephrology, 31(3): 401-408.
- Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ (2008) unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B, 112(43):13608-13619.
- Karpenko NA, Malukin YV, Koreneva EM, Klochkov VK, Kavok NS, Smolenko NP, Pochernyaeva SS (2013) The Effects of Chronic Intake of Cerium Dioxide or Gadolinium Ortovanadate Nanoparticles in Aging Male Rats. In Proceedings of the International Conference Nanomaterials: Applications and Properties, 2(4): 04NABM28 (1-4).
- Espanani HR, Fazilati M, Sadeghi L, Yousefi Babadi V,Bakhshiani S, Amraie. (2013) Investigation the Zinc Oxide Nanoparticle’s Effect on Sex Hormones and Cholesterol in Rat. International Research Journal of Biological Sciences. 2(8): 54-58.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences