The Effect of Some Plant Growth Regulators (PGRs), Coconut milk and Casein Hydrolysate on Somatic Embryogenesis of Stevia (Stevia rebaudiana) as an Anti-Diabetic Medicinal Plant
Maryam Dehestani-Ardakani, Kazem Kamali, Soghra Rezaie
1Department of Horticultural Science, Faculty of Agriculture & Natural Resources, Ardakan University, Ardakan, Iran
2Department of Soil Science, Faculty of Natural Resources, Yazd University, Yazd,Iran
Received date: August 15, 2017; Accepted date: September 27, 2017; Published date: October 04, 2017
Citation: Dehestani-Ardakani M, Kamali K, Rezaie S. The Effect of Some Plant Growth Regulators (PGRs), Coconut Milk and Casein Hydrolysate on Somatic Embryogenesis of Stevia (Stevia rebaudiana) as an Anti-Diabetic Medicinal Plant. Electronic J Biol, 13:4
Abstract
Background: Stevia (Stevia rebaudiana), a sweet herb and anti-diabetic medicinal plant, could be replaced by artificial sweeteners and even table sugar. It grows in South America. Methods and findings: Somatic embryogenesis of leaf and bud explants was studied in two experiments in a completely randomized design with 5 replications. At first experiment, different concentrations of 6-benzyladenine (BA), α-naphthaleneacetic acid (NAA) and 2,4-Dichlorophenoxyacetic acid (2,4- D) on Murashige and Skoog (MS) culture medium were tested. According to the results the highest percentage of embryogenesis callus obtained in T2 (MS medium supplemented with 1 mg/l BA+2 mg/l NAA) and T6 (0.01 mg/l BA+1 mg/l 2,4-D) (10.87%) and the lowest one (1.25%) observed in T11 (0.5 mg/l NAA+0.5 mg/l 2,4-D). As well as the highest embryogenesis percentage was obtained in MS medium supplemented with 0.01 mg/l BA and 1 mg/l 2,4-D. In second experiment, the effect of casein hydrolyzate in 5 concentrations (0, 50, 100, 150 and 200 mg/l) and coconut milk in two levels (0 and 55 mL) on stevia embryogenesis was investigated. According to the result 78.82% of bud and 25.09% of leaf explants were induced embryo in MS medium. Coconut milk and casein hydrolysate did not show significant difference on callus induction. Resulted embryos were transferred to MS medium containing 0.1 mg/l GA3 for organogenesis, and some of the embryos were grown to complete plant. Conclusion: In this study the most embryo induction was obtained by using MS medium without casein hydrolysate and the lowest one obtained by 50 mg/l casein hydrolysate. It proposed that type and concentration of different PGRs for embryogenic callus induction and somatic embryogenesis of Stevia investigated.
Keywords
Casein hydrolysate; Coconut milk; Somatic embryogenesis; Stevia rebaudiana.
Abbreviations
ANOVA: Analysis of Variance; MS: Murashige and Skoog; BAP: Benzylaminopurine; TDZ: Thidiazuron; IBA: Indole-3-butyricacid; 2, 4-D: 2,4-Dichlorophenoxyacetic acid; GA3: Gibberellic Acid; BA: 6-Benzyladenine; NAA: Naphtalin Acetic Acid; PGR: Plant Growth Regulators
1. Introduction
Stevia rebaudiana Bertoni, is a small, herbaceous, semi-bushy, perennial shrub of Asteraceae family. This plant is native to certain regions of South America-Brazil and Paraguay [1]. It is one of the important antidiabetic medicinal plants. Stevioside and rebaudioside of its leaves taste about 300 times sweeter than sugar [2]. It is a major source of sweetener and has enormous commercial importance. Stevia has various properties such as regulating blood sugar, preventing hypertension and tooth decay, anti-bacterial, anti-candidal, anti-fungal, anti-viral, diuretic, hypoglycemic, vasodilator and treatment of skin disorders [3,4].
Heterozygocity, self-incompatibility and low germination percentage are the most important problems in stevia seed propagation [5]. Because of lower number of individuals that can be obtained simultaneously from a single plant, classical vegetative propagation is not suitable [6]. To overcome all, multiplication and improvement of this medicinal plant, biotechnologically approaches specially tissue culture is the desirable alternative for rapid mass propagation of Stevia. Several studies have been reported clonal propagation of this plant [7-10].
Somatic embryogenesis and organogenesis have been the common pathways for clonal propagation of superior medicinal plant species. Somatic embryogenesis enables large numbers of plantlets to be produced within a short span of time. In Stevia, somatic embryogenesis has been reported from leaves [11-13]. Somatic embryogenesis in S. rebaudiana reported by Filho et al. [14] for the first time. Their results showed that combination of 10 or 25 mM 2,4-D and 1 mM BA in MS medium were effective for somatic embryogenesis. The embryos directly induced, without intermediate callus development. Filho et al. [15] cultured floret explants on MS medium supplemented with 2,4-D (9.05 and 18.10 mM) and kinetin (0 to 9.29 mM) and after 10 days, observed embryogenic callus. They reported that embryogenic callus was light green or light yellow color, it had compact structure and globular somatic embryos observed on its surface. Khan et al. [16] were found the highest direct regeneration frequency from leaves of stevia plant on MS medium supplemented with 1 mg/l BAP+1 mg/l NAA, while indirect regeneration from callus was obtained on MS medium fortified with 1 mg/l BAP+2 mg/l NAA. Ahmad et al. [17] after 30 days of stevia culture on MS medium+2.0 mg/l BA+2.0 mg/l 2,4-D was observed the best callogenic response.
Somatic embryogenesis of Stevia rebaudiana was successfully achieved from axillary buds using nodal and leaf as explants in basal culture medium (MS) with vitamins, sucrose (30 g/l), agar (0.9% w/v) and supplemented with 2, 4-D (2.0 mg/l)+BAP (0.2 mg/ l)+TDZ (0.2 mg/l).
Naranjo et al. [18] obtained the highest number of somatic embryos in MS medium fortified with glutamine, adenine and coconut milk and supplemented by the combination of 2,4-D (18.09 μM) and 2iP (7.38 μM) as a plant growth regulator. Somatic embryos in the regeneration medium containing gibberellic acid (GA3) (0.29 μM) and activated charcoal regenerated to the seedlings. In a small aromatic tree, Murraya koenigii, embryogenic callus was obtained from 90% zygotic embryonic axis and 70% cotyledon explants in MS basal medium supplemented with 8.88 μM BA and 2.675 μM NAA [19]. BA could increase multiplication rate, vitrification and somaclonal variation in Stevia plant [20].
Because of low energetic nature of stevia, many people especially diet conscious consumers are interested to use it as an alternative to chemical sweetners. Regeneration of S. rebaudiana by somatic embryogenesis is very important, since this technique can be used for clonal propagation of this plant within a short span of time. The goal of this study was investigation the effect of some PGRs and other additives on callus and embryo induction.
2. Materials and Methods
2.1 First experiment
S. rebaudiana explants were collected from tissue cultured plantlets. Leaf and bud explants were obtained from matured plants were grown in a primary MS medium supplemented with different concentrations of BA. The explants (leaf and bud) were transferred on MS medium basal medium. This medium fortified with different PGRs at various concentrations consisted of BA (0, 0.01, 0.5, 1 and 2 mg/l), NAA (0, 0.5, 1 and 2 mg/l), 2, 4 dichlorophenoxy acetic acid (2, 4-D) (0, 0.5, 1, 1.5, 2 and 2.5 mg/l) and supplemented with various sucrose concentrations (30, 40, 100 g/l) (Table 1). The pH of the media was adjusted at 5.8 before gelling the medium with (7.5 or 8 g/l) agar. The media were sterilized by autoclaving at a pressure of 1.2 atm. (121°C) for 15 min. Solid medium cultures were incubated either in 16/8 h photo period (25 μmol m-2 s-1) after stored in darkness for 4, 6 or 16 days (Table 1). According to Table 1, treatments designed in 13 groups.
The cultures were incubated at 25 ± 1°C with a photoperiod of 16 h at 3000 lux light intensity of cool white fluorescent light. All the experiments were performed twice with 5 cultures per treatment. Observations were made every day and data were recorded after 4 weeks of inoculation.
The secondary medium was consisted of MS medium without growth regulators with 30 g/l sucrose and 7 g/l agar for gelling. The explants were cultured for 5 weeks in primary medium and then embryogenic callus were transferred to the secondary medium to progress embryos. The color of callus was initially white in turning yellow and then after about 30 days showed green color.
2.2 Second experiment
Effect of casein hydrolysate and coconut milk on embryogenic callus of stevia was investigated. In the next step, the effect of different concentrations of casein hydrolysate (0, 100, 150 and 200 mg/l) and coconut milk (0 and 55 ml/l) their combination on the best somatic embryogenesis medium of previous experiment (MS supplemented with 0.01 mg/l BA+1 mg/l 2, 4-D) tested. These media solidified with 7.5 g/l agar; pH was adjusted to 5.8 prior to autoclaving at 121°C for 20 min. In this section, leaf and axillary buds used as explant.
The cultured were incubated 6 days in darkness then transferred to 3000 lux light intensity in 16/8 h photo period (25 μmol m-2 s-1) and temperature of 25 ± 1°C. All the experiments were performed twice with 5 cultures per treatment. After 5 weeks, embryogenic callus observed and after 8 weeks embryos formed. Embryogenic callus (in globular phase) transferred in two different media:
1) MS medium supplemented with 0.05 mg/l BA
2) MS medium contained B5 vitamins (2 mg/l glycine+1 mg/l nicotinic acid+10 mg/l thiamin+1 mg/l pyridoxine)+100 mg/l myoinositol+0.05 mg/l BA+1 g/l active charcoal
For organogenesis, embryos transferred to MS medium supplemented with 0.1 mg/l GA and darkness treatment for 10 days. Then all embryos transferred to 3000 lux light intensity in 16/8 h photo period (25 μmol m2 s-1).
2.3 Hardening
Rooted explants were exited from the glass jars, washed carefully with sterile water to remove agar media, placed in the plastic cups filled with sterilized peat moss and perlite by the 1: 3 ratio (Figure 1e). Plastic bags on the pots, used for maintaining humidity in pots. These plants incubated at 25 ± 1°C with a photoperiod of 16 h at 3000 lux light intensity of cool white fluorescent light. After hardening established plantlets, were re-potted in small pots containing sterilized garden soil and sand (1:1) (Figure 1f).
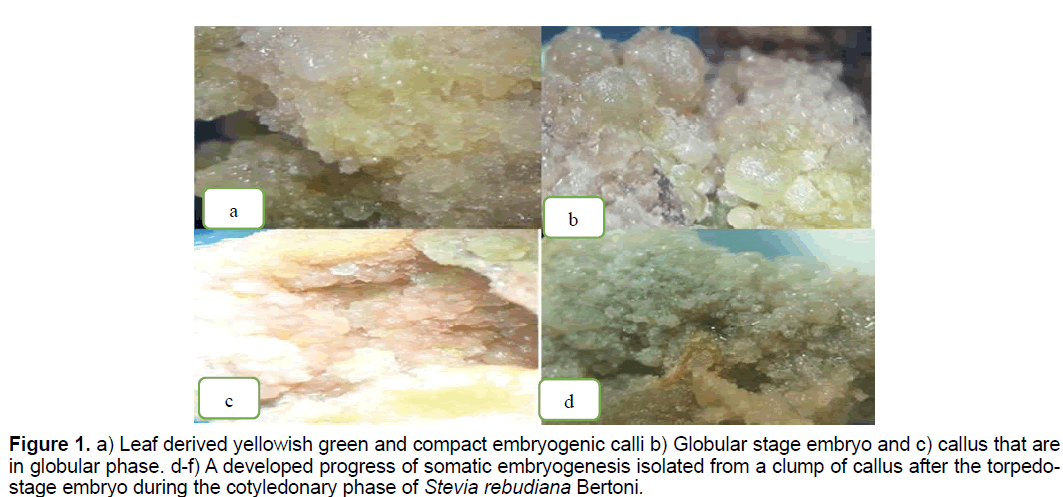
Figure 1. a) Leaf derived yellowish green and compact embryogenic calli b) Globular stage embryo and c) callus that are in globular phase. d-f) A developed progress of somatic embryogenesis isolated from a clump of callus after the torpedostage embryo during the cotyledonary phase of Stevia rebudiana Bertoni.
2.4 Observation recorded and statistical analysis
Data with regarded to frequency of multiple callus and embryo induction (mean ± SD) was recorded after 30 days of culture. Each treatment had five replicates and each single explant was considered as an experimental unit. All data are statistically analyzed by analysis of variance (ANOVA). Significant differences among the treatments were tested by Duncan‘s multiple range test at 5% level using SAS 9.1 software.
3. Results
3.1 First experiment
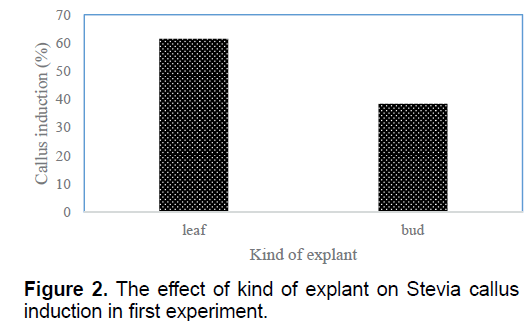
According to the results the most percentage of embryogenic callus obtained in T2 (1 mg/l BA+2 mg/l NAA) and T6 (0.01 mg/l BA+1 mg/l 2,4-D) (10.87%) and the lowest one (1.25%) observed in T11 (0.5 mg/l NAA+0.5 mg/l 2,4-D) (Table 1). These treatments showed friable proliferated callus with greenish yellow color, smooth and compact which produced somatic embryos (Figure 1). Between two kinds of explants, the highest callus induction (61.5%) observed in leaf explants (Figure 2).
The highest percentage (19.04%) of somatic embryogenesis was induced in T3 (2 mg/l BA+1 mg/l NAA), But T1 explants induced the lowest (2.27%) embryos (Table 1).
| Treatments | BA (mg/l) | NAA (mg/l) | 2,4-D (mg/l) | Sucrose (g/l) | Agar (g/l) | Ammonium nitrate (mg/l) | Darkness (days) | Callus induction | Embryo induction |
|---|---|---|---|---|---|---|---|---|---|
| T1 | 1 | 1 | 0 | 40 | 8 | - | 4 | 5.3cd | 2.27c |
| T2 | 1 | 2 | 0 | 40 | 8 | - | 4 | 10.87a | 10.88abc |
| T3 | 2 | 1 | 0 | 40 | 8 | - | 4 | 10.46ab | 19.04a |
| T4 | 0 | 0 | 2 | 40 | 7.5 | - | 6 | 9.2abc | 8.84bc |
| T5 | 0.01 | 0 | 0.5 | 40 | 7.5 | - | 6 | 4.6cd | 14.28ab |
| T6 | 0.01 | 0 | 1 | 40 | 7.5 | - | 6 | 10.87a | 12.24abc |
| T7 | 0.01 | 0 | 1.5 | 40 | 7.5 | - | 6 | 8.36abc | 8.16bc |
| T8 | 0.01 | 0 | 2 | 40 | 7.5 | - | 6 | 6.27bc | 8.84bc |
| T9 | 0.01 | 0 | 2.5 | 40 | 7.5 | - | 6 | 8.36abc | 9.52abc |
| T10 | 0.5 | 0.5 | 1 | 30 | 7.5 | - | 4 | 5.85cd | 8.16bc |
| T11 | 0 | 0.5 | 0.5 | 30 | 7.5 | - | 4 | 1.25d | 5.42c |
| T12 | 1 | 0 | 2.5 | 100 | 7.5 | - | 16 | 5.85cd | 3.40c |
| T13 | 1 | 0 | 2.5 | 100 | 7.5 | 1650 | 16 | 7.53abc | 4.00c |
Table 1. Different treatments for S. rebudiana callus and embryo induction in first experiment.
3.2 Second experiment
In the second experiment coconut milk, combination of coconut milk and cacein hydrolysate, kind of explant and their interaction didn’t show significant difference on callus induction.
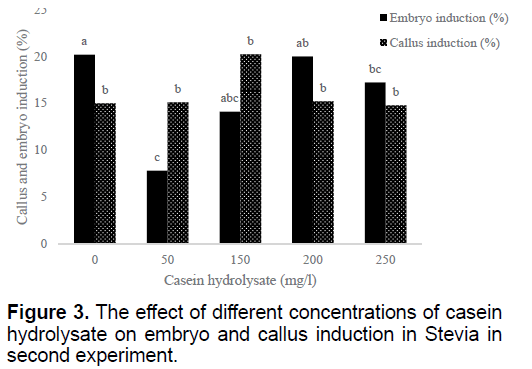
Using 100 mg/l casein hydrolysate could induce more callus than other treatments (data not shown). But the most embryos induced in MS medium without casein hydrolysate and the lowest one obtained by 50 mg/l casein hydrolysate (Figure 3). Higher concentrations of casein hydrolysate (150 and 200 mg/l) resulted with no further increase in the callus induction (Figure 3). Bud explants in MS medium showed the best result in embryo induction (Table 2).
| Explant | Coconut water (ml/l) | Culture media | |
|---|---|---|---|
| Leaf | Bud | ||
| 13.2cd | 42.35a | 0 | MS |
| 5.49cd | 5.88cd | MS+B5 | |
| 5.69cd | 16.86b | 55 | MS |
| 0.39d | 13.3cd | MS+B5 |
Table 2. The interaction between culture media, coconut milk and kind of explant on embryo induction ofStevia in second experiment.
While transferring the explants in MS medium without coconut water resulted in the highest embryos (42.35%) (Table 2) and the lowest one obtained in MS medium supplemented with B5 vitamins and coconut water (Table 2).
4. Discussion
In this study the most percentage of embryogenic callus observed in MS medium supplemented with BA+NAA or 2,4-D. By callus culture in various conditions, differentiation of adventitious shoots, roots or even embryos can take place.
Das et al. [16] investigated the callus culture of stevia on different strength of MS medium supplemented with various plant growth regulators. Their results, showed using the half strength medium supplemented with 2,4-D (1.0 mg l-1) and kin (0.2 mg l-1) was the best culture medium for callus induction and NAA (0.1 mg l-1) and BAP (2.0 mg l-1) combination was the best for maintenance.
Combination of BA+NAA resulted the maximum percentage of somatic embryogenesis. Swanson et al. [21] also yielded friable callus by culture of leaf explants of stevia in MS medium with vitamins, sucrose (30 g l-1) and agar (0.9 % W V-1) and supplemented with NAA (0.5 mg l-1) and BA (0.5 mg l-1). By eliminating the agar and modulating the medium PGRs concentration, facilitated differentiation of the callus tissue.
According to the results, embryogenesis in Stevia was affected by the concentration of casein hydrolysate and kind of explant and culture media. Coconut milk and casein hydrolysate did not show significant difference on callus induction.
Das et al. [22] found that glutamine (50 mg/l) and casein hydrolysate (100 mg/l) produced greenish, healthy, nodular calli with more embryogenic potential and less necrotic lesion in comparison with only PGR supplemented basal medium which further performed better in regeneration medium for producing large number of regenerated shoots. It was similar to our results. Casein hydrolysate contains several substances such as carbohydrates, protein, fat, several vitamins, phenolic compounds, fatty acids and mixture of up to 18 amino acids. It is found that any of these substances (single or in combination) cannot improve callus growth [23].
Coconut milk contains some growth hormones and is commonly use in tissue culture [24]. When containing auxin added to the culture medium, the coconut milk can induce plant cells to divide and grow rapidly. In the present study addition of coconut milk into the media culture didn’t show positive effect on callus induction. These results were not in agreement with [25].
According to the results, culture media contain casein hydrolysate induced 60.01% embryos, while coconut milk media produced only 30.99%. So, casein hydrolysate showed effective role in Stevia embryogenesis.
5. Conclusion
In conclusion, the present study demonstrates type and concentration of PGRs was effective in embryogenic callus induction and somatic embryogenesis of Stevia. Also kind of explant was important. Somatic embryogenesis was affected by casein hydrolysate concentration, but coconut milk didn’t show significant effect.
References
- Alhady MRAA. (2011). Micropropagation of Stevia rebaudiana Bertoni-A new sweetening crop in Egypt. Glob J Biotechnol Biochem. 6: 178-182.
- Geuns JMC. (2003). Molecules of interest: Stevioside. Phytochemistry. 64: 913-921.
- Mathur M, Begum T. (2015). Shootlets regeneration and tissue culture studies on Stevia rebaudiane Bertoni and Terminalia bellerica Roxb. Int J Rec Biotech. 3: 25-35.
- Singh SD, Rao GP. (2005). Stevia: The herbal sugar of 21st century. Sugar Technol. 7: 17-24.
- Toffler F, Orio OA. (1981). Acceni sulla pin ata tropicale ÃÆâÃâââ¬ÃâÃÅKaa-he-eÃÆâÃâââ¬Ãââ⢠ou ÃÆâÃâââ¬ÃâÃÅerba dolceÃÆâÃâââ¬Ãâââ¢. Rev Soc Sci Aliment. 4: 225-230.
- Sakaguchi M, Kan T. (1982). Japanese researches on Stevia rebaudiana. (Bert.) Bertoni and stevioside. Ci Cult. 34: 235-248.
- Patel RM, Shah RR. (2009). Regeneration of Stevia plant through callus culture. Indian J Pharm Sci. 71: 46-50.
- Gupta P, Sharma S, Saxena S. (2010). Callusing in Stevia rebaudiana. (natural sweetener) for steviol glycoside production. Int J Agric Biol Sci. 1: 30-34.
- Singh N, Yadav K, Kumari S, et al. (2011). Metabolic changes during differentiation in callus cultures of Stevia rebaudiana. (Bertoni). J Phytol. 3: 63-67.
- Singh P, Dwivedi P. (2013). Two-stage culture procedure using thidiazuron for efficient micropropagation of Stevia rebaudiana Anm. anti-diabetic medicinal herb. Biotech. 4: 431-437.
- Bespalhok JC, Vieira LGE. (1993). Fatores influenciando a micropropagacao in vitro de gemas axilares de Stevia rebaudiana. (Bert.). Revista Brasileira de fistiologia vegetal. 4: 59-61.
- Wada Y, Tamura Y. (1981). Callus culture and morphogenesis of Stevia rebudiana Bertoni. Yukagaku. 30: 215-219.
- Miyagawa H, Fujita Y. (1984). Studies on tissue culture of Stevia rebudiana Bertoni and its compound. Shoyakugaku Zashi. 38: 12-18.
- Filho JCB, Hashimoto JM, Vieira LG. (1993). Induction of somatic embryogenesis from leaf explants of Stevia rebaudiana. Braz J Plant Physiol. 5: 51-53.
- Filho JCB, Hattori K. (1997). Embryogenic callus formation and histological studies from Stevia rebaudiana. (Bert.). Bertoni floret explants. R Bras Fisiol Veg. 9: 185-188.
- Das K, Dang R, Khanam S, Rajasekharan PE. (2005). In vitro methods for production of steviosides from stevia. Indian J Nat Prod. 21: 14-15.
- Ahmad N, Fazal H, Zamir R, et al. (2011). Callogenesis and shoot organogenesis from flowers of Stevia rebaudiana. (Bert.). Sugar Tech. 13: 174-177.
- Naranjo EJ, Fernandez Betin O, Urrea Trujillo AI, et al. (2016). Effect of genotype on the in vitro regeneration of Stevia rebaudiana via somatic embryogenesis. Acta biol Colomb. 21: 87-98.
- Paul S, Dam A, Bhattacharyya A, et al. (2011). An efficient regeneration system via direct and indirect somatic embryogenesis for the medicinal tree Murraya koenigii. Plant Cell Tiss Organ Cult. 105: 271-283.
- Ibrahim IA, Nasr MI, Mohammed BR, et al. (2008). Plant growth regulators affecting in vitro cultivation of Stevia rebaudiana. Sugar Tech. 10: 254-259.
- Swanson SM, Mahady GB, Beecher CWW. (1992). Stevioside biosynthesis by callus, root, shoot and rooted-shoot cultures in vitro. Plant Cell Tissue Organ Cult. 28: 151-157.
- Das A, Mandal N. (2010). Enhanced development of embryogenic calllus in. (Stevia rebaudiana Bertoni). Biotecnology. 9: 368-372.
- Islam MO, Hiraiwa H, Ichihashi S. (1997). Effects of solidifiers, coconut water and carbohydrates on growth of embryogenic callus of Phalaenopsis. Proceedings of Nagoya International Orchid Show (NIOS 97) Japan. 43-48.
- Jayakumar S, Lingam RR. (2013). Influence of additives on enhanced in vitro shoot multiplication of Orthosiphon aristatus. (Blume). Miq, Notulae Scientia Biologicae. 5: 338-345.
- Muneppa Sridhar T, Reddy Aswath C. (2014). Influence of additives on enhanced in vitro shoot multiplication of Stevia rebaudiana. (Bert.), an important anti diabetic medicinal plant. Am J Plant Sci. 5: 192-199.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences