Synthesis and Toxicity Evaluation of Lead Oxide (PbO) Nanoparticles in Rats
Asghar Amiri, Marziyeh Mohammadi, Marziyeh Shabani
Asghar Amiri1,*, Marziyeh Mohammadi2, Marziyeh Shabani1
1 Department of Chemistry, Payame Noor University, 19395-3697 Tehran, Iran
2 Department of Chemistry, Faculty of Science, University of Vali-e-Asr, P.O. Box 77176, Rafsanjan, Iran
Received date: February 05, 2016 Accepted date: February 16, 2016 Published date: February 22, 2016
Citation: Amiri A, Mohammadi M, Shabani M, Synthesis and Toxicity Evaluation of Lead Oxide (PbO) Nanoparticles in Rats. Electronic J Biol, 12:2
Abstract
Toxicological studies have shown increasing toxicity of nanoparticles compared to micrometer particles of the same composition; this has raised concern about the impact of nanoparticles on human and animal health. In this research, lead oxide nanoparticles (PbO-NPs) have been synthesized. The tissue distribution and toxicity of administered PbO bulk and PbO-NPs (?55nm) were investigated in a situation of 100% bioavailability. Male Wistar rats were treated with a single intravenous injection of PbO and PbO-NPs in sterile saline (5 mg/kg body weight, once a week) for 8 consecutive weeks. Lead, zinc and iron concentrations in brain, liver, spleen and kidneys were measured by using ICP-OES. Biochemical parameters in serum were also assessed to determine potential pathological changes. The Pb levels were highest in the kidney, followed in decreasing order by the levels in the liver, spleen and brain. The concentration of Pb in PbO-NPs exposed rats was more than PbO bulk group and lead exposure caused significant decrease on Hb and RBC levels and platelet counts. Exposure to PbO and PbO-NPs resulted in a significant decrease in zinc concentration in kidney and liver and iron concentration was also significantly decreased in liver and spleen.
Keywords
Nano-PbO; Lead oxide; Iron parameters; Tissue distribution; Rats.
1. Introduction
Nanotechnology refers to a wide range of technologies that measure, manipulate, or incorporate materials and/or features with at least one dimension between approximately 1 and 100 nm. Despite the fact that there are a number of publications concerning the undesirable side effects of nanotechnology, the health and safety aspects of nanotechnology have lagged far behind its development. The increase in relative surface area that occurs as particle size decreases down to the nano scale gives rise to novel and enhanced material properties, but it also renders them more biologically reactive. Metal and metal oxide nanoparticles (NPs) are under development for antimicrobial, self-decontaminating and UV blocking functions for both military protection gear and civilian health products. Ag, PbO, TiO2, ZnO, and CeO2 are among the nanomaterials most widely incorporated into market goods [1-7].
Most of the production processing takes place within closed systems however during production and packaging, PbO-NPs may be released into the ambient air where they circulate for some time. During this time there is a potential for worker and public exposure via inhalation .The lead exposure is a public health concern; childhood lead exposure is estimated to contribute to 600,000 new cases of children with intellectual disabilities every year with 99 % of them living in developing countries [8].
Compounds containing lead can induce a wide variety of adverse human effects, such as genotoxicity [9], oxidative stress [10] and neurological effects [11]. It is known that lead can affect multiple systems in the body, most notably the nervous system. Cardiovascular, immune, and reproductive systems as well as bones, teeth, and kidneys are also sensitive targets [12]. Despite increased interest in PbO-NPs as industrial materials, very little is known about their biological or environmental interactions.
2. Materials and Methods
2.1 Preparation of PbO nanoparticles
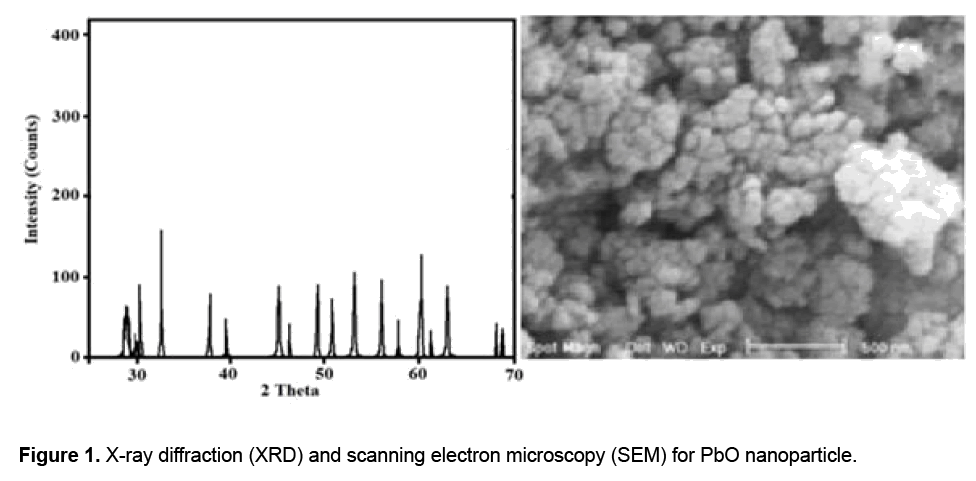
PbO nanoparticles were prepared by Chemical synthesis method [13]. Briefly, 60 ml of 1.0 M lead (II) acetate solution was prepared using deionized water and heated up to 90°C. This solution was added to an aqueous solution of 50 ml of 19 M NaOH in a beaker and stirred vigorously. Upon adding the lead (II) acetate, the solution initially became cloudy, and then turned a peach color, and finally a deep orange red. At this position, stirring was stopped, and the precipitate was allowed to settle. The supernatant was then decanted, filtered on a Buchner funnel, washed with de-ionized water repeatedly, and dried for overnight in a drying oven at 90oC. Structural characterizations were done for confirmation of PbONPS. The powder-X-ray diffraction (XRD) analyses were performed on a Philips PW-1830 X-ray diffract meter and the morphologies of the samples were characterized using scanning electron microscopy (SEM) (JEOL 6300 ). The specific surface area of samples was determined using the Brunauer-Emmet- Teller (BET) method in a volumetric adsorption apparatus (ASAP 2010 M, Micrometritics Instrument Corp). For in vivo experiments, the solutions of PbONPs in sterile 0.9 % saline were sonicated using a water bath sonicator (Saehan-Sonic, Seoul, Korea) for 10 min to breakup agglomerations.
2.2 Maintenance of the animals
Male Wistar rats (150 ± 10 g) were obtained from animal house facility of Kerman Neuroscience Research Center (Kerman, Iran). The animals were kept under a controlled light: dark (12:12 h) schedule. The rats were divided randomly to control (n=7) and treated groups and were housed in well-cleaned sterilized cages in an air-conditioned room with temperature maintained at 23°C ± 1oC and humidity 50% with water and a meal available ad libitum. Animal ethical committee of Kerman Neuroscience Research Center approved the protocols for the experiments.
2.3 Experimental groups
In order to evaluate bio-distribution of PbO and PbONPs in rats, animals were divided into 3 groups of 5 rats each and given the following treatment for 8 consecutive weeks.
• Group Control (no treatment)
• Group PbO (bulk) ( 5 mg/kg body weight, intravenously, once a week)
• Group PbO-NPs (5 mg/kg body weight, intravenously, once a week)
The concentrations of lead, zinc and iron in control group were compared with the groups that received PbO-NPs. The control animals were injected intravenously via the tail vein with 1 ml of sterile saline and the treated animals were injected intravenous via the tail vein with the test substance application preparation which contained PbO-NPs at a dose of 5 mg/kg body weight (1 ml of test substance preparation/kg of rat body weight).
All animals of each group were sacrificed under light ether anesthesia, 48 h after the last dosing. Brain, liver, spleen and kidneys samples were weighed, dried and collected for determination of lead, zinc and iron concentration. The samples were put in an oven at 60°C for 3 days. Then, 1 g of each samples were digested by 1 ml of HNO3. After digestion, the solutions were vaporized with the addition of 0.5 ml of H2O2 under the hood. Afterwards, the samples were filtered and lead, zinc and iron concentrations were measured by using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES, Model: Varian VISTA-MPX). The values are expressed as mean values (at least three separate determinations) ± standard error of the mean (SEM). The data were subjected to statistical analysis by Student’s t-test; Ρ<0.05 was considered significant.
2.4 Clinical hematological variables
Blood was collected by cardiac puncture in heparinized tubes and level of hemoglobin (Hb), platelets (PLT), red blood cell (RBC) count and white blood cell (WBC) count were measured using a Sysmex hematology analyzer (model K4500).
3. Result
At nanosize, materials may exhibit unique properties when compared with larger or bulk forms of the same material. However, the same properties that make nanomaterial desirable in these various applications have the potential to alter the biological properties that impact the environment, health, and safety of these materials. In this study ZnO-NPs have been synthesized. Scanning electron microscopy (SEM) and X-ray diffraction (XRD) were used to characterize the particle size and morphology. The PbO-NPs were in the size range 30-55 nm and had a BET of 41.6 m2/g (Figure 1).
The effects of lead concentration in the various tissues are shown in Table 1. Exposure to PbO and PbO-NPs resulted in a significant increase in lead concentration in brain, liver, spleen and kidneys. The maximum amount of lead accumulation was found in kidney and liver, respectively. The concentration of lead in PbO-NPs exposed rats was more than PbO group.
The results of iron concentrations are shown in Table 2. Iron concentration was significantly decreased in liver and spleen in PbO-NPs group. The effects of exposure to PbO and PbO-NPs on some hematological variables are shown in Tables 3. Lead exposure caused significant depletion of Hb and RBC levels and platelet counts but WBC showed no significant decrease in toxic groups. Blood parameters that are known to indicate an immune response in organ function were changed in both groups of rats.
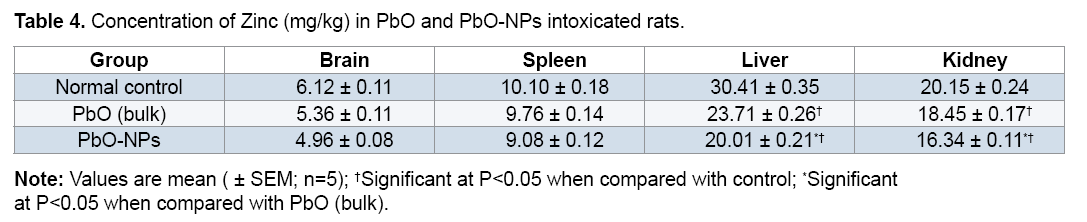
Exposure to PbO and PbO-NPs resulted in a significant decrease in zinc concentration in kidney and liver and a small decrease (no significant) in other organs, which is probably due to an interference that could take place by lead through zinc uptake mechanism (Tables 4).
4. Discussion
When nanoparticles get inside the body, they come into contact with different biomolecules, especially protein. The binding of protein with nanoparticles may trigger conformational changes in protein folding, altering its biological function and affecting the signaling pathways activated by nanoparticles. We undertook an investigative study to determine the toxicity and tissue distribution of intravenously administered nanoparticles of PbO because of the fundamental importance to obtain information on the kinetics of this widely used nanoparticle in a situation of 100% bioavailability. The Pb levels were highest in the kidney, followed in decreasing order by the levels in the liver, brain and spleen. The concentration of Pb in PbO-NPs exposed rats was more than PbO bulk group. Blood parameters that are known to indicate an immune response in organ function were changed in both groups of rats. The tissue distribution and elimination of the test substance was as expected considering the intravenous route of exposure and given the function of the liver and kidney as routes of metabolism and elimination (of PbO).
Lead irreversibly binds to the sulfhydryl group of proteins, causing impaired functions and the interaction of lead with oxy-hemoglobin has been suggested to be an important source of superoxide radical formation in RBCs [14,15]. Ercal et al. postulated that antioxidant enzymes inhibited hemoglobin auto oxidation by lead, suggesting [O2 -] and H2O2 were involved in this process [16]. It was found that as the lead concentration increased in the tissues, iron concentration decreased in spleen and liver and zinc level decreased significantly in kidney and liver of lead exposed rats which is probably due to an interference that could take place by lead through iron and zinc uptake mechanism and damage the antioxidant defense system [17].
Lead exposure caused significant decrease on Hb and RBC levels. Earlier studies have shown that toxic metals exposure causes bone marrow depression that ultimately results in decreased RBC count leading to plastic anemia. It can thus be concluded that lead induced oxidative stress might be due to the interaction of lead with oxyhemoglobin leading to peroxidative hemolysis in RBC membranes. RBCs have a high affinity for lead and they contain a majority of lead found in the blood stream, hence, they are vulnerable to oxidative damage [18]. This study might be effective for preliminary testing of PbO-NPs toxicity.
Acknowledgments
The authors would like to thank Prof. Alireza Badiei, for providing working facilities.
References
- Wang L, Ding W, Zhang F. (2010). Acute Toxicity of Ferric Oxide and Zinc Oxide Nanoparticles in Rats. J NanosciNanotechnol.10:8617-8624.
- Xie G, Sun J, Zhong G, Shi L, Zhang D. (2009).Biodistributionand toxicity of intravenously administered silica nanoparticles in mice. Arch Toxicol.84:183-190.
- Tang J, Xiong L, Wang S, et al. (2009). Distribution, translocation and accumulation of silver nanoparticles in rats. J NanosciNanotechnol. 9:4924-4932.
- Warheit DB, BrockWJ, Lee KP, Webb TR, Reed KL. (2005). Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact of surface treatments on particle toxicity. Toxicol Sci.88: 514-524.
- Karlsson HL, Gustafsson J, Cronholm P, Moller L. (2009). Size dependent toxicity of metal oxide particlesa comparison between nano- and micrometer size. ToxicolLett.188: 112-118.
- Kim IS, Baek M, Choi SJ. (2010). Comparative cytotoxicity of Al2O3, CeO2, TiO2 and ZnO nanoparticles to human lung cells. J NanosciNanotechnol.10: 3453-3458.
- Ogami A, Morimoto Y, Myojo T, et al. (2009). Pathological feature of different sizes of nickel oxide following intratracheal instillation in rats. InhalToxicol.21:812-818.
- International Lead Poisoning Prevention Week. (2013). GENEVA: WHO.
- Zeliko VJT,Li JH, Hartwig A, Wang XW, Costa M,Rossman TG. (1988).Genetic toxicology of lead compounds. Carcinogenesis.9:1727-1732.
- Sharma V, Sharma A, Kansal L. (2010). The effect of oral administration of Allium sativumextracts on lead nitrate induced toxicity in male mice. Food Chem Toxic.48:928-936.
- De Gennardo LD. (1978).The effects of lead nitrate on the central nervous system of the chick embryo I. Observations of light and electron microscopy. Growth.42:141-155.
- White D, Cory-Slechta A, Gilbert E, et al. (2007). New and evolving concepts in the neurotoxicology of lead. ToxicolApplPharmacol.22:51-27.
- H, Karimi MA, Haghdar S, Sadeghi A, MirGhasemiR,Mahdi-Khani S. (2008).Synthesis of lead oxide nanoparticles by Sonochemical method and its application as cathode and anode of lead-acid batteries.Materials Chemistry and Physics.108:337-344.
- Song MM, Song WJ, Bi H, et al. (2010). Cytotoxicity and cellular uptake of iron nanowires. Biomaterials.31:1509-1517.
- Gurer H, Ercal N.(2000). Can antioxidants is beneficial in the treatment of lead poisoning? Free Rad Biol Med.29:927-945.
- ErcalN, Gurer-OrhanH, Aykin-Burns N. (2001). Toxic metals and oxidative stress. Part I. Mechanism involved in metal induced oxidative damage. CurrTopMedChem.1:529-539.
- Flora SJS, Pande M, Mehta A.(2003).Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiolchelators in the treatment of chronic lead intoxication. Chemico Biological Interactions.145:267-280.
- Hughes MF.(2002). Arsenic toxicity and potential mechanism of action. Toxicology Letters.133:1-16.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences