Synapsin III in Differentiated SHSY5Y Cell Line as a Potential Tool for Neuropharmacological Evaluations in Schizophrenia
Anjali Janardhanan, Anjana Sadanand, Arambakkam Janardhanam Vanisree*
Anjali Janardhanan, Anjana Sadanand, Arambakkam Janardhanam Vanisree*
Department of Biochemistry, University of Madras,Guindy Campus, Chennai-600 025, Tamilnadu, India.
- Corresponding Author:
- Arambakkam Janardhanam Vanisree
Department of Biochemistry
University of Madras,Guindy Campus
Chennai-600 025, Tamilnadu, India
Tel: 91-44-22202732
E-mail: journalsvuom@gmail.com
Received date: March 26, 2018; Accepted date: April 07, 2018; Published date: May 03, 2018
Citation: Vanisree AJ, Sadanand A, Janardhar A. Synapsin III in Differentiated Shsy5y Cell Line as a Potential Tool for Neuropharmacological Evaluations in Schizophrenia. Electronic J Biol, 14:2
Abstract
Purpose: Development of in vitro cell culture model is on foremost call considering the requirement of neurological disease management. This is particularly relevant in the case of neuropsychiatric diseases especially, schizophrenia where there is a lack of proper in vitro model.
Methods: In the present study, the properties of differentiation were assessed when SHSY5Y cell line were exposed to all trans-retinoic acid at a concentration varying from 0-100 μM for a period of 0-7 days for which average neurite length was calculated. Following this primary observation, we analysed the expression pattern of synapsin III and other key molecules like MAPK, TrkB, BDNF and synaptic markers like synaptophysin, drebrin and PSD-95 on every alternate day from 0-7 days when SHSY5Y culture was conferred to 20 μM RA.
Results: We observed that a presentation of 20 μM RA could exert a maximal significant impact on neurite length on Day 7. A significant and gradual amplification of synapsin III both at mRNA and protein level was observed and attained maximal significance on Day 7. Similar to Synapsin III, MAPK, TrkB, BDNF, synaptophysin, drebrin and PSD-95 also displayed highest expression on Day 7.
Conclusion: Our observations are important as these would assist various investigations that employ SHSY5Y cells for appreciating in vitro studies and thus could fill the lacunae in the molecular features of disease models especially like schizophrenia, where synapsin III is a candidate gene altered in this disease. To conclude we demonstrate that differentiated SHSY5Y cells serve as a platform for neuropharmacological evaluations where there is a paucity of models.
Keywords
BDNF; TrkB; MAPK; Synaptophysin; Drebrin.
Introduction
Though studies using experimental animals have provided invaluable information on the pathogenesis and pathophysiology of neurological disorders but development of in vitro cell culture model is on major demand considering the requirement for further disease management at cellular level [1] and it is more taskful for neuropsychiatric disorder like schizophrenia.
The neuroblastoma cell line SHSY5Y has been used for studying various neurodegenerative pathways. Owing to its neurological characteristics on differentiation, SHSY5Y is subjected to many differentiating factors for various investigations and one such prominent factor being all-trans retinoic acid (RA) - a natural morphogen involved in development and differentiation of nervous system and their receptors are expressed in distinct regions in the adult central nervous system [2,3]. The differentiation process is initiated with a decrease in cell line proliferation and extension of neurites and several signalling pathways like TrkB-MAPK are triggered for the survival of cell and differentiation at this timeline [4-8].
Synapsins are proteins which are encoded by three genes- SynI, SynII and SynIII that code for synapsin I, synapsin II and synapsin III in mammals. Data on the former two synapsins-I and II were abundantly available while the role of lately discovered synapsin III is still uncharacterised [9]. The role of synapsin III in axon differentiation in the central nervous system and its localisation to extra-synaptic sites like soma and growth cones in the adult brain was described by Ferreira et al. [10] and Porton et al. [11]. Synapsin III is identified as a candidate gene in schizophrenia and was found to be associated to synaptic vesicles and participates in the neurotransmission [12,13]. The current data still remains fragmentary owing to the fact that a few amount of data is available regarding the role of synapsin III, and still ahead, the factors that influence its role is not available. TrkB activation by its ligand BDNF that plays fundamental roles in cell survival, neuronal cell proliferation is assumed to modulate the interactions of synapsin III with MAPK/ ERK during neuronal differentiation [14].
Drebrin is a neuronally expressed actin filament binding protein that has been entailed in the regulation of neuronal morphology [15,16]. It is a major protein expressed in the brain during neuronal development that plays a role in neuronal migration and formation of neurite-like processes [15-18]. Synaptophysin is one of the most abundant polypeptide components of synaptic vesicles and is believed to be essential for synaptic plasticity [19]. Both drebrin and synaptophysin has been implicated in schizophrenia and was selected in this study.
The present study was intended to evaluate the properties of differentiation of SHSY5Y cell line on exposure to RA but in relevance to synapsin III and the key factors of TrkB pathway along with pre-synaptic (synaptophysin) and post-synaptic markers (drebrin and PSD-95). Such an understanding is necessary so as to design the SHSY5Y cells pertaining to various disease models in vitro, especially schizophrenia, where synapsin III is a candidate gene altered in this disease situation [12]. To accomplish this, the whole study was planned in two steps. The first part concentrated on fixing the time and concentration of RA which can give a maximal significance in neurite length on SHSY5Y differentiation and the latter part dealt with the assessment of specific molecules implicated in schizophrenia.
Materials and Methods
Cell line and culture maintenance
SHSY5Y cells were procured from National Centre for Cell Sciences, Pune, India and were cultured in DMEM/HAM’s F-12 (HiMedia) containing 10% Foetal bovine serum (Gibco), 100 units/mL penicillin G, 100 mg/mL streptomycin (HiMedia). After attaining 80-90% of confluence, cells were sub-cultured according to the need of the experiments. The cells were incubated under standard conditions (37°C in 5% CO2 with 95% humidity) in Eppendorf - New Brunswick Galaxy CO2 incubator.
Preparation of RA stock
All-trans retinoic acid (RA) was purchased from Sigma Aldrich (Cat No-R2625). A 5 mg/ml stock solution was prepared in DMSO and stored in light protected vials at -80°C and diluted with tissue culture medium right before use. The final DMSO concentration never exceeded 0.1% in cell culture.
Differentiation of SHSY5Y using different concentrations of RA for a period of 0-7 days
Cells were seeded at an initial density of 104cells/ cm2 in poly L-lysine coated six well plates and grown in previous conditions. After 60-70% confluence was attained, RA was added in test groups at a concentration ranging from 5 μM-100 μM in DMEMHams F12 and maintained in dark. The cells which did not receive RA served as control and vehicle control were the ones that were treated with 0.1% DMSO alone. After incubation with RA at the end of experimental period, cells were washed thrice with medium and further studies were carried out. The experiments were conducted in triplicate, i.e., N=3 wells.
Neurite length assay
To analyse the average neurite length, cells were observed using an inverted microscope and images captured using a digital camera connected to the microscope at 100x. An average of 35 cells per field was counted and the neurite length was measured using Magnus Pro software on the captured images. Average neurite length was calculated as the total neurite length normalised to the number of cells included in the analysis. Independent cell culture experiments were conducted in triplicate.
Analysis of mRNA and protein levels of synapsin III, ERK, BDNF, TrkB, synaptophysin and drebrin in RA (20 μM) differentiated SHSY5Y
The next aim was to check the expression level of synapsin III, ERK, BDNF, TrkB, synaptophysin and drebrin at various time intervals after they were conferred to RA (20 μM). Cells were seeded at an initial density of 104 cells/cm2 in six well plates and grown in previous conditions. After 70-80% of confluence was attained, 20 μM RA was added in all of the test groups grown in DMEM-Hams F12. The test groups were analysed every alternate day from a period of 0-7 days after RA treatment. The cells which did not receive RA served as control.
qRT–PCR studies
After the respective time interval, cells were washed with the medium thrice, harvested using TRIsoln (Merck-Genei) to isolate total RNA. cDNA was obtained from mRNA using cDNA synthesis kit (Thermo Scientific).Genes for GAPDH, BDNF, TrkB, ERK, synapsin III, synaptophysin, drebrin, PSD-95 were amplified from 20 ng of cDNA from each group using their corresponding primers (Sigma).
The primers used for qRT-PCR are listed in Table 1:
| S.NO | Primers | Sequence (5’to3’) |
|---|---|---|
| 1 | BDNF | Fp: TTTCATTGTGTGCTCGCGTT3 Rp: TGCTTCTTTCATGGGGGCAG |
| 2 | TrkB | Fp: GGGACTACTGTTGCCTATCCC3 Rp: GTCACAGCTCACAACAAGCA |
| 3 | ERK | Fp: CAGTGAGCTCTAGCAAGGGAG Rp: CCTTCCAATAAGGAGCTTGGA |
| 4 | Synapsin III | Fp:TGTCTTCTGGGCCTCACCTA Rp: TAGCCATTAGGCAGGTTGGC |
| 5 | Synaptophysin | Fp: AGCTCTCGAATGGAAATCTGAC Rp: AATGCGGTAGGATACCACTTTC |
| 6 | Drebrin | Fp: ACTCAAAAGGAGGGGACCCA Rp: TACAGGAGGCGGAACCTTTG |
| 7 | PSD-95 | Fp: TACGACAAGACCAAGGACTGC Rp: GGAATGAAGCCAATGTCGTCG |
| 8 | GAPDH | Fp: AGAAGGCTGGGGCTCATTTG Rp: AGGGGCCATCCACAGTCTTC |
Table 1: Primers used for qRT-PCR.
qRT-PCR was performed in 10 μl reaction mixture containing 1 μg of cDNA, 5pmol of forward primer, 5 pmol reverse primer and 5 μl SYBER Green master mix (2x) and made up with MQ water. All samples were initially denatured at 95°C for 10 min before cycling and subjected to melt curve analysis after cycling. The amplification conditions are as follows: a denaturing step at 95°C for 45 s, annealing step at appropriate Tm (°C) for 45 s and an extension step at 72°C for 45 s. The optimized number of cycles for specific genes was 33-38 cycles. GAPDH served as intrinsic control. All qRT-PCR experiments were performed in triplicate and qRT-PCR data was normalized to GAPDH expression. The comparative threshold cycle (CT) values were derived from qbasePLUS Software. The ΔΔCT method of relative quantification was used to determine the fold changes in expression. Expression fold value=2-ΔΔCT.
Western blot studies
Cells were washed with PBS and lysed with 1x lysis buffer containing 50 mM Tris-HCl (pH-8.0), 150 mM NaCl, 0.02% sodium azide, 100 μg/ml phenylmethanesulfonyl fluoride, 1 μg/ml aprotinin and 1% TritonX 100 on ice. Solubilized cells were centrifuged at 10,000x g for 20 min and the supernatant was collected. Protein concentrations were determined by Bradford assay. Protein samples (20-40 μg) were loaded onto a 12%-15% SDS-polyacrylamide gel separated by electrophoresis and then transferred to PVDF membranes. Membranes were blocked with 10% fat-free milk in Tris-buffered saline (TBS) at room temperature for 1 h. Membranes were then probed with primary antibodies against target proteins- BDNF, TrkB, PI3K, Akt, mTOR, MAPK, Synapsin III, synaptophysin, drebrin (Santa Cruz Biotechnology) overnight at 4°C. Table 2 shows primary antibodies at different dilutions were used for Western blot.
| Antibody | Cat no., Company | Dilution for Immunoblot |
|---|---|---|
| BDNF | sc-20981,Santa cruz biotechnology | 0.736111111 |
| TrkB | sc-8316,Santa cruz biotechnology | 0.736111111 |
| PI3K | sc-7177,Santa cruz biotechnology | 0.388888889 |
| Akt | sc-1619, Santa cruz biotechnology | 0.388888889 |
| mTOR | sc-8319, Santa cruz biotechnology | 0.388888889 |
| MAPK | sc-292838,Santa cruz biotechnology | 0.388888889 |
| Synapsin III | sc-30098,Santa cruz biotechnology | 0.319444444 |
| Synaptophysin | sc-9116, Santa cruz biotechnology | 0.736111111 |
| Drebrin | sc-366274,Santa cruz biotechnology | 0.736111111 |
| β-Actin | Santa cruz biotechnology | 1.083333333 |
Table 2: Primary antibodies at different dilutions.
After overnight incubation, the membrane was washed with TBS-T followed by probing the membranes with the corresponding secondary antibody (Cell signaling technologies, USA) for 2 h at room temperature. After washing membranes with TBS, the membrane was developed by enhanced chemiluminescence (Biorad).
Statistical analysis
Results were analysed as Mean ± SD (Mean ± Standard Deviation). Statistical analysis of the data was performed with Student’s t-test and one-way analysis of variance (ANOVA). Bonferroni correction was performed and p values ≤ 0.05 were considered as significant.
Results
Neurite length analysis
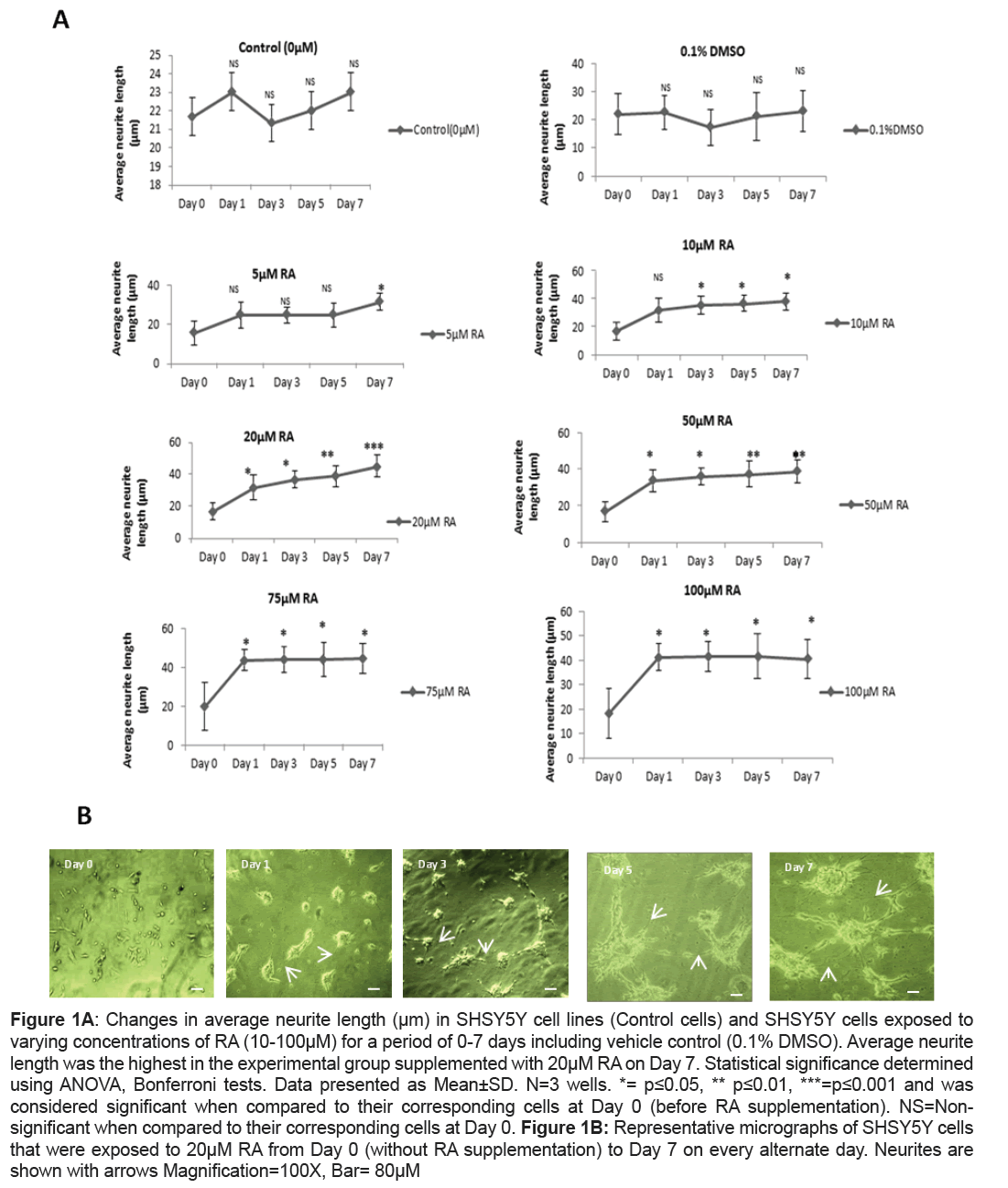
A significant increase in average neurite length in RA supplemented SHSY5Y culture when compared to the control cells that did not receive RA supplementation were observed. However, the level of significance varied both with the concentration of RA and the period of exposure. A concentration of 5 μM RA enhanced average neurite length at significant level when compared to their corresponding control cells (Day 0) only at Day 7 (p=0.05) while a similar significant change was brought on Day 3 itself when the culture was subjected to a higher concentration of 10 μM of RA (p=0.05). Unlike the previous concentrations of RA, it was found that when SHSY5Y cells were supplemented with 20 μM RA a significant increase in neurite outgrowth when compared to their corresponding control cells was recorded as early on Day 1 (p=0.04).The increase was at its maximum with a remarkable significance on Day7 (p=0.001) (Figures 1A and 1B). On exposure to 50 μM, 75 μm, 100 μM RA, the average neurite length was significantly higher when compared to their corresponding control cells on Day 7 (p=0.01 for 50 μM RA, p=0.05 for both 75 μM and p=0.04 for 100 μM RA) but was less significant when compared to that of 20 μM RA treatment on Day7. Interestingly, 100 μM RA exposures to SHSY5Y were accompanied with the loss of normal morphology in cells with their drifting towards round morphology by Day 7. Unlike RA supplemented cells, 0.1% DMSO treated cells showed no significance from their corresponding control cells even after 7 days (Figure 1A).
Figure 1A: Changes in average neurite length (μm) in SHSY5Y cell lines (Control cells) and SHSY5Y cells exposed to varying concentrations of RA (10-100μM) for a period of 0-7 days including vehicle control (0.1% DMSO). Average neurite length was the highest in the experimental group supplemented with 20μM RA on Day 7. Statistical significance determined using ANOVA, Bonferroni tests. Data presented as Mean±SD. N=3 wells. *= p≤0.05, ** p≤0.01, ***=p≤0.001 and was considered significant when compared to their corresponding cells at Day 0 (before RA supplementation). NS=Nonsignificant when compared to their corresponding cells at Day 0. Figure 1B: Representative micrographs of SHSY5Y cells that were exposed to 20μM RA from Day 0 (without RA supplementation) to Day 7 on every alternate day. Neurites are shown with arrows Magnification=100X, Bar= 80μM
qRT-PCR Studies
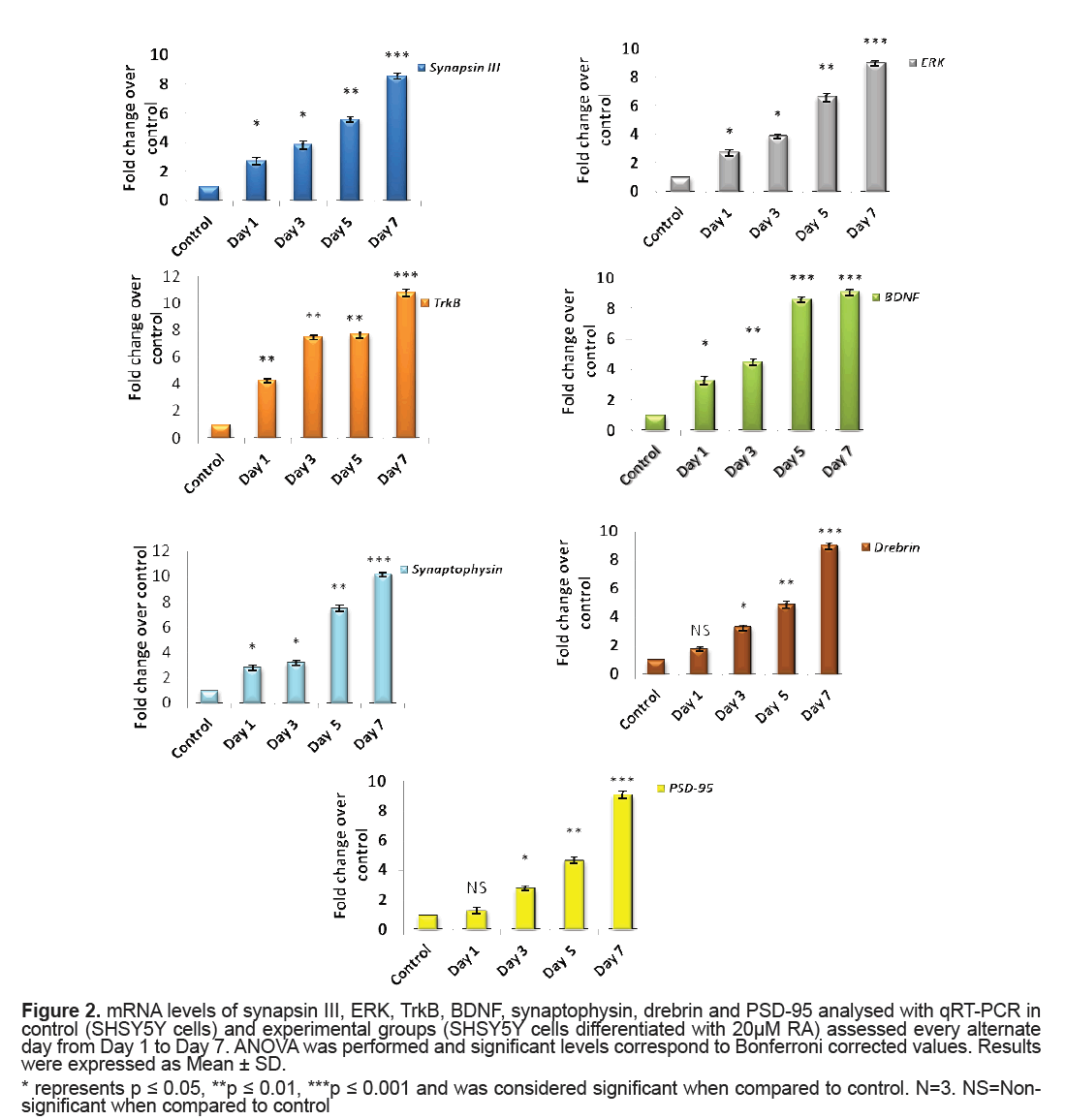
The mRNA levels of synapsin III, ERK, BDNF, TrkB, synaptopysin and drebrin in control and experimental groups were analysed using qRT-PCR. A significant elevation in levels of synapsin III from Day 1 to Day 5 (p=0.04, p=0.02, p=0.009 for Day 1, Day 3 and Day 5, respectively) of RA treatment when compared to that of control cells was observed and the expression was highly significant on Day 7 (p=0.001). Concordantly, there was a significant hike in the levels of ERK expression level on RA treatment from Day 1 (p=0.04, p=0.02, p=0.003 for Day1, Day 3 and Day 5, respectively) and displayed maximum significance on Day 7 (p=0.001) when compared to that of control cells (Figure 2). A significant rise in the level of BDNF from Day 1 of RA supplementation (p=0.03 on Day 1) was recorded which rose to a high significance from Day 5 to Day 7 (p=0.001). Unlike the ligand BDNF, its receptor Trk B presented a marked significant increase in its mRNA levels right from Day 1 (p=0.01) and it was also highly significant on Day 7 (p=0.001) (Figure 2).
Figure 2: mRNA levels of synapsin III, ERK, TrkB, BDNF, synaptophysin, drebrin and PSD-95 analysed with qRT-PCR in control (SHSY5Y cells) and experimental groups (SHSY5Y cells differentiated with 20μM RA) assessed every alternate day from Day 1 to Day 7. ANOVA was performed and significant levels correspond to Bonferroni corrected values. Results were expressed as Mean ± SD.
* represents p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and was considered significant when compared to control. N=3. NS=Nonsignificant when compared to control
Synaptophysin did exhibit higher level that was revealed from Day 1 (p=0.04) of RA application when compared to that of control cells and was remarkably significant on Day 7 (p=0.001). Both PSD95 and drebrin was shown to significantly express by Day 3 (both p=0.03) with much prominence on Day 7 (both p=0.001) (Figure 2).
Western blot studies
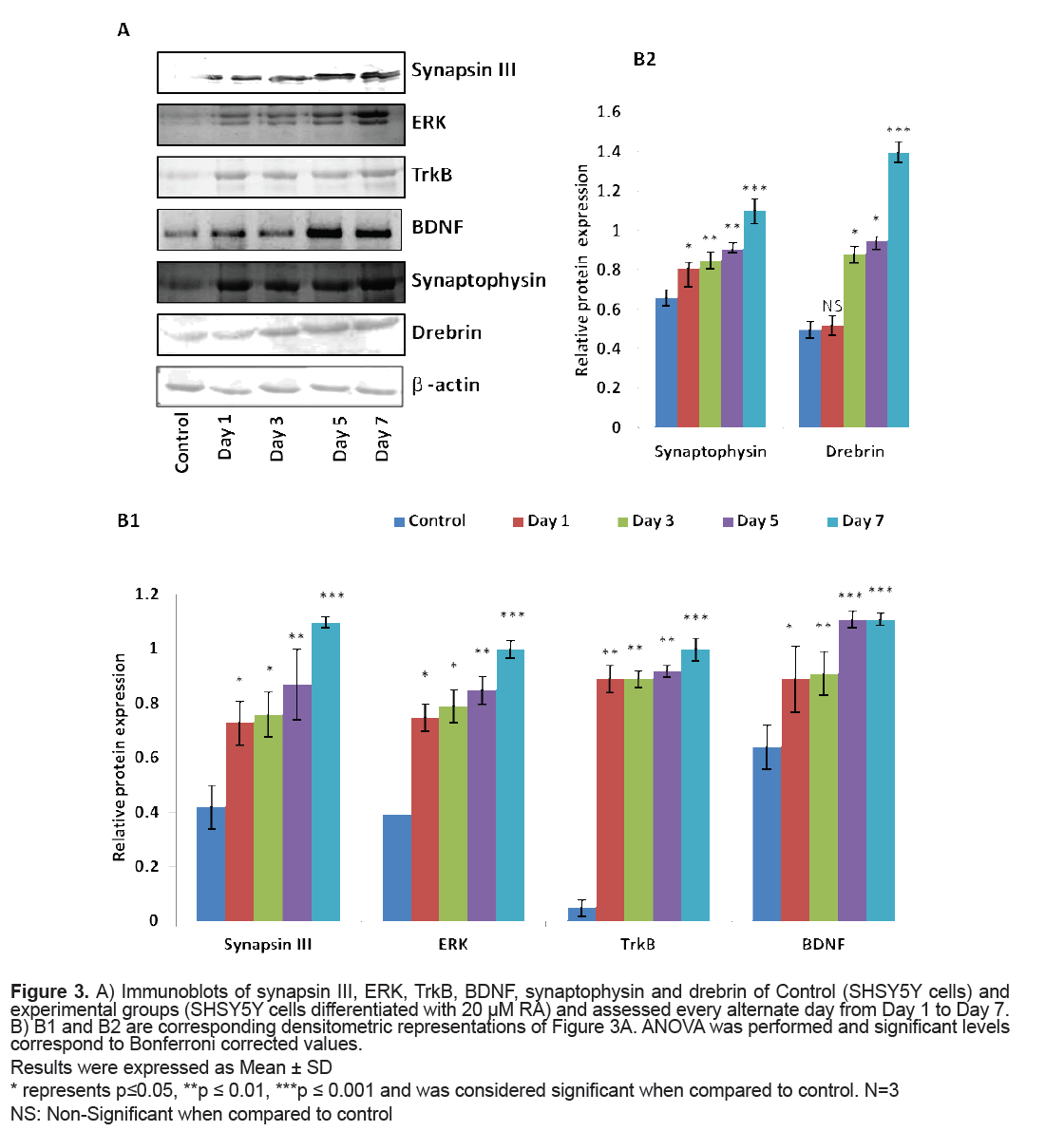
The levels of proteins viz. synapsin III, ERK, TrkB, BDNF, synaptophysin and drebrin were analysed through Western blot. The expression of synapsin III was significantly higher on RA induction (p=0.04 on Day 1) when compared to that of control cells and attained maximum significance on Day 7 (p=0.001). ERK also presented a similar pattern of expression with a significantly higher expression on Day 7 (p=0.001) when compared to that of control cells. The levels of TrkB were significantly elevated on Day 1 (p=0.01) when SHSY5Y cells were conferred with 20 μM RA and the expression was maximum on Day 7 (p=0.001). The levels of BDNF were significantly elevated from Day 1 of RA induction (p=0.04) with remarkable levels from Day 5 to Day 7 (p=0.001). Synaptophysin had also displayed a significantly higher expression on Day 1 of RA treatment (p=0.03) when compared to that of control cells but a marked increase in the level was found on Day 7 (p=0.001).
Drebrin level did show a significant rise by Day 3 and was highly significant by Day 7 (p=0.001) (Figure 3).
Figure 3: A) Immunoblots of synapsin III, ERK, TrkB, BDNF, synaptophysin and drebrin of Control (SHSY5Y cells) and experimental groups (SHSY5Y cells differentiated with 20 μM RA) and assessed every alternate day from Day 1 to Day 7. B) B1 and B2 are corresponding densitometric representations of Figure 3A. ANOVA was performed and significant levels correspond to Bonferroni corrected values.
Results were expressed as Mean ± SD
* represents p≤0.05, **p ≤ 0.01, ***p ≤ 0.001 and was considered significant when compared to control. N=3 NS: Non-Significant when compared to control
Discussion
In the present study, SHSY5Y cells were differentiated with varying concentrations of RA for seven days to inspect the changes in morphology as there is insufficient literature uncovering the effect of different concentrations of RA on SHSY5Y in relevance to average neurite length. Further, this part of the work of determination of the effect of different concentrations of RA from Day 1 to Day 7 could be a work plan in choosing d-SHSY5Y cells for assessing the expressional variations of the proteins involved in neuronal differentiation, survival and synaptic plasticity and hence in disease conditions like schizophrenia. It is well-known that RA binds to its nuclear retinoic acid receptors to regulate the transcription of neurotrophin receptor genes and thereby it was reported to enhance neurite outgrowth [20]. The role of RA in adult neurogenesis by enhancing the ability of multipotent progenitor cells of neurons and glial cells in hippocampal culture to differentiate to neuronal phenotype was also described earlier [21,22]. It was observed in the present study that the neurite outgrowth was triggered on Day 1 and was extensive after seven days in cultures subjected to 20 μM of RA and was highly significant than any other concentrations of RA at any time period. This implicated that SHSY5Y cells when differentiated for seven days with 20 μM RA serve a better representation for neurological studies with augmented neurite outgrowth. At highest dose of 100 μM of RA there was a change in morphology of SHSY5Y probably owing to the accumulation of substrate adherent S type cells at higher concentration as also observed by Arcangeli et al. [23].
When SHSY5Y cells were supplemented with 20 μM RA, a significant upregulation of synapsin III (both mRNA and protein level) was observed. This might reflect the result of cellular processes following neurite outgrowth on RA treatment as this was the stage where there could be an active process of axon and dendrite elongation. The results were similar to the observations made by Ferreira et al. [10] showing a cardinal expression of synapsin III in cultured mice hippocampal neurons after few days of plating and identified the expression of synapsin III as fine puncta of axonal spots during hippocampal neuron maturation. This was also evident from another study in cultured human NTERA-2cl.D1 cells that documented a significant transcriptional upregulation of all synapsins of which synapsin III revealed a magnificent augmentation on differentiation with RA [24]. According to the current observations, synapsin III also finds its role in synapse maturation owing to its very high significant expression in high neuritic connectivity after seven days of RA treatment when the process of establishing of a refined neuronal network happens as observed in the average neurite length in seven days (Figure 1).
A striking report by Jovanovic et al. [25] highlighted the existence of presumed sites for MAPK/ERK for synapsin III and it was later reported that a tuning of phosphorylation and dephosphorylation at this site regulates synaptic vesicle trafficking within the presynaptic terminal so as to maintain proper synaptic connections [9]. The significantly very high levels of ERK observed in the current study was also supported by the observations of activated MAPK/ ERK pathway made in SK-N-BE(2)C neuroblastoma on RA induction [26] (Figures 2 and 3B1). MAPK/ ERK regulates translation of synaptic proteins and hence the protein related synaptic plasticity [27]. The concordant expression pattern of synapsin III and MAPK observed in the current study suggest that synapsin III act downstream to MAPK and hence may be involved in maintaining the synaptic connections at high neurite length (Figures 2 and 3B1). There are also evidences suggesting the role of neurotrophins and their receptors in neurite outgrowth and their survival. It was reported that treatment of BDNFantisense oligonucleotides reduced neuronal survival by 35% in neuroblastoma culture and the addition of BDNF rescues cells from death [28]. Contradictory to the almost null expression of TrkB in control cells there was a gradual increment in TrkB on RA induced differentiation showing the maximal protein expression on Day 7(p=0.001) (Figures 2 and 3B1). The significant increase in BDNF as observed in the study on RA induction would have probably activated its receptor TrkB to proceed to downstream and activate its docking sites to hire various intermediates of intracellular signaling pathway like MAPK/ERK signaling pathway (Figures 2 and 3B1). The expression pattern of BDNF-TrkB at different intervals of RA treatment was comparable to that of the expression frame work of synapsin IIIMAPK and it may be speculated that TrkB activated by BDNF also play a major role during RA induced differentiation as both the levels of mRNA and protein were found to be parallel to the maximal neurite connections Supporting the current observation, BDNF-TrkB signalling was also thought to play a role in inducing neuritis through PI3K according to a recent study [29]. The variations in the level of presynaptic and neuronal differentiation marker like synaptophysin is involved in several crucial aspects of synaptic vesicle trafficking, including the initiation of neurotransmitter release [30,31] and hence was assessed in the present study owing to its implications in schizophrenia [32,33]. The changes in neurite length with corresponding changes in the expression of synaptophysin following differentiation of SHSY5Y by RA were reported earlier [34,35]. A marked significance in the mRNA and protein level of synaptophysin by Day 7 following RA supplementation (20 μM) in the present study indicates that synaptic vesicles are more mature by Day 7 as also supported by Sarkanen et al. [36]. It is quiet noteworthy that this increase in the level of synaptophysin coincides with the time course in which maximal neurite length was observed.
Drebrin, which is a post-synaptic marker supports polarization of actin filament and plays a role in the modulation of synaptic plasticity [37,38]. It has been shown that diminution of drebrin expression in developing hippocampal neurons had an impact on the width and density of filopodia spines [39]. Drebrin may also guide microtubule tips to actin rich filopodia which is a fundamental step in the formation of a neurite [40,41]. It was shown to play a role in the formation of axonal filopodia by regulating the emergence of filopodia from actin patches [42]. The observation of a marked significance in the mRNA level of PSD- 95 (Figure 2), a major scaffolding protein involved in dendritic spine density, on Day 7 in SHSY5Y cells after RA presentation and the expression levels of MAPK is consistent with the reports of Yoshii and Constantine- Paton [43] who suggested that MAPK/ERK regulates transcription of PSD-95 in neuronal cultures (Figures 2 and 3). Corroborating these findings along with the expressional variations of BDNF-TrkB it is suggested that BDNF increases spine density through PSD- 95 mediated through MAPK/ERK (Figures 2 and 3). The argument holds good as per the reports made by Alonso et al. 2004 who stated that ERK activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Though there is a huge data of evidence regarding RA induction in neuroblastoma cells and its role in neurite outgrowth and establishing synaptic connections, the fate of synapsin III in such conditions is not yet described. It may be recalled that synapsin III was expressed to the maximum when SHSY5Y cells were differentiated with 20 μM RA for 7 days, the time point at which neurite length were largest in conjunction with increased expression of key molecules like BDNF, TrkB and MAPK/ERK (Figures 1-3). Based on the current data it may be presumed that BDNF-TrkBERK pathway can influence the expression pattern of synapsin III as observed in the similarity of expression pattern of the same to that of synapsin III. Further analyses should provide additional insights into the participation of synapsin III in the mechanisms of its role in neurite outgrowth and how the BDNF-TrkBERK pathway tunes the expression of synapsin III and leads to the stabilization of synaptic machinery which is dysregulated in many neurological disorders including schizophrenia.
Conclusion
More studies devoted to the role of cytoskeletal proteins during neuronal extensions are essential to describe their interplay during the sprouting and elongation of neuronal extensions. Such an understanding is necessary so as to design the SHSY5Y cells pertaining to various disease models in vitro, especially schizophrenia, where synapsin III is a candidate gene altered in this disease situation [12]. According to the above observations, upregulated levels of BDNF, Trk B and synapsin III in 20 μM RA differentiated SHSY5Y for 7 days may serve as best in vitro representation for use in various neurotoxicological and pharmcological investigation and this can be chosen to mimic neurological conditions that are characterized by altered neurotrophins and synapsins as in schizophrenic conditions. Meanwhile, the authors do agree that there are some constraints to the elucidation of the data. This is an in vitro cell line model and the results accounted here need further confirmation in primary neuronal cultures.
Conflict of Interest
The authors indicate there is no potential conflict of interest in this study.
Acknowledgement
The authors thank University Grants Commission, New Delhi, India for the necessary financial support in the form of JRF and SRF (F.17-42/O8(SA-I)- Sanction no dated 26-7-2011.
References
- Krishna A, Biryukov M, Trefois C, et al. (2014). Systems genomics evaluation of the SH-SY5Y neuroblastoma cell line as a model for Parkinson's disease. BMC Genomics.15: 1154.
- Zetterstrom RH, Lindqvist E, Mata de Urquiza A, et al. (1999). Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur J Neurosci. 11: 407-416.
- Gofflot F, ChartoireN, Vasseur L, et al. (2007). Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell. 131: 405-418.
- Pang L, Sawada T, Decker SJ, et al. (1995). Inhibition of MAP kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 270: 13585-13588.
- Pan J, Kao YL, Joshi S, et al. (2005). Activation of Rac1 by phosphatidylinositol 3-kinase in vivo: Role in activation of mitogen-activated protein kinase (MAPK) pathways and retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Neurochem. 93: 571-583.
- Encinas M, Iglesias M, Llecha N, et al. (1999). Extracellular regulated kinases and phosphatidylinositol 3-kinase are involved in brain-derived neurotrophic factor-mediated survival and neuritogenesis of the neuroblastoma cell line SH-SY5Y. J Neurochem. 73: 1409-1421.
- Kaplan DR, Matsumoto K, Lucarelli E, et al. (1993). Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Eukaryotic signal transduction group. J Neuron. 11: 321-331.
- Kobayashi M, Kurihara K, Matsuoka I. (1994). Retinoic acid induces BDNF responsiveness of sympathetic neurons by alteration of Trk neurotrophin receptor expression. FEBS Lett. 356: 60-65.
- Perlini LE, Szczurkowska J, Ballif BA, et al. (2015). Synapsin III acts downstream of semaphorin 3A/CDK5 signaling to regulate radial migration and orientation of pyramidal neurons in vivo. Cell Rep. 11: 234-248.
- Ferreira A, Kao HT, Feng J, et al. (2000). Synapsin III: Developmental expression, subcellular localization and role in axon formation. J Neurosci. 20: 3736-3744.
- Porton B, Wetsel WC, Kao HT. (2011). Synapsin III: Role in neuronal plasticity and disease. Semin Cell Dev Biol. 22: 416-424.
- Porton B, Wetsel WC. (2007). Reduction of synapsin III in the prefrontal cortex of individuals with schizophrenia.Schizophr. 94: 366-370.
- Feng J, Chi P, Blanpied TA, et al. (2002). Regulation of neurotransmitter release by synapsin III. J Neurosci. 22: 4372-4380.
- Jha SK, Jha NK, Kar R, et al. (2015). p38 MAPK and PI3K/AKT signalling cascades in Parkinson’s disease. Int J Mol Cell Med. 4: 67-86.
- Dun XP, Chilton JK. (2010). Control of cell shape and plasticity during development and disease by the actin-binding protein Drebrin. Histol Histopathol. 25: 533-540.
- Dun XP, de Lima TB, Allen J, et al. (2012). Drebrin controls neuronal migration through the formation and alignment of the leading process. Mol Cell Neurosci. 49: 341-350.
- Hayashi K, Ishikawa R, Ye LH, et al. (1996). Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J Neurosci. 16: 7161-7170.
- Mizui T, Kojima N, Yamazaki H, et al. (2009). Drebrin E is involved in the regulation of axonal growth through actin-myosin interactions. J Neurochem. 109: 611-622.
- Kwon SE, Chapman ER. (2011). Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 70: 847-854.
- Lane MA, Bailey SJ. (2005). Role of retinoid signalling in the adult brain. Prog Neurobiol. 75: 275-293.
- Palmer TD, Takahashi J, Gage FH. (1997). The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 8: 389-404.
- Takahashi J, Palmer TD, Gage FH. (1999). Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J Neurobiol. 38: 65-81.
- Arcangeli A, Rosati B, Crociani O, et al. (1999). Modulation of HERG current and herg gene expression during retinoic acid treatment of human neuroblastoma cells: Potentiating effects of BDNF. J Neurobiol. 40: 214-225.
- Leypoldt F, Flajolet M, Methner A. (2002). Neuronal differentiation of cultured human NTERA-2cl.D1 cells leads to increased expression of synapsins. NeurosciLett. 324: 37-40.
- Jovanovic JN, Sihra TS, Nairn AC, et al. (2001).Opposing changes in phosphorylation of speciÃÆïÃâìÃâÃÂc sites in synapsin I during Ca2+ dependent glutamate release in isolated nerve terminals. J Neurosci. 21: 7944-7953.
- Lee JH, Kim KT. (2004). Induction of cyclin-dependent kinase 5 and its activator p35 through the extracellular-signal-regulated kinase and protein kinase A pathways during retinoic-acid mediated neuronal differentiation in human neuroblastoma SK-N-BE(2)C cells. J Neurochem. 91: 634-647.
- Kelleher RJ, Govindarajan A, Jung HY, et al. (2004). Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 116: 467-479.
- Ho R, Eggert A, Hishiki T, et al. (2002). Resistance to chemotherapy mediated by TrkB in neuroblastomas.Cancer Res.62: 6462-6466.
- Sainath R, Gallo G. (2015). Cytoskeletal and signaling mechanisms of neurite formation. Cell Tissue Res. 359: 267-278.
- Valtorta F, Pennuto M, Bonanomi D, et al. (2004). Synaptophysin: Leading actor or walk-on role in synaptic vesicle exocytosis? BioEssays. 26: 445-453.
- Dayem AA, Kim B, Gurunathan S, et al. (2014). Biologically synthesized silver nanoparticles induce neuronal differentiation of SH-SY5Y cells via modulation of reactive oxygen species, phosphatases and kinase signaling pathways. Biotechnol J. 9: 934-943.
- Egbujo CN, Sinclair D, Borgmann-Winter KE, et al. (2015). Molecular evidence for decreased synaptic efficacy in the postmortem olfactory bulb of individuals with schizophrenia. Schizophr Res. 168: 554-562.
- Rao JS, Kim HW, Harry GJ, et al. (2013). Increased neuroinflammatory and arachidonic acid cascade markers and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophr Res. 147: 24-31.
- Cheung YT, Lau WK, Yu MS, et al. (2009). Effects of all-trans retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 30: 127-135.
- Bai X, Strong R. (2014). Expression of synaptophysin protein in different dopaminergic cell lines. J Biochem Pharmacol Res. 2: 185-190.
- Sarkanen JR, Nykky J, Siikanen J, et al. (2007). Cholesterol supports the retinoic acid-induced synaptic vesicle formation in differentiating human SH-SY5Y neuroblastoma cells. J Neurochem. 102: 1941-1952.
- Galkin VE, Orlova A, Kudryashov DS, et al. (2011). Remodeling of actin filaments by ADF/cofilin proteins. Proc Natl Acad Sci U S A. 20: 20568-20572.
- Mikati MA, Grintsevich EE, Reisler E. (2013). Drebrin-induced stabilization of actin filaments. J Biol Chem. 288: 19926-19938.
- Takahashi H, Sekino Y, Tanaka S, et al. (2003). Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis. J Neurosci. 23: 6586-6595.
- Bazellie`res E, Massey-Harroche D, Barthelemy-Requin M, et al. (2012). Apico-basal elongation requires a drebrin-E-EB3 complex in columnar human epithelial cells. J Cell Sci. 125: 919-931.
- Geraldo S, Khanzada UK, Parsons M, et al. (2008). Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat Cell Biol. 10: 1181-1189.
- Ketschek A, Spillane M, Dun XP, et al. (2016). Drebrin coordinates the actin and microtubule cytoskeleton during the initiation of axon collateral branches. Dev Neurobiol. 76: 1092-1110.
- Yoshii A, Constantine-Paton M. (2014). Postsynaptic localization of PSD-95 is regulated by all three pathways downstream of TrkB signaling. Front Synaptic Neurosci. 6: 6.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences