Successful In Vitro Maturation of Oocytes Collected from Whole Porcine Ovaries Vitrification
Van Hanh N, Viet Linh N, Nghia Son H
Van Hanh N1,*, Viet Linh N1, Nghia Son H2
1Laboratory of Embryo Biotechnology, Institute of Biotechnology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi, Vietnam
2Department of Animal Biotechnology, Institute of Tropical Biology, Vietnam Academy of Science and Technology, 9/621 Xa Lo Ha Noi Street, Linh Trung Ward, Thu Duc District, Ho Chi Minh City 70000, Vietnam
Received date: August 03, 2016; Accepted date: August 31, 2016; Published date: September 07, 2016
Citation: Hanh NV, Liet NV, Son HN. Successful In Vitro Maturation of Oocytes Collected from Whole Porcine Ovaries Vitrification. Electronic J Biol, 12:4.
Abstract
Background: Ovarian cryopreservation has become more and more important not only as a tool of genetic resource and biodiversity conservation but also as a bio-medical application. Objective: The aim of the present study is to survey on slow freezing and vitrification method towards a simple method to cryopreserve intact ovary which can be applied in the field condition. The result of method base on evaluation of viability and maturation potential of immature oocytes were collective from frozen–thawed ovaries. Materials and methods: The whole porcine ovarian were vitrified using same cryoprotective solution in three groups (V1, V2 and V3) which the treatment time in ES solution is 15, 30 and 45 min, respectively comparing to slow freezing program. After three weeks of storage, frozen samples were thawed and immature oocytes were collected for in vitro maturation. FDA staining of immature oocytes was used to conï¬ÂÂrmed the viability and morphology of intracellular structures. The oocytes matured were evaluated by extrusion of the first polar body. Results: The percentage of viability oocytes was the highest in group V2 (7.03%) compare with that in other group V1; control group and V3 group (6.47; 0.91 and 0.65%, respectively). The maturation rate did differ in two groups V1 (6.47%) and V2 (7.03%), but it was not in control and V3. Conclusion: whole ovarian vitrified is already a feasible technique, it is possible to collect viable immature oocytes that have the ability to mature in vitro.
Keywords
Whole ovarian cryopreservation; IVM; Porcine; Vitrification; Slow freeze.
Introduction
Cryopreservation of endangered species represents a challenging perspective regarding the quick decline of biodiversity [1]. This technique is not only the benefit of human medicine but also helpful for agronomic research and development. A wide range of reproductive biotechnologies are now available to facilitate conservation of mammals threatened with extinction. In human, the different cryopreservation options available for fertility preservation in cancer patients are embryo cryopreservation, oocyte cryopreservation and ovarian tissue cryopreservation [1]. Ovarian grafting commenced in the first half of last century and contributed greatly to advances in our understanding of endocrinology and graft rejection [2]. The first baby was delivered from a patient, who was auto-transplantation of cryopreserved ovarian tissue and having the presence of early growing follicles and ovulation (24). The mature oocyte was retrieved and fertilized, embryo transferred after four days. The patient became pregnant and gave a healthy infant after receiving these embryos [3]. The ovarian tissue cryopreservation was not so well developed in compare to oocyte and embryo In Vitro culture and cryopreservation. After that, because of the demand in using ovarian cryopreservation as a genetic material banking strategy for laboratory, companion and domestic species as well as rare and endangered species and humans, ovarian cryopreservation was reemerged [4,5].
Whole ovarian tissue sections have been proved to have the ability in surviving after cryopreservation with normal follicle growth and ovulation following xeno-transplantation to a host with suppressed immune system so that the foreign ovary tissue would not be rejected [2]. Oocytes collected from such frozen-thawed sections are not only viable, but also able to result live births in mice and sheep after autologous transplants of ovarian grafts stored in liquid nitrogen [2,5,6]. Whole ovary freezing has also been investigated, with the aim of achieving vascular anastomoses and a functioning organ after transplantation. The method has been shown to restore fertility in rats and sheep but a high rate of follicle loss is still a concern. Directional freezing combined with microvascular anastomosis have improved outcomes, and long lasting ovarian function has thus been obtained in sheep [7-9].
Ovarian preantral follicles were considered very important in conservation of endangered species dued to their enormous number, small size and maturation status. Preantral follicles represent 90% of the follicular population in mammals [10]. Therefore, small preantral follicles recovered from the ovaries of post-mortem or convalescent animals are really a promising oocyte source, especially when their viability and ability to develop to maturity In Vitro were proved [11]. On the other hand, whole ovarian cryopreservation techniques were all developed in laboratories with complex instrument and through slow freezing processes [12]. Methods for ovarian tissue vitrification followed by In Vitro growth (IVG), In Vitro maturation (IVM) after thawing or those of IVM followed by cryopreservation, have practical and theoretical advantages in many species, but these techniques also need to be improved for the whole ovarian [13].
It is a challenge to effectively cryopreserve such a large organ as the ovary [4]. The organ is more complex, the less success would be achieved in freezing [14]. Attempts were made to both minimizing toxic injuries caused by cryprotectants and protecting tissue from freeze-induced injury at the same time [15]. Whole ovary cryopreservation followed by vascular transplantation is suggested to be more effective in compare to avascular transplantation of ovarian cortical tissue due to shorter period of warm ischemia at ovarian cortex transplantation [16]. As a result, it has a higher proportion of survived ovarian cortex after transplantation than the calculated around 30% [17]. An advantage of whole ovary transplantation is that the entire follicle pool of one ovary would be transplanted, as compared to the limited number of follicles at ovarian cortex transplantation [18]. However, in whole ovary sheep model, fairly poor follicular survival rates (0- 8%) were achieved after vascular transplantation of frozen-thawed ovaries [8,19]. In those studies, follicular survival was controlled 12-18 months after re-transplantation. It might be good for follicular pool survived the first weeks after transplantation; however other mechanisms might lead to a later follicular loss, regardless of that the vascular anastomosis may have worked. It is known from other studies of whole sheep ovary auto-transplantation that were common [15].
Although ovarian tissue cryopreservation was successful with IVM and IVG, until now there was no report on successful In Vitro maturation oocytes from whole ovarian cryopreservation. The aim of the present study is to survey on slow freezing and vitrification method to underline the major improvements and prospective directions towards application in field condition for immature oocyte production to be In Vitro matured from the whole ovary cryopreservation.
Materials and Methods
Ovaries preparation
The porcine ovaries were obtained from pre-pubertal crossbred gilts (Landrace × Large White) at a local slaughterhouse and transported to the laboratory at 33°C. The ovary was submerged into PBS (4°C) immediately after retrieval and a cutting to open the ovarian artery and tied in place with dries paper.
Ovarian slow freezing
Whole ovaries were cryopreserved using the slow freezing method described by Imhof et al. [20] with some minor modification. Group 1-slow freezing: Three ovaries per each time treat which was plunged a Falcon 50 mL Conical Centrifuge Tubes (Nalgene Nunc, USA) with 20 ml of the cryo-solution which is containing 10% DMSO (Sigma-Aldrich, USA) with supplementation of 0.1 M sucrose and 15% bovine serum albumin (Sigma-Aldrich, USA) in M199 (Gibco, UK), keep 1hr at RT, 1 h at 4oC, 1 h at -20°C, 1 h at liquid nitrogen vapor (3 cm above liquid nitrogen surface), then plunged into liquid nitrogen. The ovaries were stored in liquid nitrogen (>2 weeks) until thawing.
Ovarian vitrification
The ovarian vitrification method was according to Courbiere et al. [8]. In brief, after being prepared as mentioned above, ovaries in group 2 (V1) in set of three were plunged in 30 ml equilibration solution (ES) (M-199 containing 7.5% (v/v) EG (Sigma, USA), 7.5% (v/v) DMSO (Sigma, USA), and 20% fetal calf serum (FCS; Sigma), at room temperature (RT) in 15 min in then exposed 2 min in vitrification solution (VS) in M-199 that contained 15% (v/v) EG, 15% (v/v) DMSO, 20% FCS and 0.5 M sucrose. In groups 3 and 4, ovaries were treated similar to group 2, but with different time in ES: 30 and 45 min, respectively (Group V2 and V3). Ovaries were immediately plunged into liquid nitrogen and stored (>2 weeks) until thawing. The control group was the fresh ovaries without treatment.
Ovarian thawing
For thawing, ovaries were placed in warming solution 1.0 M sucrose for 10 min at 37°C incubation. Icemelted ovaries were further incubated for 10 min in diluting solution, in M-199 that contained 0.5 M, 0.25 M sucrose containing 20% FCS, and final in the washing solution was 20% FCS in M-199 for oocytes collected.
Evaluation of oocyte viability
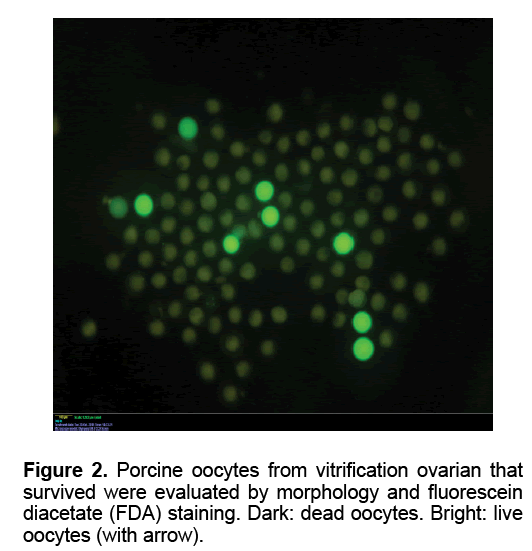
The oocytes were collected as reported previously [21]. To assess viability, oocytes were estimated by FDA staining. The oocytes were transferred in PBS supplemented with 5 mg/ml BSA containing 1mg/ ml fluorescein diacetate (FDA, Sigma, USA) and incubated for 2 min. After that, oocytes were examined under UV light with an axioplan 2 epifluorescence microscope (Carl Zeiss, Germany). Live oocytes accumulate intracellular fluorescein when exposed to FDA. After using the simultaneous staining technique, the cytoplasm of live oocytes appeared green (FDA positive). The cytoplasm of dead oocytes was FDA negative. A few oocytes were not stained with FDA and had blue metaphase plate, which were considered to have an intact plasma membrane but with compromised esterase activity thus they were classified as dead.
In Vitro maturation
Collection and IVM of porcine oocytes were carried out as reported previously [21]. The oocytes were collected from follicles 2-6 mm in diameter in were evaluated for maturation status under stereo microscope. Those with a polar body extruded are considered matured.
Data analysis
The average numbers of oocytes per ovaries were represented as the mean ± SEM. All data were subjected to one-way ANOVA, and the significance of difference among means was determined by Tukey’s multiple range tests. Differences at P<0.05 were considered significant.
Results
Viability of oocytes after cryopreservation
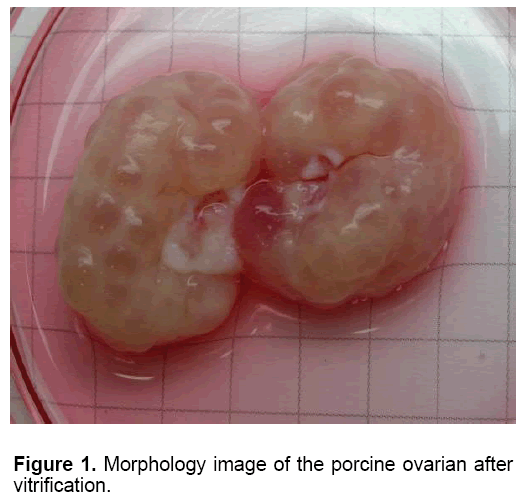
Table 1 showed the oocytes collection and their viability in each group. The mean number of oocyte collected slow freezing and V1 groups (27.80 and 27.20, respectively) was higher than those in other Medium 199 (M199; with Hanks’ salts, Sigma, USA) supplemented with 5% (v/v) fetal bovine serum (Sigma, USA), 20 mM HEPES (Dojindo Laboratories, Kumamoto, Japan), 100 U/ml penicillin G potassium (Sigma) and 0.1 mg/ml streptomycin sulfate (Sigma). About 40 live oocytes were cultured for 24 h in four well dishes (Nunc, Thermo Fisher Scientific, Denmark) containing 500 μl of IVM medium, which is a modified form of North Carolina State University (NCSU) solution, containing 10% (v/v) porcine follicular fluid, 0.6 mM cysteine, 50 TM β1 mercaptoethanol, 1 mM dibutyryl cAMP (Sigma, USA), 10 IU/ml eCG (Serotropin, Japan) and 10 IU/ml hCG (Puberogen 1500 U; Novartis Animal Health, Tokyo, Japan). They were subsequently cultured in IVM medium without dibutyryl cAMP and hormones for a further 22 h. Maturation culture was carried out under an atmosphere of 5% CO2, at 38,5ºC and saturated humidity. Porcine follicular fluid was collected from ovaries of pre-pubertal crossbred gilts by aspiration with a syringe, centrifuged at 1,800 × g for 1.5 h, and the supernatant was stored at −20ºC in advance. Then about 1 L of the stock was thawed. The basic solution (M-199) was TCM-199 containing 2.5 mg/ mL HEPES (Sigma, USA), 2.47 mg/mL Na-HEPES (Sigma, USA), 0.35 mg/mL NaHCO3 and 0.05 mg/mL kanamycin sulfate. After removal of cumulus cells by gentle pipetting in M-199 with hyalunidase, oocytes groups. Oocyte viability in the control group (fresh - 100%) was significantly the highest in all experimental groups (P<0.05). The oocyte viability in group V1 and V2 (6.47 and 7.03%, respectively) was higher than those in other experimental groups (P<0.05). The mean of oocyte viability in group V1 (1.87) was the highest in all experiment group (P<0.05). Similar morphology of ovaries was observed after in five fresh and frozen-thawed groups (Figure 1). However, almost of oocytes collected from ovarian freezing was without the surrounded granulosa cells. The oocytes collected from freeze-thawed ovaries showed their viability when stained by FDA (Figure 2).
| Group | No. of ovarian | Mean number of oocytes collected (Mean ± SEM) | Mean number of viable oocytes (Mean ± SEM) | Rate of viability (Mean ± SEM) |
|---|---|---|---|---|
| Fresh oocytes | 30 | 13,99 ± 1,91a | 13.73±1,32a | 100a |
| Slow freeze | 50 | 27.80 ± 3.44b | 0.86±0.38b | 0.91±0.35b |
| Vitrification 1 | 33 | 27.20 ± 3.77b | 1.87±0.39c | 6.47±0.52c |
| Vitrification 2 | 36 | 13.75 ± 1.37 a | 1.25 ± 0.44c | 7.03 ± 2.07c |
| Vitrification 3 | 40 | 27.27 ± 3.15b | 0.17 ± 0.06b | 0.65 ± 0.25b |
Table 1: The numbers of oocytes derived from porcine ovaries fresh or after freezing.
The potential of oocytes after IVM
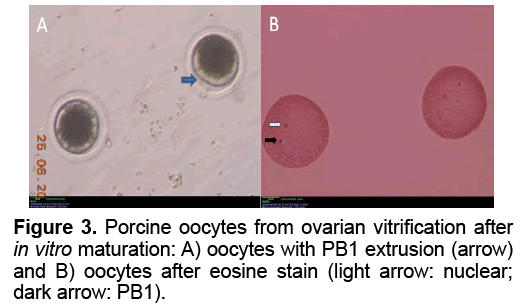
The results of oocyte In Vitro maturation were show in Table 2 and Figure 3. A total of 123 viability oocytes female cancer victims or gemates genetic resource of animal [22]. Cryopreservation of whole ovary will certainly play a direct role in preserving genetic diversity, conserving individuals, breeds or species, as well as be used for adding fundamental knowledge on reproductive biology [23]. Beside transplantation, isolation of follicles from ovaries after whole ovarian cryopreservation and In Vitro culture of them to mature stage was important tool to evaluate follicular development and function after cryopreservation procedures. Despite of that, the objective of the present study was only to evaluate simple cryopreservation methodologies that may be used to develop better cryopreservation protocols usable in field condition without complicated laboratory conditions. We chose the pig ovary model because its size is similar to human’s ovary which made pig as a good model for ovarian cryopreservation research as well as the possibility to get fresh pig ovaries from slaughter houses [22]. Since 2004, first whole ovarian cryopreservation of the pig ovaries was reported [20]. In this research, the ovary was cryopreserved with a slow freezing protocol, using 1.5 M DMSO and evaluation of viability of cryopreserved whole ovaries was under conventional light microscopy [20]. The result of cryopreservation was evaluated by viablility of primordial follicles stained with trypan blue test. The primordial follicular viability after cryopreservation of whole ovary using slow freezing method as assessed with trypan blue test was 83% in ovine and 84% in porcine, higher than that of 76% in human frozen ovary [18,20,24]. Ovarian tissue vitrification could achieved viability of oocytes (92%) as high as that in four experiment groups were cultured. In control group, the rate of PB1 extrusion and MII stage (60.95% and 60.71%) was significantly different of those in experiment groups. There was no significant difference in the rates of PB1 extrusion in group V1 and V2 (8.33% and 9.09%, respectively). However, the nuclear maturation rate (MII) of the group V2 (9.09%) was significantly higher than that of group V1 (6.67%). The GV stage oocytes in slow freezing group (17.78%) were significantly higher than those in group V1 and V2 (11.76% and 11.36%, respectively). In group V3, the rate of damaged oocytes after IVM was 100%. Figure 3 shows the PB1 extrusion and MII nuclear stage after IVM in group V1.
| Group | No. of oocytes | P1 extrusion n (%) | Nuclear stages - n (%) | |||
|---|---|---|---|---|---|---|
| GV | MI | MII | Damage | |||
| Fresh oocytes | 420 | 256 (60.95)a | 144 (34.29)a | 12 (2.86)a | 255 (60.71)a | 9 (2.14)a |
| Slow freeze | 45 | 0 (0)c | 8 (17.78)c | 0 (0)b | 0 (0)c | 37 (82.22)b |
| Vitrification 1 | 60 | 5 (8.33)b | 7 (11.67)b | 0 (0)b | 4 (6.67)b | 49 (81.66)b |
| Vitrification 2 | 44 | 4 (9.09)b | 5 (11.36)b | 0 (0)b | 4 (9.09)b | 35 (79.55)b |
| Vitrification 3 | 7 | 0 (0)c | 0 (0)d | 0 (0)b | 0 (0)c | 7 (100)c |
Table 2: Oocyte status after IVM.
Discussion
Ovarian cryopreservation research is naturally to maintain the potential of reproduction procedure in of control (fresh) specimens [25]. This might indicate that the freezing process had minimal effected into this cell type [25]. In another study, viablity of fresh and vitrified tissue showed no significant difference, but that of slow freeze-cryopreserved tissue was less than one-half (42%; P<0.01). Transmission electron microscopy has also been used to analyze ovarian tissue that had been either cryopreserved by slow freezing or vitrified by ultrarapid freezing, showing that vitrification seemed to be superior with less tissue and cellular damages [26]. In our research, other method for evaluation of viability is FDA staining and the follicular was colleted from 2-6 m for immature oocytes. Immatured oocytes were subjected for evaluation of viablity and maturation of ovarian materials after vitrification. Viablity as well as maturation rates of oocytes after thawing were therefore lower than those of other studies. Higher viability in group V2 might be due longer exposure to cryoprotectant, revealing a higher chance for it to be absorbed into each oocytes.
In Vitro culture of follicle is the basic method to assess function and viability of ovarian tissue [27]. Immature oocytes isolation from ovarian after thawing method was eatablisted and the percentages of morphologically normal and viable follicular oocytes from cryopreservation ovarian were significantly higher than those from the cryo-solution toxic test group [28]. There was not any major difference between subcellular structures in primordial follicles of fresh and properly frozen-thawed ovaries when obsevation under eclectron microscopy [20]. However, in this report, there is an important conclusion that morphological criteria can not be used as the sole methods to evaluate the viability of ovaries that have been cryopreserved [20]. Our results, on the other hand, not only contribute information on the morphology of viable follicular oocytes, but also the developmental ability after In Vitro maturation. The GV stage appear in all slow freeze and vitrification groups showed the potential of the frozed-thawed immature oocytes before IVM. Moreover, in the present study we also evaluate the developmental ability of oocytes collected from frozen-thawed ovaries through In Vitro maturation, not only oocyte viability and morphology right after collecting. In 2002, Al-Aghbari and Menino reported successful isolation of follicles from vitrified sheep ovarian tissue and obtained metaphase II oocytes after IVM [29]. To our knowledge, there is no report concerning of the MII oocytes after In Vitro maturation following whole ovarian cryopreservation until now. In the present study, we report the development In Vitro of immature oocytes from whole ovarian cryopreservation to MII stage for the first time.
Whole ovary cryopreservation and auto-graft has been described in small animal such as: Rats and mice and in large animal such as: ewes, sheep [12,30-33]. However, interests in ovarian cryopreservation re-emerged as its practical value as a strategy for banking genetic material for maintain the genetic resource of animal laboratory, domestic species as well as rare and endangered species and humans was realised [2,34]. Cryopreserved ovaries may be used as a source of competent oocytes if appropriate IVM protocols are developed [18]. Beside orthotopically grafting in a recipient animal in order to restore fertility in that animal, oocytes may be harvested from cryopreserved whole ovaries, IVMed to be subsequently used in an IVF procedure to produce embryos [35,36]. However, until now there was no report applying this technique with the whole ovarian cryopreservation. The risk of whole ovarian cryoprservation include the size of samples effective to cryoprotective dilivery into sample [8]. We have used an empirical model of impregnation by ovarian vitrification solution, based on another team’s previous study in sheep ovaries to establish a system and try to see whether there is an ability of oocytes to develop until MII [37]. This is a simple method of ovarian impregnation which would enable the cryopreservation in field condition. However, it should be further assessed and optimized to be effectively applied for IVF of oocytes. This enable the development of the necessary conditions for embryo production from oocytes collected from ovary vitrification.
In conclusion, freezing method with different conditions has been surveyed. After thawing, 7.03% of oocytes maintained viable appearance in vitrification method with 30 min in ES medium and 9.09% of them were able to develop to MII stage after IVM. The method was a promising technique in cryopreservation of the whole ovarian to establish a female gamete bank to conserve precious domestic and wild animal female reproductive tissue/organ even if collection of samples might be carried out at a field trip site, far from the laboratory.
Acknowledgement
This research is funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.16-2011.43.
References
- Donnez J, Martinez Madrid B, Jadoul P, et al. (2006). Ovarian tissue cryopreservation and transplantation: A review. Human Reprod Update.12: 519-535.
- Salle B, Demirci B, Franck M, et al. (2002). Normal pregnancies and live births after autograft of frozenthawed hemi-ovaries into sheep. FertilSteril.77: 403–408.
- Migishima F, Suzuki-Migishima R, Song SY, et al. (2003). Successful cryopreservation of mouse ovaries by vitrification. BiolReprod.68: 881–887.
- Fahy GM, Wowk B, Wu J (2006). Cryopreservation of complex systems: the missing link in the regenerative medicine supply chain. Rejuvenation Res.9: 279-291.
- Gunasena KT, Villines PM, Critser ES, et al. (1997). Live births after autologous transplant of cryopreserved mouse ovaries. Hum Reprod. 12: 101-106.
- Salle B, Demirci B, Franck M, et al. (2003).Long-term follow-up of cryopreserved hemi-ovary autografts in ewes: Pregnancies, births and histologic assessment. FertilSteril.80: 172–177.
- Yin H, Wang X, Kim SS, et al. (2003). Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischaemia and genotype. Hum Reprod.18: 1165-1172.
- Courbiere B, Caquant L, Mazoyer C, et al. (2009). Difficulties improving ovarian functional recovery by microvascular transplantation and whole ovary vitrification. FertilSteril.91: 2697 - 2706.
- Arav A, Gavish Z, Elami, et al. (2010). Ovarian function 6 years after cryopreservation and transplantation of whole sheep ovaries. Reprod Biomed Online.20: 48-52.
- Figueiredo JR, Rodrigues APR,Amorim CA. (2002).Manipulação de oócitosinclusosemfolículosovarianos. In: BiotécnicasAplicadas à Reprodução Animal, (Eds.) Gonsalves PBD, Figueiredo JR, Freitas VJF, Varella, São Paulo: 227–260.
- Fatehi R, Ebrahimi B, Shahhosseini M, et al. (2014). Effect of ovarian tissue vitrification method on mice preantral follicular development and gene expression. Theriogenology.81: 302-308.
- Campbell BK, Hernandez-Medrano J, Onions V, et al. (2014). Restoration of ovarian function and natural fertility following the cryopreservation and autotransplantation of whole adult sheep ovaries. Hum Reprod. 29: 1749-1763.
- Rahimi G, Isachenko E, Isachenko V, et al. (2004). Comparison of necrosis in human ovarian tissue after conventional slow freezing or vitrification and transplantation in ovariectomized SCID mice. Reprod Biomed Online. 9: 187-193.
- Woods EJ, Benson JD, Agca Y, et al. (2004). Fundamental cryobiology of reproductive cells and tissues. Cryobiology. 48: 146-156.
- Milenkovic M, WallinA, Ghahremani M, et al. (2011). Whole sheep ovary cryopreservation on: Evaluation of a slow freezing protocol with dimethylsulphoxide. J Assist Reprod Genet.28: 7-14.
- Arav A, Revel A, Nathan Y, et al. (2005). Oocyte recovery, embryo development and ovarian function after cryopreservation and transplantation of whole sheep ovary. Hum Reprod.20: 3554–3559.
- Baird DT, Webb R, Campbell BK, et al. (1999). Long term ovarian function in sheep after ovariectomy and transplantation of autografts stored at -196ºC. Endocrinology.140: 462-471.
- Bedaiwy MA, Hussein MR, Biscotti C, et al. (2006). Cryopreservation of intact human ovary with its vascular pedicle. Hum Reprod. 21: 3258-3269.
- Imhof M, Bergmeister H, Lipovac M, et al. (2006) Orthotopicmicrovascularreanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. FertilSteril. 85: 1208–1215.
- Imhof M, Hofstetter G, Bergmeister H, et al. (2004). Cryopreservation of a whole ovary as a strategy for restoring ovarian function. J Assist Reprod Genet.21: 459-465.
- Kikuchi K, Onishi A, Kashiwazaki N, et al. (2002). Successful piglet production after transfer of blastocysts produced by a modified in vitro system. BiolReprod.66: 1033−1041.
- Milenkovic M, Díaz-Garcia C,Brännström M. (2012). Review on ovarian cryopreservation in large animals and non-human primates. In: Current Frontiers in Cryopreservation, InTech Europe, University Campus STePRi: 187-204.
- Santos RR, Amorim C, Cecconi S, et al. (2010). Cryopreservation of ovarian tissue: An emerging technology for female germ line preservation of endangered species and breeds. AnimReprodSci122: 151-163.
- Bedaiwy MA, Jeremias E, Gurunluoglu R, et al. (2003). Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. FertilSteril.79: 594–602.
- Silber S, Kagawa N, Kuwayama M, et al. (2010). Duration of fertility after fresh and frozen ovary transplantation. FertilSteril.94: 2191–2196.
- Keros V, Xella S, Hultenby K, et al. (2009).Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod.24: 1670–1683.
- Kondapalli LA. (2012). Ovarian tissue cryopreservation and transplantation. In: Oncofertility Medical Practice: Clinical Issues and Implementation. (Eds) Gracia C & Woodruff TK, Springer Science+Business Media, New York: 63–75.
- Abd-Allah SM. (2009). Successful cryopreservation of buffalo ovaries using in situ oocyte cryopreservation, Vet Ital. 45: 507-512.
- Al-aghbariAM,Menino AR. (2002). Survival of oocytes recovered from vitrified sheep ovarian tissues. AniReprod Sci.71: 101–110.
- Sugimoto M, Maeda S, Manabe N, et al. (2000). Development of infantile rat ovaries autotransplanted after cryopreservation by vitrification. Theriogenology.53: 1093–1103.
- Salehnia M. (2002).Autograft of vitrified mouse ovaries using ethylene glycolascryoprotectant. Exp Anim.51: 509 –512.
- Tan X, Song E, Liu X, et al. (2012). Successful vitrification of mouse ovaries using less-concentrated cryoprotectants with Supercool X-1000 supplementation. In Vitro Cell DevBiol Anim.48: 69-74.
- Bordes A, Lornage J, Demirci B, et al. (2005). Normal gestations and live births after orthotopicautograft of vitrified–warmed hemi-ovaries into ewes. Hum Reprod. 20:2745–2748.
- Zhang JM, Sheng Y, Cao YZ, et al. (2011). Cryopreservation of whole ovaries with vascular pedicles: Vitrificationor conventional freezing?JAssist Reprod Genet.28: 445-452.
- Meirow D, Levron J, Eldar-Geva T, et al. (2005). Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemother. N Engl J Med.353: 318-321.
- Van-den Hurk R, Santos R. (2009). Development of fresh and cryopreserved early stage ovarian follicles, with special attention to ruminants. AniReprod.6: 72-95.
- Courbiere B, Odagescu V, Baudot A, et al. (2006). Cryopreservation of the ovary by vitrification as an alternative to slow-cooling protocols. FertilSteril.86: 1243–1251.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences