Response of Relative Water Content and Cell Membrane Stability to Mycorrhizal Biofertilizer in Maize
Mohammadreza Naghashzadeh
Department of Agricultural and Natural Resources Science and Research Branch,Islamic Azad University,Tehran,Iran
- Corresponding Author:
- Mohammadreza Naghashzadeh
Department of Agricultural and Natural Resources Science and Research Branch
Islamic Azad University,Tehran,Iran
Tel: +98(0)916-6614904
Fax: +98(0)661-2202202
E-mail: naghashzadeh4@yahoo.com
Abstract
Maize (Zea mays L.) is an effective host of mycorrhiza in infertile and drought conditions. In order to study the effects of arbuscular mycorrhizal fungi on relative water content (RWC) and cell membrane stability (CMS) of maize two field experiments were conducted in 2011 and 2012. The experiments were carried out as split-plot factorial based on randomized complete block design with three replications. Irrigation was imposed at three levels based on 70, 50 and 30% field capacity. Mycorrhizal biofertilizer was applied at two levels; control and 100 kg ha-1. Phosphorus fertilizer was applied at three levels; 0, 75 and 150 kg ha-1 triple superphosphate. The results of combined analysis showed that different irrigation treatments and mycorrhizal biofertilizer application have significantly affected RWC and CMS, but different P fertilizer levels have not significantly affected measured traits. RWC and CMS as affected by different irrigation regimes were decreased by increasing drought stress. The data showed that the mycorrhizal biofertilizer application improved RWC and CMS in maize plant as a consequence of enhancing nutrient uptake, extension of the root system and water status of the plants. Generally, AM plants have a greater effect than non-AM plants.
Keywords
Maize,Mycorrhizal biofertilizer,Water stress.
1. Introduction
The term mycorrhiza literally derived from the Greek words,mykes and rhiza,which mean fungus and root respectively [1]. It was used at the first time by Albert Bernhard Frank in 1885. Mycorriza is a symbiosis between soil fungi and plant roots [2].
Water deficit and drought are the most serious obstacles to agricultural development [3]. Arbuscular mycorrhizal symbiosis can protect host plants against detrimental effects of water scarcity [4] through direct uptake,transfer of water by the fungal hyphae to the host plant [5],retention properties through changes in soil water [6] and better osmotic adjustment [7]. Arbuscular mycorrhizal fungi symbiotically associated with plant roots are known to enhance plant growth under drought conditions by increasing nutritional status and water uptake. However,osmotic stresses may reduce microbial activities and as a consequence,to reduce also plant productivity. Limitation in soil water content causes a series of reactions in plants like stomatal closure that limits CO2 fixation. After some time,lack of water in the growing medium causes poor nutrients diffusion in soil which has important detrimental effects on plant growth [8]. Tong-jian et al. [9] reported that the mycorrhizal fungi and plant roots symbiosis can promote plant uptake water and improve plant growth. Smith and Read [10] reported that under drought conditions,mycorrhizal colonization promotes water relations of the host plants through stimulated plant nutrition as a direct effect and possibly through enhanced direct water uptake. Arbuscular mycorrhizal (AM) fungi are beneficial to improve the soil structure and aggregate stability [11]. Thus,improved soil aggregation can be expected to increase absorption of water by plants,which can also enhance plant growth [12]. Auge [13] has reported about the effects of AM symbiosis on plant water relations in numerous host species colonized by various fungal symbionts,with a particular emphasis on these effects under drought conditions.
When cells expose to osmotic stress,solution metabolites increase to prevent water deficit and turgor pressure reduction. When osmotic adjustment occurs,the metabolites accumulate,which include nitrogen ingredients,such as proline and other amino acids,poly amines and ammonium [14]. Organic solutions accumulate in cytosol and play an important role in osmotic adjustment and cell retention,while water deficit is increased [15].
The primary site of injury under drought stress conditions is the plasmalemma [16]. The membrane integrity is altered by drought stress. A logical reason of this is the increase of the cell permeability accompanied by electrolyte leakage from the cell [17]. The experiment to detect the integrity of cell membrane is called Cell Membrane Stability (CMS) and was used to demonstrate drought resistance in plants [18].
The aim of this study was to assess the effects of mycorrhizal biofertilizer and drought stress on relative water content and cell membrane stability to water resources appropriately.
2. Materials and Methods
2.1. Region of experiment
Two experiments were conducted at the Agricultural Research Station in Khorramabad (Iran) in 2011 and 2012 (June 7th),with Lat. 33˚,29΄ N; Long. 48˚,21΄ E; Alt. 1171 m above sea level; mean temperature at the during growth season in first and second year were 24.90°c and 25.92° c respectively.
2.2. Experimental design and agronomic applications
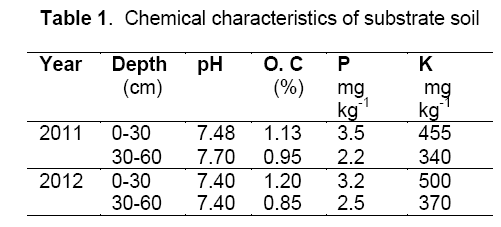
Two experiments were carried out as split-plot factorial based on randomized complete block design with three replications. Irrigation was imposed at three levels; (a) well-watered conditions (I1),based on 70% field capacity; (b) moderate drought stress conditions (I2),based on 50% field capacity; (c) severe drought stress conditions (I3),based on 30% field capacity,as the main plot. Mycorrhizal biofertilizer (species Glomus intraradices) was applied at two levels; (a) control or without application of mycorrhizal biofertilizer (M1); (b) application of mycorrhizal biofertilizer (m2) 100 kg ha-1,as the sub plot. Phosphorus fertilizer was applied at three levels; (a) control (P1); without application of phosphorus fertilizer; (b) application of 75 kg ha-1 triple superphosphate (P2); (c) application of 150 kg ha-1 triple superphosphate (P3),as the sub plot (values were used according to the soil testing). According to the mycorrhizal biofertilizer testing by department soil biology research,ten average mycorrhizal biofertilizer segments had 31 spores per cm3. The experimental field was ploughed in fall and disked twice in spring. Each plot was 8 m in length and consisted of 4 rows separated by 0.75 m,with a distance of 0.20 m between the plants in each row. The studied hybrid was NS-640. According to the soil testing (Table 1) nitrogen and potassium fertilization were determined,including 250 kg ha−1 urea and 100 kg ha−1 potassium sulfate. One third of nitrogen (N),all of mycorrhizal biofertilizer,phosphorous (P) and potassium (K) fertilizers were applied at planting and the remaining N was applied during the vegetative growth. Farm operations for two years were same.
2.3. Soil water content measurement
Soil water content was measured by weighing the soil before and after drying at 105°C for 24 h. Moisture weight percentage was calculated by using the following equation proposes by Kirkham [19].
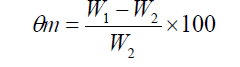
where Өm,W1 and W2 are water content (moisture content) percentage,soil wet weight (g) and soil dry weight (g) respectively. Samples were collected from the 0 – 30 and 30 – 60 cm depths. Bulk density was 1.35 g cm-3. Moisture weight percentage in field capacity was 26.5 and 24.2 in 2011 and 2012 respectively. Hydrogen ion concentration (pH) was 7.5
Irrigation time was determined by weighting soil samples (taken by Auger from the root extension depth) to obtain moisture weight percentage. Then by using the following equation proposes by Doorenbos and Pruitt [20] irrigation water volume was calculated.
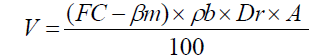
where V is the irrigation water volume (m3),FC is the gravimetric soil water content at field capacity (%),βm is the soil water content before irrigation by weight (%),ρb is the bulk density of the soil (g cm-3),Dr is the root extension depth (m),A is the irrigated area (m2).
Irrigation duration was determined by using the following equation:
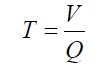
where T is the Irrigation duration (s),V is the irrigation water volume (m3) and Q is the discharge (m3 s-1).
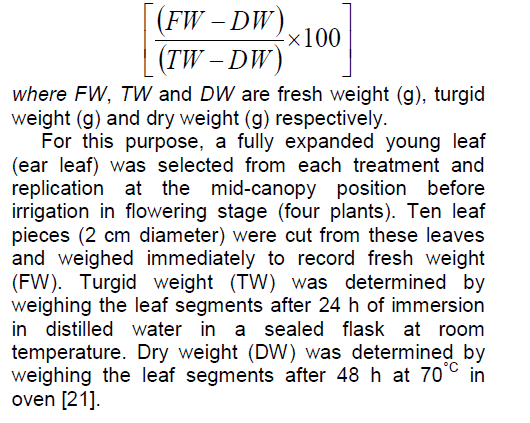
2.4. Relative water content measurement
Relative water content (RWC) was calculated by using the following equation:
2.5. Electrolyte leakage measurement
Cell membrane stability as measured by the electrolyte leakage technique has been used as a tolerance index for abiotic stresses. Electrolyte leakage was determined on leaflet disks removed from the material used for osmotic potential measurements as described by Tas and Basar,(2009). For this purpose,a fully expanded young leaf (ear leaf) was selected from each treatment and replication at the mid-canopy position before irrigation in flowering stage (four plants). Ten leaf pieces (2 cm diameter) were cut from these leaves and washed with distilled water to remove the debris from tissue. The leaf segments were put into glass bottles,then added 20 ml distilled water. Prepared bottles were left in shaker for 24 h and after this procedure solutions in bottles were transferred into test tubes and C1 value was measured in EC meters. Solutions were again transferred into bottles and put in autoclave at 120˚C for 20 min. Afterwards C2 value was measured in room temperature [22]. Electrolyte leakage percentage was calculated by the following equation proposes by Tas and Basar [22].
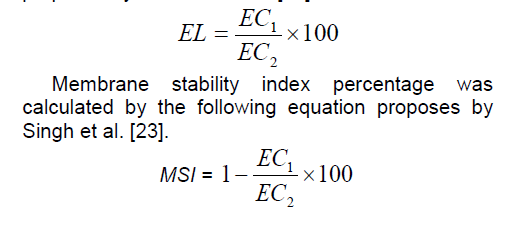
2.6. Statistical analysis
The recorded data were statistically analyzed using the software MSTAT-C. Mean comparisons were calculated using Duncan’s Multiple Range Test at P≤ 0.05.
3. Results and Discussion
The results of combined variance analysis showed that different irrigation treatments and mycorrhizal biofertilizer application have significantly affected relative water content (RWC) and cell membrane stability (CMS) but different P fertilizer levels have not significantly affected measured traits (Table 2). The results of mean comparisons showed that RWC and CMS were decreased by increasing drought stress. There was not significantly different in RWC between well-watered (70% field capacity) and moderate drought stress (50% field capacity). Severe drought stress (30% field capacity) was 26% lower than well-watered conditions. Well-watered had the highest CMS of all irrigation regimes. Severe drought stress was 28% lower than well-watered conditions. AM plants have increased about 3.8% RWC to their leaves compared with non-mycorrhizal. The CMS of AM plants were 4% higher than non- AM plants. Generally,relative water content and cell membrane stability in inoculated plant were higher than non-inoculated plant (Table 3).
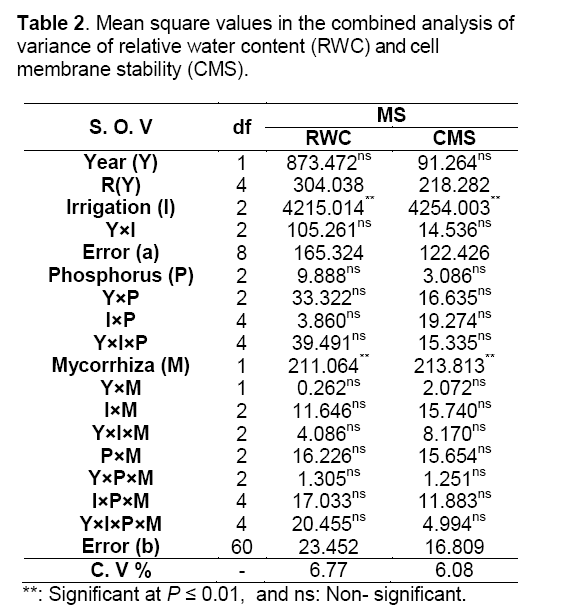
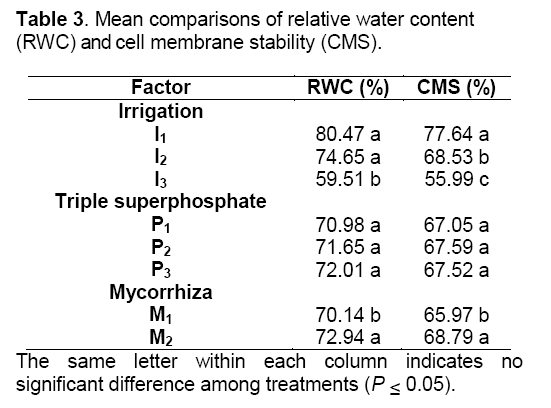
Arbuscular mycorrhizal fungi postpone reductions in leaf water potential during periods of drought stress and hasten returns to control levels upon the reduction of water-limiting conditions [13] through direct uptake and transfer of water by the fungal hyphae to the host plant [5]. The results of present study are in agreement with the conclusions of Auge et al. [5] that fungal hyphae were obtained water by direct uptake and transfer to the host plant,so that protection plants against drought stress. Also,osmotic adjustment occurs to decrease their water potential to maintain a beneficial gradient for water flow from soil into plant roots. The results of this study are in agreement with the conclusions of Porcel and Ruiz-Lozano [7] that AM plants have a greater osmotic adjustment than non-AM plants. On the one hand fertilizers increased RWC under drought stress conditions [24],on the other hand Singh et al. [25] reported that there was no significant effect of soil-P on RWC by increasing P fertilizer,which is somehow in accordance with the results of this experiment.
Among the physiological traits that are correlated with performance under drought stress conditions,membrane stability has recognized as a useful measure of drought tolerance [18]. Water limitation in the growing medium reduces diffusion,uptake by roots and transport of nutrients from roots to shoots due to altered membrane permeability [26]. However,water limitation has been accepted as one of the major causes of increased cell membrane permeability [27]. Arbuscular mycorrhizal plants have higher electrolyte concentration than non-mycorrhizal plants by improvement integrity and stability of the membrane [28].
Water stress leads to increase accumulation of reactive oxygen species [29] such as superoxide (O2•−),hydrogen peroxide (H2O2) and the hydroxyl radical (•OH). They cause damage to cell membrane structure by injuring cell components,that ultimately electrolyte leakage is increased [30]. Khodary [31] concluded that water stress caused to change in phospholipid membranes and increased unsaturated acids and therefore,increased electrolyte leakage. The results of the present study are in agreement with the conclusions of Janda et al. [29],Foyer [30] and Khodary [31] associated with the role of water stress to increase electrolyte leakage.
4. Conclusion
The results obtained in this study show that relative water content (RWC) and cell membrane stability (CMS) in maize plants have been affected greatly by water stress conditions. The data showed that the mycorrhizal biofertilizer application improved RWC and CMS in maize plants as a consequence of enhancing nutrient uptake and water status of the plants. Generally,AM plants have a greater effect than non-AM plants. With respect to environmental problem associated with fertilizer and water limitation in future,it is essential that we apply water resources appropriately and decrease fertilizers application in order to improve soil fertility,productivity and water quality.
Acknowledgements
I thank the staff of Lorestan Agricultural and Natural Resources Research Center for their assistance in conducting the field experiment and for providing the equipments.
References
- Bardgett R. D. (2005) The Biology of Soil A Community and Ecosystem Approach. Oxford University Press,UK. p: 242.
- Muchovej R. M. (2009) Importance of Mycorrhizae for Agricultural Crops. The Institute of Food and Agricultural Sciences (IFAS). pp: 1-5.
- Wilhite D. A (2005). Drought and Water Crises Science,Technology,and Management Issues. CRC Press,Taylor & Francis Group. p: 406.
- Ruiz-Lozano J. M.,Porcel R.,Aroca R.,et al. (2008) Evaluation of the possible participation of drought induced genes in the enhanced tolerance of arbuscular mycorrhizal plants to water deficit. In: Varma,A. ed. Mycorrhiza: state of the art,genetics and molecular biology,eco-function,biotechnology,eco-physiology,structure and systematics,(3rd ed). Germany: Springer-Verlag. pp: 185-205.
- Auge R. M.,Moore J. L.,Stutz J.C.,Sylvia D. M.,Al-Agely A. K.,Saxton A. M.,et al. (2003) Relating foliar dehydration tolerance of mycorrhizal Phaseolus vulgaris to soil and root colonization by hyphae. J Plant Physiol. 160:1147-1156.
- Auge R. M.,Stodola A. J. W.,Tims J. E.,Saxton A. M.,et al. (2001) Moisture retention properties of a mycorrhizal soil. Plant Soil. 230: 87-97.
- Porcel R.,Ruiz-Lozano J. M. (2004) Arbuscular mycorrhizal influence on leaf water potential,solute accumulation and oxidative stress in soybean plants subjected to drought stress. J Exp Bot. 55:1743-1750.
- Benabdellah K. Abbas Y.,M.,Aroca R.,Azcon R.,et al. (2011) Abourouh Influence of two bacterial isolates from degraded and non-degraded soils and arbuscular mycorrhizae fungi isolated from semi-arid zone on the growth of Trifolium repens under drought conditions: Mechanisms related to bacterial effectiveness. Eur. J. Soil Biol. 47: 303-309.
- Tong-jian X.,Qing-song Y.,Wei R.,Guo-hua X.,Qi-rong S. H.,et al. (2010) Effect of Inoculation with Arbuscular Mycorrhizal Fungus on Nitrogen and Phosphorus Utilization in Upland Rice-Mungbean Intercropping System. Agric Sci China. 9(4): 528-535.
- Smith S.,Read D. J. (2008) Mycorrhizal Symbiosis. Academic Press Publishers. London. p: 605.
- Jeffries,P.,Barea J. M. (2000) Arbuscular mycorrhiza – a key component of sustainable plant- soil ecosystems. In: Hock ,B. (Ed.),The Mycota IX,Fungal Associations. Springer,Berlin. pp: 95-113.
- Kohler J.,Caravaca F.,Alguacil M. M.,Roldan A.,et al. (2009) Elevated CO2 increases the effect of an arbuscular mycorrhizal fungus and a plant-growth-promoting rhizobacterium on structural stability of a semiarid agricultural soil under drought conditions. Soil Biol. Biochem. 41:1710-1716.
- Auge R. M. (2001) Water relations,drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 11: 3-42.
- Tamura,T.,Hare K.,Yamaguchi Y.,Koizumi N.,Sanaa H.,et al. (2003) Osmotic stress tolerance of transgenic tobacco expressing a gene encoding a membrane-located receptor- like protein from tobacco plants. Plant Physiol. 131: 454-462.
- Pinheiro C.,Chaves M. M.,Ricardo C. P.,et al. (2001) Alterations in carbon and nitrogen metabolism induced by water deficit in the stems and leaves of Lupinus Albus. L. J. Exp. Bot. 52:1063-1070.
- Levitt J. (1980) Responses of Plant to Environment Stresses Water,Radiations,Salt and other stresses. Vol. 2 Academic Press,New York.
- Blum A.,Ebercon A. (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 21: 43-47.
- Farooq S.,Azam F. (2002) Co-existence of salt and drought tolerance in Triticeae. Hereditas. 135: 205-210.
- Kirkham M. B. (2005) Principles of soil and plant water relations. Elsevier Academic Press. p: 500.
- Doorenbos J.,Pruitt WO. (1975) Crop Water Requirements. Irrigation and Drainage Paper No. 24. FAO Rome. pp: 179.
- Efeoglu B.,Ekmekci Y.,Cicek N.,et al. (2009) Physiological responses of three maize cultivars to drought stress and recovery. S Afr J Bot. 75:34-42.
- Tas B.,Basar H. (2009) Effects of various salt compounds and their combinations on growth and stress indicators in maize (Zea mays L.). African J Agric Res. 4:156-161.
- Singh A.,Kumar J.,Kumar P.,et al. (2008) Effects of plant growth regulators and sucrose on post harvest physiology,membrane stability and vase life of cut spikes of gladiolus. Plant Growth Regul. 55:221-229.
- Graciano C.,Guiamet J. J.,Goya J. F.,et al. (2005) Impact of nitrogen and phosphorus fertilization on drought responses in Eucalyptus grandis seedlings. Forest Ecol Manage. 212:40–49.
- Singh V.,Pallaghy C. K.,Singh D.,et al. (2006) Phosphorus nutrition and tolerance of cotton to water stress II. Water relations,free and bound water and leaf expansion rate. Field Crop Res. 96:199-206.
- Sardans J.,Penuelas J.,Ogaya R.,et al. (2008) Drought’s impact on Ca,Fe,Mg,Mo and S concentration and accumulation patterns in the plants and soil of a Mediterranean evergreen Quercus ilex forest. Biogeochemistry. 87:49-69.
- Tabaei-Aghdaei S.,Harrison P.,Pearee R. S.,et al. (2000) Expression of dehydration-stress related genes in crown of wheat grass species having contrasting acclimation to salt,cold and drought. Plant Cell Environ. 23: 561-571.
- Kaya C.,Ashraf M.,Sonmez O.,Aydemir S.,Tuna A. L.,Cullu M. A.,et al. (2009) The influence of arbuscular mycorrhizal colonization on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci Hortic. 121:1–6.
- Janda T.,Szala G.,Antunovics Z.,Hovart E.,Paldi E.,et al. (2000) Effect of benzoic acid and aspirin on chilling tolerance and photosynthesis in young mice. Plants Mydica. 45:29-33.
- Foyer C. H. (2002) The contribution of photosynthetic oxygen metabolism to oxidative stress in plants. In: Inze,D. And M. V. Montage,ed. Oxidative stress in plants. New York,USA: Taylor and Francis Publishers. pp: 33-68.
- Khodary S. E. A. (2004) Effect of salicylic acid on the growth,photosynthesis and carbohydrate metabolism in salt-stressed maize plants. Intl. J. Agri. Biol. 6:5-8.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
