Quality control parameters, antioxidant activity and chemometrics of Brazilian honey
Maria Cristina Marcucci*, Daniella Barretto Toledo, Alexandra Christine Helena Frankland Sawaya, Begoña Giménez-Cassina López, Ivair D. Gonçalves, Thaiana Cristina de Camargo, Carolina Passarelli Gonçalves
Maria Cristina Marcucci1*, Daniella Barretto Toledo1, Alexandra Christine Helena Frankland Sawaya2, Begoña Giménez-Cassina López2, Ivair D. Gonçalves1, Thaiana Cristina de Camargo1, Carolina Passarelli Gonçalves3
1Postgraduate Program in Pharmacy and Biotecnology, Universidade Anhanguera de São Paulo UNIAN-SP), Raimundo Pereira de Magalhães, 3305 São Paulo, 05145-200, SP, Brazil
2Program of Bioscience and Technology of Bioactive Products, Institute of Biology and Faculty of Pharmaceutical Science, Universidade Estadual de Campinas (UNICAMP), Cidade Universitaria Zeferino Vaz, distr. Barão Geraldo, Campinas, 13083-970, SP, Brazil
3Pharmacy Department, Anhanguera University of São Paulo, Avenida dos Autonomistas, 1325 Osasco, 06020-010, SP, Brazil
- *Corresponding Author:
- Maria Cristina Marcucci
Postgraduate Program in Pharmacy and Biotecnology
Universidade Anhanguera de São Paulo UNIAN-SP)
Raimundo Pereira de Magalhães, 3305 São Paulo, 05145-200, SP, Brazil
Tel: +55 11 2967-9147
E-mail: cris.marcucci@yahoo.com.br
Received Date: January 23, 2019; Accepted Date: February 16, 2019; Published Date: February 25, 2019
Citation: Marcucci MC, Toledo DB, Sawaya AC, Lopez B, Gonçalves ID, Camargo TC, Gonçalves CP. Quality control parameters, antioxidant activity and chemometrics of Brazilian honey Electronic J Biol, 15:1
Abstract
Bee honey presents beneficial properties for human health and is considered a high quality food. However, if the producer does not take the necessary care related to extraction and storage, honey suffers changes in its chemical composition reducing its quality. In order to evaluate the quality of commercial honey, some physicochemical analyses (moisture, electrical conductivity, soluble solids, ash, hydroxymethylfurfural (HMF), proline, color, antioxidant activity, Lund and Lugol reactions) of eleven honey samples from different producers and one corn glucose sample were performed. Honey samples were generally considered of good quality, displaying results within the standards allowed by Brazilian laws. The only analyses that were able to distinguish honey from corn glucose were: Lugol, HMF and proline, was well as the Principal Component Analysis (PCA) of the total results. Good correlation was found between electric conductivity versus ash, electric conductivity versus colour and ash versus colour; ED50 versus ash and colour versus ED50 presented an inverse correlation. All honey’s samples presented good quality and are within the legal parameters. The essential analyzes for the differentiation of good quality honeys against the corn glucose sample were: Lugou, HMF and Proline.
https://tipobette.com https://vdcasinoyagiris.com https://venusbetting.com https://sahabetting.com https://sekabete.com https://sahabete-giris.com https://onwine-giris.com https://matadorbet-giris.com https://casibomkayit.com https://casibomba.com https://casiboms.com https://casinoplusa.org https://casibomlink.com https://yenicasibom.com https://jojobetegiris.com https://jojoguncel.com https://jojobetyeni.com https://girisgrandbetting.com https://pashabetegiris.com https://grandbettingyeni.com
Keywords
Honey quality and adulteration; Antioxidant activity; Principal component analysis; Correlations.
Introduction
Beekeeping in Brazil is changing from artisanal and amateur, becoming constantly more entrepreneurial, technical and productive. The Brazilian Service of Support to Micro and Small Companies has been supporting honey producers in very well established cooperatives [1]. Throughout the country, thousands of jobs are generated relating to beekeeping as well as the manufacture and commerce of related equipment [2]. Honey is considered a high-quality food and is of great nutritional importance, rich in numerous substances considered to be beneficial to our health [3]. Honey has antianemic, emollient, conservative, digestive, laxative and diuretic, anticancer and prebiotic properties [4]. It is a complex food, both from the biological and the analytical points of view, as its composition changes depending on its geographic and floral origin, as well as due to climatic conditions [5,6].
Brazil has an abundant biodiversity and the rugged Africanized bees, both presenting great potential for obtaining high quality honey and other derived apicultural products. However, there is still a lot to learn and develop related to the properties and characteristics of our bee products [7,8].
Honey is a viscous, aromatic and sugary substance. Its aroma, taste, color, viscosity and medicinal properties are directly related to its source of nectar and the species of bee that produced it. Honey is composed of sugars, water, enzymes, vitamins, flavonoids and minerals [9]. A series of other organic compounds, such as organic acids, and even bacteria contribute to its colour, odor and flavor [10]. The composition of honey is mainly dependent on its source(s) of nectar, but variation in the type of soil, species of bee, physiology of the colony, state of maturity and climatic conditions when the honey is obtained may also affect its composition, as well as its final quality. Therefore honeybees produce better honey in regions where the flora and climate favor the collection of nectar [7].
Besides sugars, honey contains dextrin, gum and small quantities of compounds containing nitrogen and phosphor. It also contains small quantities of minerals, organic acids, vitamins, pigments and aromatic substances. The ash content is usually below 0.5%. Most nectar sources are acid (pH 2.7-6.4), as a result of approximately 0.57% organic acids [11] but some may be alkaline (pH up to 9.1). The vitamin content is low, with the presence of: thiamin, riboflavin, pyridoxine, nicotinic acid, pantothenic acid, folic acid, biotin and ascorbic acid (the only vitamin found in reasonable concentrations in nectar and honey) [12]. Table 1 presents the basic composition of a sample of commercial honey produced by Apis mellifera Adansonii honeybees from Brazil. The standards of honey approved by the Brazilian Legislation are only met by honeys produced by Apis species.
| Components | Mean | Standard deviation | Variation |
|---|---|---|---|
| Ash (%) | 0.169 | 0.15 | 0.020 - 1.028 |
| Diastase* | 20.8 | 9.76 | 2.1 – 61.2 |
| Free acidity (meq/kg)* | 22.03 | 8.22 | 6.75 - 47.19 |
| Fructose (%)* | 38.19 | 2.07 | 27.25 – 44.26 |
| Glucose (%)* | 31.28 | 3.03 | 22.03 – 40.75 |
| Lactose (meq/kg) | 7.11 | 3.52 | 0.00 – 18.76 |
| Lactose/Free acidity | 0.335 | 0.135 | 0.000 - 0.950 |
| Maltose (%) | 7.31 | 2.09 | 2.74 - 15.98 |
| Nitrogen (%) | 0.041 | 0.026 | 0.000 - 0.133 |
| Other sugars (%) | 3.1 | 1.97 | 0.0 - 13.2 |
| pH* | 3.91 | - | 3.42 - 6.10 |
| Sucrose (%)* | 1.31 | 0.95 | 0.25 – 7.57 |
| Total acidity (meq/kg)* | 29.12 | 10.33 | 8.68 - 59.49 |
| Total sugars (%) | 1.5 | 1.03 | 0.13 – 8.49 |
| Water (%)* | 17.2 | 1.46 | 13.4 – 22.9 |
*Analysis recommended for unifloral honey [13-14,39].
Table 1. Basic composition of a Brazilian honey sample, EMBRAPA [16].
For these reasons, the purpose of this study was to analyze the physicochemical parameters of honey samples and their antioxidant activity in order to evaluate which analyses could more clearly separate pure honey from adulterated or false samples.
2. Materials and Methods
2.1 Chemicals
Tannic acid, iodine and potassium iodate were purchased from Synth (Brazil), 2,2'-diphenyl- 1-picrylhydrazyl radical, proline and hydroxymethylfurfural were from Sigma Chemical Co. (St. Louis, MO, USA). For HPLC analysis we used solvents HPLC grade, methanol from Merck (Darmstadt, Germany), HPLC filters were minisart RC 15, 0.45 μm (RC-mebrane) from Sartorius (Germany).
2.2 Honey sample
Eleven samples of commercial honey and one sample of corn glucose were analyzed. All samples were kept in their original containers, wrapped in plastic to guarantee isolation from contamination, at room temperature. The predominant floral sources of these samples were: 1 (cipó-uva, Paullinia rubiginosa-a Brazilian plant), 2 (eucalyptus, genus Eucalyptus), 3 (cashew, Anacardium occidentale), 4 (Amazon flowers), 5 (orange, Citrus aurantium), 6 (wild flowers), 7 (honey plus propolis), 8 (eucalyptus), 9 (wild flowers), 10 (orange), 11 (cipó-uva) e 12 (corn glucose).
2.3 Analytical procedures for honey samples
Some physicochemical parameters were evaluated according to literature (water insoluble solids, ash, proline, humidity (water content), electric conductivity and hydroxymethylfurfural (HMF) [13-15]. All samples were analyzed in triplicate.
Lund reaction was performed using an aliquot of 2 g of honey and 20 mL of distilled water placed in a graduated tube of 100 mL and homogenized. 5 mL of a 0.95% solution of tannic acid were added and, in sequence, 40 mL of water. This solution was shaken and the left to rest for 24 hours. After this period of time the amount of precipitate was measured in millimeters.
Lugol solution was prepared by adding 0.5 g of iodine to 1.5 g of potassium iodate and dissolving in water, then completing the volume to 25 mL. In a 50 mL beaker, 10g of the honey sample were dissolved in 10 mL of water, then 1 mL of Lugol solution was added. This test is to evaluate the reaction of Lugol reactive with starch. If hydrolyzed starch is present, the solution turns reddish-brown.
Colour determination was carried out using Pfund Honey Color Analyzer (Hanna Instruments, Rhode Island, USA). A cuvette containing 5 g of honey without air bubbles was inserted and color for each sample analyzed in triplicate. Colour grades based on Pfund scale are: water-white ≤ 8.0 mm, extrawhite 8-16 mm, white 17-34 mm, extra light-amber 35-50 mm, light-amber 51-85 mm, amber 86-114 mm and dark ≥ 114 mm [6].
2.4 Antioxidant activity
This method is based on the reduction of the stable free radical 2,2'-diphenyl-1-picrylhydrazyl (DPPH) in ethanol solution (absorption maximum at 515- 528 nm). When it reduced, it changes color and its absorption at 517 nm diminishes. After the reaction reaches equilibrium, the amount of an antioxidant substance needed to reduce active DPPH in 50% can be calculated. The honey samples were diluted ten times in distilled water and then eleven tubes with different dilutions of the samples were added to the same volume of 60 μM DPPH solution, at one minute intervals. Individual readings were done at 517 nm after 30 minutes (equilibrium time) at one min intervals. Negative control was a tube containing DPPH and the solvents used. A curve of % absorbance versus concentration of honey (mg/ mL) was built for each sample and the concentration at 50% reduction of the DPPH (ED50) was calculated by the minimal squares method.
2.5 Analysis of variance
The analysis of variance test (ANOVA) followed by Tukey-Kramer multiple comparison test (parametric data) and Kruskal-Wallis (nonparametric data) (GraphPad, Prism 6.0, San Diego, CA, USA) was used to determine significant difference between sample results.
2.6 Chemometric analysis
The chemometric method used was principal component analysis (PCA) where a matrix containing the data for all the samples is prepared, with the lines representing the samples and the columns representing the variables-the results of all the analyses. This multi-dimensional information is transformed into graphics composed of two or three dimensions known as principal components (PC) which is the limit of human capacity to visualize and analyze. The “scores” graph represents the position of the samples in this new bi- or tridimensional space, while “loadings” graph represents the variables. As the results of the analyses are given in different units, all the results were auto-scaled and mean centered before analysis. Pirouette software, version 3.11 (Infometrix, Woodinville, WA, EUA) was used.
3. Results and Discussion
The water-insoluble solids, ashes, proline, HMF and humidity contents are presented in Table 2, along with the electric conductivity of the samples.
| Sample | Water-insoluble solids | Ash (% w/w) | Proline (mg/kg) | Humidity-Water content (%w/w) | Eletric condutivity (µS/cm) | HMF (mg/kg) |
|---|---|---|---|---|---|---|
| Cipó-uva (1) | 0.0203 ± 0.0004A | 0.061 ± 0.010 A | 111.82 ± 1.37 A | 12.37 ± 0.06 A | 160 ± 1 A | 11.02 ± 1.32 A |
| Eucalyptus (2) | 0.0378 ± 0.0025 A | 0.390 ± 0.032 | 128.83 ± 0.64 | 15.75 ± 0.05 | 712 ± 4 | 1.86 ± 0.70 A |
| Cashew (3) | 0.0483 ± 0.0308 A | 0.343 ± 0.149 | 1136.05 ± 0.70 | 15.93 ± 0.06 | 560 ± 10 | 11.01 ± 0.63 A |
| Amazon flowers (4) | 0.2013 ± 0.0322 | 0.178 ± 0.025 | 79.93 ± 3.63 | 18.20 ± 0.10 | 370 ± 90 | 9.60 ± 0.54 A |
| Orange (5) | 0.0220 ± 0.0007 | 0.105 ± 0.060 | 264.28 ± 2.36 | 16.17 ± 0.06 | 215 ± 20 | 46.35 ± 2.28 |
| Wild (6) | 0.0185 ± 0.0007 | 0.280 ± 0.068 | 486.24 ± 0.90 | 16.50 ± 0.10 | 542 ± 15 | 6.29 ± 0.47 |
| Honey + propolis (7) | 0.1748 ± 0.0053 | 0.264 ± 0.105 | 441.38 ± 0.64 | 17.13 ± 0.15 | 520 ± 90 | 14.65 ± 0.32 |
| Eucalyptus (8) | 0.0470 ± 0.0014 | 0.434 ± 0.005 | 309.21 ± 4.22 | 15.30 ± 0.17 | 764 ± 10 | 8.27 ± 2.18 |
| Wild (9) | 0.0320 ± 0.0007 | 0.197 ± 0.056 | 268.75 ± 0.30 | 15.73 ± 0.06 | 400 ± 20 | 18.61 ± 0.67 |
| Orange (10) | 0.0213 ± 0.0004 | 0.225 ± 0.021 | 207.09 ± 1.75 | 17.27 ± 0.06 | 401 ± 30 | 4.09 ± 0.02 |
| Cipó-uva (11) | 0.0170 ± 0.0021 | 0.084 ± 0.059 | 192.85 ± 1.56 | 15.80 ± 0.10 | 180 ± 1 | 8.91 ± 0.02 |
| Corn glucose (12) | 0.0083 ± 0.0004 | 0.246 ± 0.025 | 0.33 ± 0.10 | 19.25 ± 0.07 | 412 ± 25 | 1484.60 ± 25.25 |
Data are means ± S.D. of three independent determinations. Means within a column sharing the letter A are not significantly different by Student’s t-test (P >0.05, One-Way ANOVA) (intergroup evaluation). The averages which are not identified by a letter were significantly different from the other averages P < 0,05 by P<0,005 to P <0,0001, ( One-Way – ANOVA).
Table 2. Water-insoluble solids (0.1g/100g), ash (% w/w), proline (mg/kg), humidity-water content (% w/w), electric conductivity (µS/cm) and hydroxymethylfurfural (HMF) (mg/kg) of tested honeys.
3.1 Water-insoluble solids
Samples 4 and 7 present results which are higher than the 0.1% legislation permits, however sample 4 has a honey comb and sample 7 is mixed with propolis. No parameters in Brazilian legislation are found pertaining to honey mixed with other products (7) or containing combs (4). Therefore, in spite of presenting irregular results, neither sample was adulterated.
Brazilian legislation permits a maximum of waterinsoluble solids of 0.1 g/100 g (0.1%), except for pressed honey, where up to 0.5 g/100 g (0.5%) is accepted [16]. The percentages of water insoluble solids found in the samples are within the legal standards. For corn glucose (12), a value of 0.008% was found.
3.2 Ash content
The ash content (%) of the honey samples (Table 2) fall within the range presented in Table 1 (0.020 to 1.028% for commercial honey). The minimum value for ash contents was 0.061% and the maximum was 0.434%. Sample 8 had the highest inorganic (ash) content but was within the legal parameters, as was the corn glucose sample. Correctly processed pure honey presents low ash contents, with a maximum of 1%. This parameter is therefore commonly used to identify irregularities such as the presence of insects, paint residue, pieces of wood or wax, indicative of lack of proper filtration or hygiene. Brazilian legislation permits a maximum of 0.6 g/100 g (or 0.6%) for floral honey. For honeydew and its mixtures with honey, up to 1.2 g/100 g is accepted [16].
3.3 Proline
Of the various amino acids found in honey, proline, which derives from the glandular secretions of honeybees, presents the highest contents. Proline is commonly the main free amino acid in honey and is almost invariably present in concentrations exceeding 20 mg/kg [17].
The proline contents of honey samples are presented in Table 2. The corn glucose sample (12) showed a much lower value of proline (0.33 mg/kg of honey). Sample 3 presented the highest proline content (1136.05 mg/kg of honey) whereas sample 4 (79.93 mg/kg of honey) showed the lowest content of the honey samples.
Czipa et al. investigated the proline content of 143 honey samples with different flower origin, produced in Hungary and from different countries of the world, e.g., Tasmania, New-Zealand, Malaysia, Thailand, South-Africa, Finland, and others, reporting a range of concentration of this amino acid similar to our findings [18].
It has been known for a long time that proline in honey is derived from the bee itself, but it has been difficult to explain why such a variation exists in the content of proline in honeys [19]. Truzzi et al. reported that proline is the main amino acid present in honey and is employed as a measure of the amount of total amino acids. Its content is one of the criteria for assessing the quality and antioxidant activity of honey [15]. It can also be used as a tool to determine its botanical origin [20].
3.4 Humidity (water content)
As honey is highly hygroscopic, it easily absorbs humidity from the air. Beekeepers should avoid collecting honey on rainy or highly humid days and only collect operculated honey, which is protected from ambient humidity by a layer of wax that is also a sign that honey is ripe and has low water content. High water content favors the growth of microorganisms, leading to fermentation and makes it improper for human consumption [21]. Brazilian legislation permits a maximum of 20% water content for floral honey and honeydew [16]. European legislation permits up to 18% water content [22,23]. Therefore all the honey samples (1-11) are within the limits established by both Brazilian and European legislation. The water content (%) of the honey samples varied between 12.37% (1) and 18.20% (4). Sample 12 is corn glucose, not honey, and presents the highest water content, 19.25%, which is within the legal parameters for commercial honey in Brazil even though it is not an apicultural product (Table 2).
The moisture content influences the taste, viscosity, conservation and crystallization, among other parameters of honey quality and also contributes to the development of fermenting microorganisms [7].
3.5 Electric conductivity
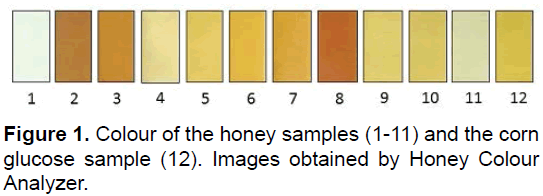
The values for electric conductivity varied between 160 (1) and 764 (8) (μS/cm). The conductivity of the corn syrup sample was 412 (12) (μS/cm). The samples which presented the highest conductivity also presented the highest level of ashes [9]. According to Acquarone et al. ash content is determined mainly by soil and climate characteristics. The samples grouped in Figure 1, demonstrate that the ash content was one of those responsible for the hierarchical separation, as discussed later [24]. The floral origin may also determine the values of ash content.
3.6 Hydroxymethylfurfural
All the honey samples (1-11) presented hydroxymethylfurfural (HMF) values within the legal maximum of 60 mg/kg [16] and excepted sample 5, for international guidelines [25] that establish the maximum of 40 mg/kg. The HMF content measures the quality of the honey. Several factors influence its content, such as temperature and heating time during processing, storage conditions, floral sources and others [26].
Sample 12, as expected for a corn glucose sample, presented a high HMF value of 1484.60 mg/kg. Some ways to fraud honey involve the addition of simple and complex sugars. One of the cases is the addition of corn glucose syrup [22,23] among others, as well as high molecular weight sugars. The addition of corn glucose increases the HMF content considerably, as demonstrated in the results found with the sample 12. Detection of honey adulteration by sugars can also be determined by the 13C/12C ratio, as previously reported [27], but this is a far more expensive and complex procedure.
3.7 Other parameters
The samples in this study presented the following results of Lund precipitation (in cm):1.00 (1), 3.70 (2), 0.80 (3), 1.20 (4), 4.10 (5), 2.00 (6), 0.20 (7), 1.60 (8), 0.20 (9), 1.30 (10), 1.30 (11) and for corn glucose 2.30 (12). For the Lund reaction, these results of precipitation of albuminous substances (proteins and their precursors-which are natural components of honey) are considered normal for pure honey. Previous studies have reported that honey commercialized in the region of Botucatu (São Paulo, Brazil) presented average precipitation of 1.7 cm [28] and honey produced mainly from field mint flowers in the Pantanal region (Brazil) presented average Lund precipitation of 1.50 cm [29]. Samples 2 and 5 presented the highest precipitation of albuminous substances; however, legislation does not stipulate values in relation to this analysis. Sample 7 presented a low result, possibly due to its mixture with propolis, which does not contain proteins.
The results of all the honey samples (1-11) were negative for the Lugol test, clearly showing they were not mixed with starch based syrup. The result of the Lugol test for sample 12 was positive, as could be expected for corn glucose.
The evaluation of sample colour showed results expressed in Pfund mm: 21 (1), 120 (2), 107(3), 63(4), 53(5), 82(6), 125(7), 135(8), 66(9), 68(10), 26(11) and 71(12) (Figure 1). The predominant colour of the honey samples was light amber (45%), followed by amber (27%), 18% white and 9% dark amber. Our results are similar to those reported in the literature, for Brazilian honey [30]. Various colour pigments deriving from vegetable sources (nectar and pollen) such as anthocyanins, phenolic acids, proanthocyanidins, flavonoids and minerals that constitute the basic colour of honey [31] which are also known to have antioxidant activity [26].
3.8 Antioxidant activity
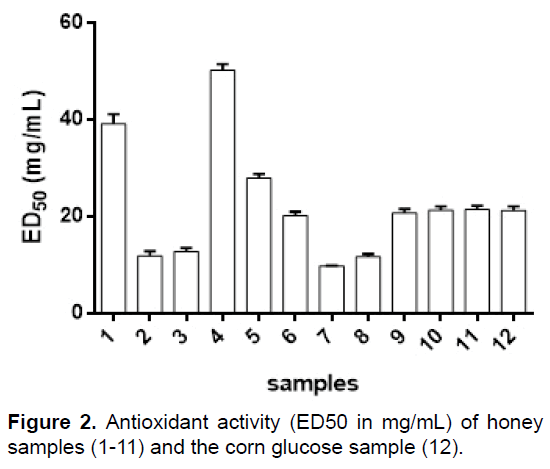
The antioxidant activity of the honey samples expressed as ED50 in mg/mL (the lower ED50 value means the higher sample activity) was: 39.19 (1), 11.88 (2), 12.80 (3), 50.22 (4), 27.93 (5), 20.21 (6), 9.81 (7), 11.74 (8), 20.80 (9), 21.40 (10), 21.45 (11) and 21.25 (12) (Figure 2). Antioxidant activity of the honey samples varied greatly, between 9.81 mg mL-1 and 50.22 mg/mL. Sample 7 presented the highest antioxidant activity, as expected, as it contains propolis. Propolis is known for its antioxidant activity, with ED50 values of approximately 10 μg/mL [32]. It was surprising to note that honey sample 4 had lower antioxidant activity than corn glucose (12), so the antioxidant activity of honey cannot be correlated to its purity. Honey sample 4, showed the lowest antioxidant activity, possibly due to this high water content and the low antioxidant potential of sample 4 may be related to low proline concentration, which has been studied as an osmoprotectant, which can remove reactive oxygen species (ROSs), and similar results for samples 6, 9, 10 and 11 to the value of sample 12 are related to the content of water insoluble solids (Table 1). Our results are in agreement with those of Beretta et al and Can who analyzed African and Turkish honeys, respectively, finding similar range of ED50 values [31,33]. Özcan and Ölmez reported much lower ED50 values for Turkish honey [10]. The weak antioxidant activity of honey could be attributed to its phenolic acid and flavonoid content [34].
3.9 Multivariate statistical analysis
Multivariate analysis, such as PCA, is a practical tool used to compare the results of diverse analytical methods applied to a group of samples. The multi-dimensional information of the results was transformed into a two dimensional graphic in which the similarities or differences between samples were easily visualized and correlation between variables was detected.
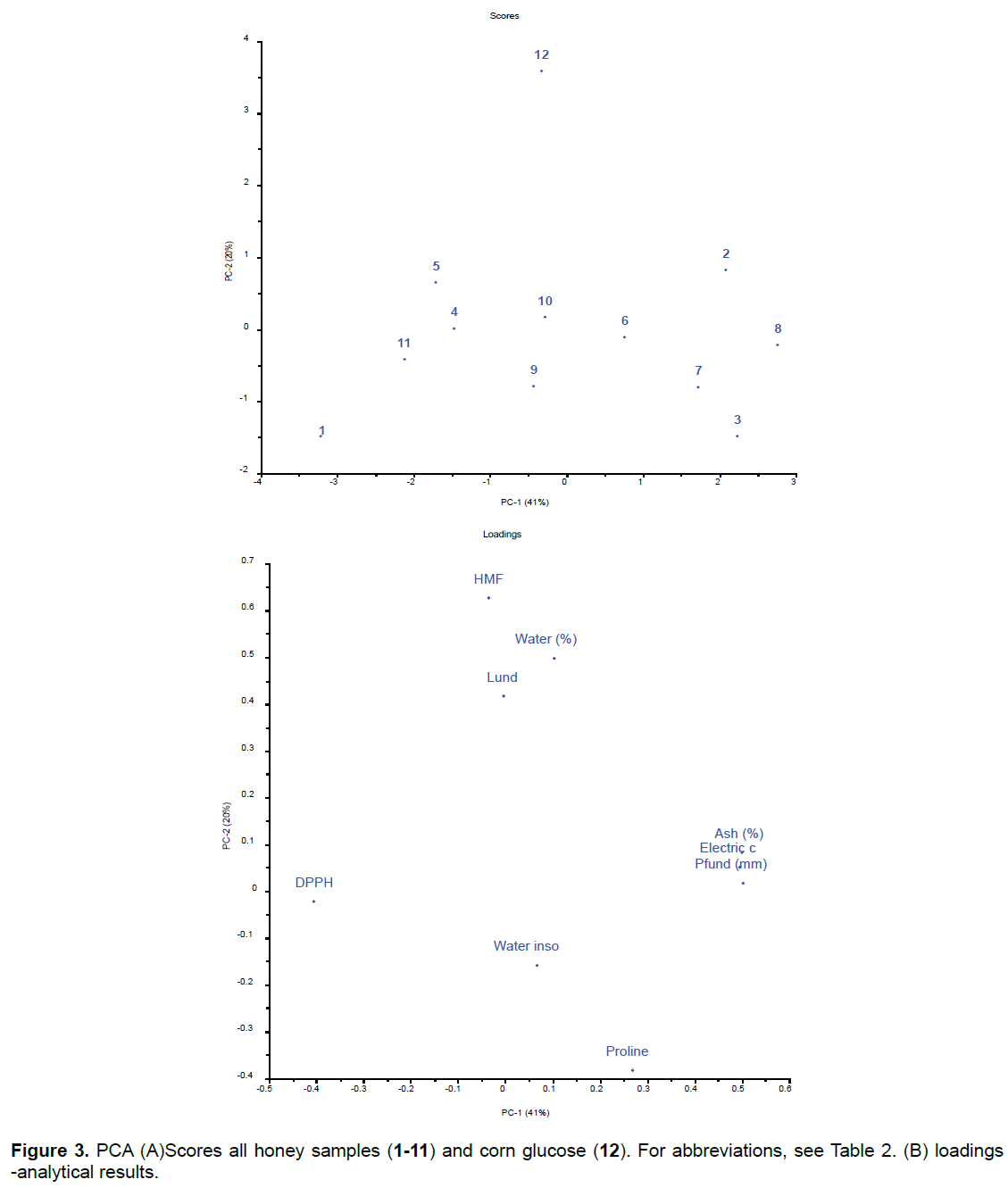
Although individual analyses did not always show differences between honey and corn glucose, when the results of all the physicochemical analyses and antioxidant activity were analyzed together using PCA, the corn glucose sample was clearly separated from the honey samples (Figure 3).
The honey samples were grouped according to the similarity in their results. One group (samples 2, 3, 6, 7 and 8) presented the highest results for ash, conductivity and colour. Samples 2 and 8 are from the same floral source (eucalyptus) and sample 3 was a cashew honey; these types of honey have a characteristically amber color [8]. Samples 6 and 7 (wild flowers and honey plus propolis, respectively) are in the same group. The presence of these two samples in this group is justified because normally the wild honeys are dark and the mixture of honey with propolis also gives to the honey a darker color. A second group (samples 4, 5, 9, 10 and 11) shows the highest results for the water insoluble and proline analysis. Sample 4 is from Amazon flowers, 5 and 10 are from orange, 9 wild flowers and 11 from cipóuva. The two samples of cipó-uva are in different groups because they are probably not exclusively originated from the nectar of this plant. For this, a pollen analysis would be necessary. Sample 5 and 10 were from orange flowers, which may be the only floral source for this honey. Samples 6 and 9 are wild honey and sample 10 was declared to be orangeflower honey. However, these floral origins consider only the main floral source, so these results indicate that this sample of orange honey may also have the contribution of nectar of other flowers. A third group (only with sample 1) presented higher DPPH values. Sample 1 is cipo-uva honey is from a native Brazilian plant which grows in the wild.
Kuchla analyzed for the first time, 31 honeys samples from different mesoregions of the state of Paraná, Brazil, evaluating physicochemical parameters and their correlations by PCA [35]. They found characteristic clusters, depending on the content of HMF, humidity, color, pH, electrical conductivity and free acidity. Yücel and Sultanoglu also found clusters of honey with similar physicochemical characteristics [36].
Sample 12, corn glucose was separated from the others not only by the high HMF content, which is formed during the excessive heating of the honey, but also by the higher percentage of moisture and higher precipitation of albuminoid substances, which probably came from maize.
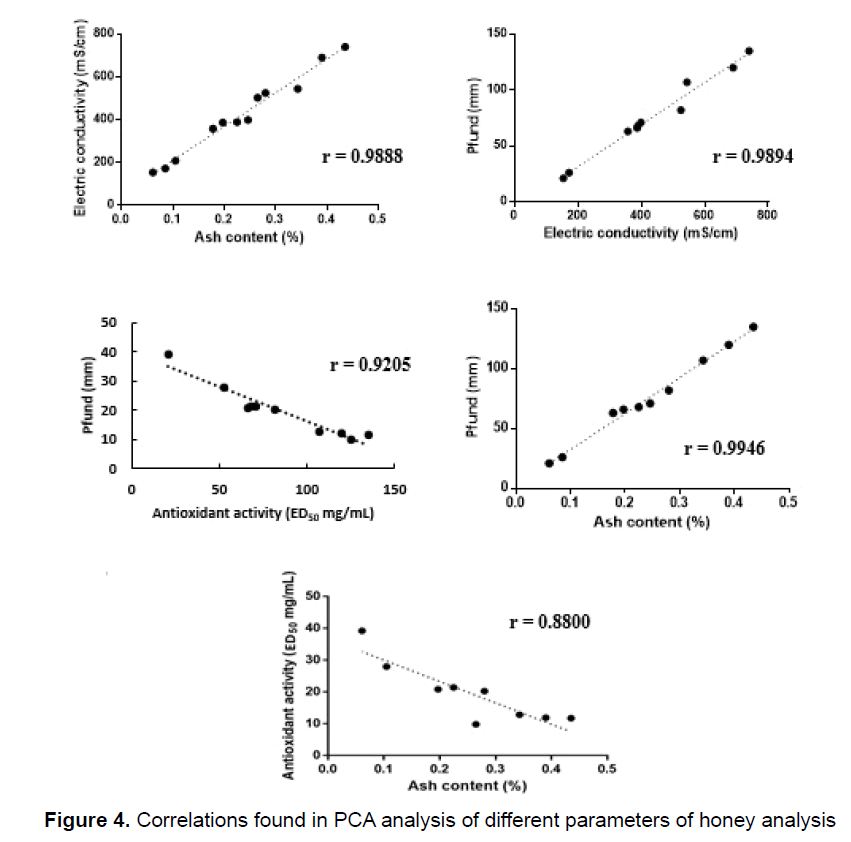
The PCA in Figure 3 indicated that some of analytical results were covariant and, therefore, the linear regression coefficient (R) of these variables was determined, confirming the positive correlation between electric conductivity versus ash, electric conductivity versus color and ash versus color. It was found an inverse correlation between ED50 versus ash and color versus ED50 (Figure 4).
There is a strong linear correlation between the results of the conductivity and ash analyses. The ash content is correlated to the electric conductivity as these ashes are in fact the oxides of inorganic compounds found in honey. These inorganic elements, in turn, are responsible for the electric conductivity in water. There is also a good correlation between the results of the conductivity and color analyses (Figure 4). Salts, mainly iron salts, usually have a brown to yellow color, which lend color to honey, which explains the correlation between these two parameters. Following this logic, the correlation between ashes and color can also be explained. The results of this study are in accordance with those of Persano Oddo and Piro [14] for color, electric conductivity and proline for unifloral honey as with those of Maia Neto et al. [8] for humidity, ash and insoluble solids. The minimum value for humidity was 12.4% and the maximum was 18.2%. Table 1 (data from the government agency, Embrapa) shows that ash contents between 0.020 and 1.028% and humidity between 13.4 and 22.9% [16] are acceptable, indicating that all samples fall within the limits established by Brazilian Law.
The correlation between color (mm Pfund) and electrical conductivity (r=0.621) was reported previously [37-39] for monofloral honeys. We found the same correlation with r=0.9894.
Good correlation was found between ash versus colour (r=0.9946). An inverse correlation between colour versus ED50 (r=0.9205) and ED50 versus ash (r=0.8800) was found.
All the honey samples were considered to be of good quality, presenting results within the legal parameters. The only analyses that were able to distinguish corn glucose from honey were Lugol, HMF and proline. For these tests, the results of the corn sucrose sample were different from all the honey samples. Therefore, we suggest that these analyses be executed whenever there is the possibility that a honey sample is really a glucose sample or that commercial glucose has been added to the honey sample. For all the other tests, the results of the corn glucose were considered within the legally acceptable parameters for honey, furnishing no indication of forgery or adulteration. Principal component analysis was also capable of separating real honey containing propolis and containing a honey comb from other samples of real honey, due to slight differences in the results of the physicochemical and antioxidant analyses. Therefore the use of several analyses plus chemometric evaluation of the data is suggested for future studies of honey quality.
4. Acknowledgments
Thanks to the beekeepers that gave us the samples, especially Novo Mel Enterprise. ACHF would like to thank FAPESP 2015/06215-4 for support.
References
- SEBRAE. https://www.sebrae.com.br/sites/PortalSebrae, 2017 (accessed in May, 2017).
- Franchin M, Freires IA, Lazarini LG, et al. (2017). The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur J Med Chem. 150: 49-55.
- Kadri SM, Zaluski R, Orsi RO. (2017). Nutritional and mineral contents of honey extracted by centrifugation and pressed processes. Food Chem. 218: 237-241.
- Miguel MG, Antunes MD, Faleiro ML. (2017). Honey as a complementary medicine. Integr Med Insights. 12: 1-15.
- Nguyen HTL, Panyoyai N, Paramita VD, et al. (2018). Physicochemical and viscoelastic properties of honey from medicinal plants. Food Chem. 241: 143-149.
- Silvano MF, Varela MS, Palacio MA, et al. (2014). Physicochemical parameters and sensory properties of honeys from Buenos Aires region. Food Chem. 152: 500-507.
- Ananias KR, De Melo AAM, De Moura CJ. (2013). Analysis of moisture content, acidity and contamination by yeast and molds in Apis mellifera L. honey from central Brazil. Braz J Microbiol. 44: 679-683.
- Maia Neto JA, de Oliveira ENA, Santos DC. (2014). Physico-chemical characterization of Apis mellifera L. honey from microregion of Pau dos Ferros, RN. Rev Bras Med Vet. 21: 268-272.
- Sohaimy SAE, Masry SDH, Shehata MG. (2015). Physicochemical characteristics of honey from different origins. Ann Agric Sci. 60: 279-287.
- Özcan MM, Ölmez C. (2014). Some qualitative properties of different monofloral honeys. Food Chem. 163: 212-218.
- Karabagias IK, Badeka A, Kontakos S, et al. (2017). Characterisation and classification of Greek pine honeys according to their geographical origin based on volatiles, physicochemical parameters and chemometric. Food Chem. 146: 548–557.
- Ranieri A, Benelli G, Castagna A, et al. (2017). Freeze-drying duration influences the amino acid and rutin content in honeybee-collected chestnut pollen. Saudi J Biol Sci. 26: 252-255.
- Persano O, Piro R. (2004). Main European unifloral honeys: descritive sheets. Apidologie. 35: 38-81.
- Persano O, Bogdanov S. (2004). Determination of honey botanical origin: problems and issues. Apidologie. 35: 2-3.
- Truzzi C, Annibaldi A, Illuminati S, et al. (2014). Dtermination of proline in honey: Comparison between official methods, optimization and validation of the analytical methodology. Food Chem.150: 477-481.
- MAPA - Ministério da Agricultura e do Abastecimento do BRASIL - Instrução Normativa n° 11, de 20 de outubro de 2000. Regulamento Técnico de identidade e qualidade do mel. https://e-legis.bvs.br/leisref/public/showAct.php?id=144&word=, 2000 (accessed in May, 2017).
- Li Y, Zhou J, Xue X, et al. (2015). Fluorometric determination of proline in honey by high-performance liquid chromatography after precolumn derivatization with7-fluoro-4-nitrobenzo-2-oxa-1,3-diazole (NBD-F). Anal Methods. 7: 7625-7630.
- Czipa N, Borbély M, Győri Z. (2012). Proline content of different honey types. Acta Aliment. 41: 26-32.
- Davies AMC. (1978). Proline in honey: an osmoregulatory hypothesis. J Apic Res.17: 227-233.
- Wen YQ, Zhang J, Yi L, et al. (2017). Characterization of chinese unifloral honeys based on proline and phenolic content as markers of botanical origin, using multivariate analysis. Molecules. 22: 1-13.
- Daniel ELD, Avellaneda ZE, Castillejos FV, et al. (2017). Effect of high hydrostatic pressure applied to a Mexican honey to increase its microbiological and functional quality. Food Bioprod Process. 102: 299–306.
- Gregorio LS, Vargas M, Chiralt A, et al. (2017); Thermal properties of honey as affected by the addition of sugar syrup. J Food Eng. 213: 69-75.
- Boussaid A, Chouaibi M, Rezig L, et al. (2014). Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab J Chem. 11: 265-274.
- Acquarone C, Buera P, Elizalde B. (2007). Pattern of pH and electrical conductivity upon honey dilution as a complementary tool for discriminating geographical origin of honeys. Food Chem.101: 695-703.
- European Economic Community - EEC Council directive of 20 December 2001 relating to honey. (2002). Official Journal of the European Communities. 110: 47-50.
- Habib HM, Al Meqbali FT, Kamal H, et al. (2014). Physicochemical and biochemical properties of honey from arid regions. Food Chem. 153: 35-43.
- Tosun M. (2013). Detection of adulteration in honey samples added various sugar syrups with 13C/12C isotope ratio analysis method. Food Chem. 138: 1629-1632.
- Salgado TB, Orsi RO, Funari SRC, et al. (2008). Análise físico-química de méis de abelhas Apis mellifera L. comercializados na região de Botucatu, São Paulo, Brasil. Cienc Rural. 2: 232-248.
- De Camargo RCR. Sistemas de produção de mel. Boletim Técnico da Embrapa, Empresa Brasileira de Agropecuária. 136pp.
- Almeida-Muradian LB, Sousa RJ, Barth OM, et al. (2014). Preliminary data on Brazilian monofloral honey from the northeast region using FT-IR-ATR spectroscopic, palynological, and color analysis. Quim Nova. 37: 716-719.
- Can Z, Yildiz O, Sahin H, et al. Na investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profile. Food Chem. 180: 133-141.
- Sawaya ACHF, Cunha IBS, Marcucci MC. (2011). Analytical methods applied to diverse types of Brazilian propolis. Chem Cent J. 5: 2-10.
- Beretta G, Granata P, Ferrero M, et al. (2005). Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal Chim Acta. 533: 185-191.
- Wilczyńska A. (2014). Effect of filtration on colour, antioxidante activity and total phenolics of honey. Food Sci Technol. 57: 767-774.
- Kuchla M, Araújo MDM, Soares AF, et al. (2015). Classification of Wild Honeys of Different Mesoregions from Paraná State, Brazil, by Principal Component Analysis. Rev Virtual Quim. 7: 2301-2313.
- Yücel Y, Sultanoglu P. (2013). Characterization of honeys from Hatay region by their physicochemical properties combined with chemometric. Food Bio Sci. 1: 16–25.
- EMBRAPA. https://sistemasdeproducao.cnptia.embrapa.br, 2003 (accessed in May, 2017).
- Mazanares AB, García ZH, Galdón BR, et al. (2017). Physicochemical characteristics and pollen spectrum of monofloral honeys from Tenerife, Spain. Food Chem. 228: 441-446.
- Silva PM, Gauche C, Gonzaga LV, et al. (2016). Honey: Chemical composition, stability and authenticity. Food Chem. 196: 309-323.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences