Purification and Characterization of Peroxidase from Broccoli (Brassica oleracea l. Var. Italica) Stems
Qurratulain Ahmad* Amna Mehmood, Zainab Saeed, Madiha Fayyaz
Institute of Molecular Biology and Biotechnology, University of Lahore, Lahore, Pakistan
- *Corresponding Author:
- Qurratulain Ahmad
Institute of Molecular Biology and Biotechnology
University of Lahore, Lahore, Pakistan
Tel: 00923356996644
E-mail: q_ain83@hotmail.com
Received date February 27, 2019; Accepted date March 25, 2019; Published date April 01, 2019
Citation: Ahmad Q, Mehmood A, Saeed Z, Fayyaz M. Purification and Characterization of Peroxidase from Broccoli (Brassica oleracea l. Var. Italica) Stems. Electronic J Biol, 15:1
Abstract
Background: Enzyme activity was increased during purification which could exert beneficial effect on plants. Methods and Findings: Enzyme purification included extraction, (NH4)2SO4 precipitation, dialysis followed by sequential chromatographies with Sephadex G-75 and Sephadex DEAE A-25. The purified enzyme was characterized with time, pH stability, metal ions, thermostability, and substrate kinetics was determined using guaiacol as substrate. The purification fold for purified broccoli stem peroxidase was 72.83 with 1.5% yield. The optimum time for enzyme activity was 6 min. and it remained stable between pH 4 to 8 having optimum pH of 6 using guaiacol as substrate. The enzyme showed maximum activity at 30°C and remained stable upto 50°C. The Km value of Broccoli Peroxidase was 0.35 m.mol/ml and 33 U/ml for guaiacol substrate by using Lineweaver-Burk graph and similarly using Michaelis Menten graph values was 0.34 m.mol/ml. Metals such as Na+, Ca2+, K+, Mg2+ and Zn2+ exhibited no effect on enzyme activity. Conclusion: These properties recommend that peroxidase could be auspicious tool for various applications in different analytical determination as well as in treatment of industrial waste.
Keywords
Peroxidase; Brassica oleracea L. var. italic; Chromatography; Dialysis; Purification.
1. Introduction
Peroxidases are large group of enzyme family called oxidoreductase that catalyze the reduction of peroxides, such as hydrogen peroxide (H2O2) and are responsible for the oxidation of various organic and inorganic compounds [1]. Peroxidase binds with other substrates such as ascorbate and ferricyanides and break them into harmless substances by donating electrons. Peroxidase catalyzes the oxidation of phenolic compounds and aromatic rings which occur naturally in plant tissues.
Peroxidases represent group of specific enzymes, such as iodine peroxidase, glutathione peroxidase and NADH peroxidase as well as a wide range of non-specific enzymes simply called as peroxidases. The molecular weight of peroxidases ranges from 30 to 150 kDa [2]. Peroxidases are associated with cell wall biosynthesis of plants. Their activity increases with plant age, with mechanical grazes and after vaccination with viruses, so they provide host resistance mechanism to plants [3].
In research areas such as biochemistry, medicine, genetics, enzymology and physiology, Peroxidases as a major enzyme group have occupied specific position in heat processing of vegetables [4]. The analysis of this enzyme have not only showed its negative effect on flavor, color and degradation of pigments of vegetables but also have positive energetic effect on vegetarian food [5].
The wide range of plant peroxidases have been purified and studied from different sources, such as Oil palm oil, Orange peel, Turnip root, Olives, Wheat, Sweet potato tuber, Green asparagus, Melon , Strawberry, Apple, Papaya fruit, Vanilla bean, Spinach, Red cabbage and cabbage leaves, Red beet, Spanish broom, Lettuce stem, Pearl millet, Sprouted green gram roots, Horseradish roots, Cauliflower, Drumstick tree leaves, Banana, Avocado, Rosemary leaves and Sweet gourd [6-32]. Multiple isoenzymes have been purified from all these sources which differ in their thermal stability, molecular mass, substrate specificity, pH optimization and their physiological role.
Peroxidases are also produced from microbial sources such as bacteria (Pseudomonas spp., Bacillus subtilis, Bacillus sphaericus, Citrobacter spp.), fungi (Chrysosporium, Candida krusei, Coprinopsis cinerea, actinomycetes, phanerochaete), Cyanobacteria (Anabaena spp.) and yeast (Pichia pastoris) [33] have various industrial applications [2]. Despite the purification of peroxidase from these sources, one of the most important source rich in peroxidase is broccoli (Brassica oleracea l. Var. Italica). Broccoli is dicotyledonous plant vegetable belong to the family Brassicaceae or Cruciferae. Peroxidase purified from the broccoli (Brassica oleracea. L. var. italica) processing waste (mostly stem) have many clinical and industrial applications [34].
2. Materials and Methods
2.1 Study design
This research work was carried out at the Lab of Institute of Molecular Biology and Biotechnology (IMBB) and the Centre for Research in Molecular Medicine (CRiMM) at The University of Lahore.
2.2 Sampling
Fresh broccoli (Brassica oleracea l. Var. Italica) was purchased from a super market in Lahore.
Sample preparation: Broccoli stems and florets were separated by cutting and only stems were washed with distilled water. Juice of broccoli stems was extracted of about 100 ml by using juicer machine and homogenized with 200 ml of 0.1 M sodiumphosphate buffer, pH 7.0, in a ratio 1:2 respectively in a beaker. The homogenized extract was centrifuged at 5000 rpm for 20 min. and only supernatant was used for further purification. Supernatant was filtered using wet Whatman filter paper no.3 for further purification. A clear purified solution of broccoli extract was stored at -20°C for further study.
2.3 Enzyme assay
The activity of peroxidase enzyme was determined by the method described by Faizyme Laboratory Manual and Zia [35].
Lowry protein assay or Hartree-Lowry assay: Method: 50 0μl (0.5 ml) of crude sample was added from the pure extract of broccoli in 5000 μl (5 ml) of alkaline copper solution. The solution was placed at room temperature for 10 minute. Then 500 μl (0.5 ml) of Folin’s reagent solution, diluted with distilled water in 1:1 ratio was rapidly added, mixed well and placed for 30 minutes at room temperature. Absorbance was noted at 600nm wavelength by using distilled water as blank on spectrophotometer [36].
3. Ammonium Sulfate Precipitation
Peroxidase (POD) enzyme was precipitated by ammonium sulfate (NH4)2SO4 salt for partial purification of enzyme. Different concentrations of 30%, 40%, 50%, 60%, 70% and finally 80% were prepared. The suspension was stirred on magnetic stirrer for two hours at room temperature. After adequate shaking, the solution was centrifuged at 6,000 rpm for 20 min and resulting supernatant and pellet were separated. Pellet was dissolved in 1 ml of 0.1 M phosphate buffer (pH 7) and enzyme assay of both the pellet and supernatant was performed using spectrophotometer following above mentioned method [37].
4. Dialysis
Maximum amount of enzyme obtained in 80% concentration of ammonium sulfate precipitation was dialyzed for desalting using dialysis bag also called as dialysis membrane. Before dialysis, the dialysis membrane was boiled in 0.1 M sodium carbonate (Na2CO3) till boiling point of solution and then retained the solution containing dialysis membrane for overnight. After that, precipitated sample was poured in dialysis bag and it was dialyzed against 200 ml, 0.1 M phosphate buffer (pH 7) on magnetic stirrer for 2 hours [38].
5. Gel Filtration Chromatography
Sephadex G 75 was used for gel filteration chromatography. After overnight soaking in water, gel was poured in the column (1 × 30 cm) and wait until it settled down. The packed column was washed with 0.1 M phosphate buffer (pH 7). After washing, 4 mL of dialyzed extract was applied on the column and fractions each of 4 ml were collected by pouring 0.1 M phosphate buffer (pH 7). Each fraction was then assayed for enzyme activity and the fraction containing maximum peroxidase activity was assayed for total protein estimation [39].
6. Ion Exchange Chromatography
After soaking, Sephadex DEAE A-25 was poured into the column (1 × 30 cm) and wait until gel get settled down. The buffer was removed and the packed column was washed first with 0.05 N HCl and then with 0.05 M NaOH solutions each of 100 ml. After washing, fractions from gel filtration chromatography containing maximum amount of peroxidase were pooled and applied in the column. Different molar concentration solution of NaCl ranging from 0.1 M to 1.0 M were applied and fractions were collected each of 5 ml. Each fraction was than assayed for enzyme activity and only the fraction containing maximum amount of peroxidase was assayed for total protein estimation [40].
7. Characterization
7.1 Effect of time
The influence of time on activity of broccoli peroxidase was determined by giving different intervals of time such as after 3, 6, 9, 12 and 15 minute. Enzyme activity was determined after adding enzyme to the substrate for these different intervals of time and then absorbance was measured.
7.2 Effect of PH
Peroxidase activity was analyzed in the range of pH 4 ̶ 9 using following buffer:
Na-acetate buffer (pH 4, 5), Na-phosphate buffer (pH 6, 7) and Tris-HCl buffer (pH 8, 9). The activity assay included H2O2, guaiacol and purified fractions of enzyme. The pH stability was investigated by measuring the activity of enzyme after buffer substrate solution had been kept overnight in various pH conditions and then the activities were tested under standard conditions.
7.3 Effect of temperature
The impact of temperature on broccoli peroxidase activity was determined by using 0.1 M phosphate buffer (pH 6). The reaction mixture of buffer substrate and enzyme extract was incubated at various temperatures such as 30, 40, 50, 60, 70 and 80°C for 6 minute in a thermostatic water bath. Enzyme activity was determined at these temperatures using spectrophotometer.
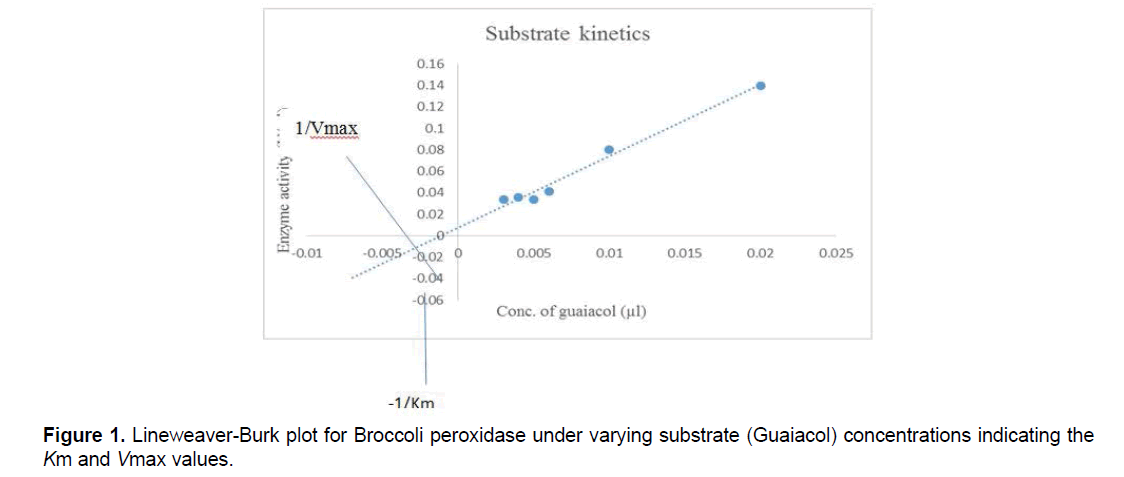
7.4 Determination of Km and Vmax:
Different concentrations of guaiacol ranging from 0.2 m.mol/ml to1.2 m.mol/ml were mixed with fixed saturated concentration of H2O2 along with 0.1M phosphate buffer and 40 μL of purified broccoli peroxidase. The solution was incubated and enzyme assay was performed. Vmax and Km values were determined for peroxidase using Lineweaver-Burk graph and Michaelis Menten graph. The kinetic data was analyzed by the LineweaverBurk plots and Michaelis Menten graph. Reaction was performed at room temperature.
7.5 Effect of metal Ions
The effect of various metal ions on the enzyme activity was verified by addition of 10 mM chloride salt of Na+ and K+ for monovalent and Mg2+, Ca2+ and Zn2+ for divalent. The activity of peroxidase was determined after 6 min incubation under standard assay conditions in the presence of metal ions.
8. Results
8.1 Crude extract
The crude sample of broccoli along with 0.1 M phosphate buffer (pH 7) was exposed to spectrophotometric exploration at 470 nm wavelength in order to obtain enzyme activity, which is given in Tables 1-3.
| Enzyme fraction | Absorbance (OD) | Activity (U/ml) | Protein content (mg/ml) | Specific activity (U/mg) |
|---|---|---|---|---|
| Crude enzyme | 2.804 | 13.1788 | 1.999 | 6.592 |
Table 1. Enzyme activity of crude sample of Broccoli peroxidase at pH 7.
8.2 Ammonium sulfate precipitation
Different concentration of ammonium sulfate salt added in crude sample of broccoli to precipitate the proteins. Addition of 80% (NH4)2SO4 salt could precipitate maximum amount of total protein in crude sample. Hence, appropriate broccoli peroxidase precipitation was found at 80% (NH4)2SO4 Maximum activity was 60.06 U/ml in 80% (NH4)2SO4 which directs the presence of enzyme (Tables 2 and 3).
| Ammonium sulfate Concentration (%) | Absorbance (OD) | Activity (U/ml) |
|---|---|---|
| 30 | 4.605 | 21.64 |
| 40 | 1.802 | 8.46 |
| 50 | 4.112 | 19.32 |
| 60 | 1.144 | 5.37 |
| 70 | 1.993 | 9.36 |
| 80 | 1.136 | 5.33 |
Table 2. Enzyme activity of supernatant after ammonium sulfate precipitation.
| Ammonium sulfate Concentration (%) | Absorbance (OD) | Activity (U/ml) |
|---|---|---|
| 30 | 0.554 | 2.6038 |
| 40 | 2.078 | 9.76 |
| 50 | 2.745 | 12.878 |
| 60 | 8.319 | 39.09 |
| 70 | 8.828 | 41.49 |
| 80 | 12.78 | 60.06 |
Table 3. Enzyme activity of pellet after ammonium sulfate precipitation.
8.3 Dialysis
80% ammonium sulfate precipitated solution was dissolved in 0.1M phosphate buffer (pH 7) dialyzed against the same buffer (Table 4).
| Enzyme fraction | Absorbance (OD) | Activity (U/ml) | Protein content (mg/ml) | Specific activity (U/mg) |
|---|---|---|---|---|
| Dialysis | 11.32 | 53.204 | 1.20 | 44.3367 |
Table 4. Dialysis of 80% ammonium sulfate precipitated solution.
8.4 Gel filtration chromatography
Dialyzed sample was subjected to sephadex G-75 for gel filtration chromatography. Out of 25 fractions 5th one showed the maximum enzyme activity of 26.818 U/ml (Table 5).
| Enzyme fraction | Absorbance (OD) |
Activity (U/ml) |
|---|---|---|
| 1 | 0.014 | 0.0658 |
| 2 | 0.006 | 0.0282 |
| 3 | 0.002 | 0.0094 |
| 4 | 1.899 | 8.925 |
| 5 | 5.706 | 26.818 |
| 6 | 4.62 | 21.714 |
| 7 | 4.596 | 21.601 |
| 8 | 2.832 | 13.31 |
| 9 | 2.64 | 12.408 |
| 10 | 2.11 | 9.917 |
| 11 | 1.73 | 8.131 |
| 12 | 0.593 | 2.787 |
| 13 | 0.583 | 2.7401 |
| 14 | 0.522 | 2.594 |
| 15 | 0.425 | 1.997 |
| 16 | 0.392 | 1.842 |
| 17 | 0.272 | 1.278 |
| 18 | 0.16 | 0.752 |
| 19 | 0.042 | 0.197 |
| 20 | 0.02 | 0.094 |
Table 5. Determination of broccoli peroxidase activity through analysis of gel filtration chromatography.
8.5 Ion exchange chromatography
Fraction of gel filtration chromatography having maximum enzyme activity was subjected to sephadex DEAE A-25 for ion exchange chromatography. Out of 20 fraction 6th (0.6M NaCl) showed maximum activity of 12.003 U/ml (Tables 6 and 7).
| Enzyme fraction | Absorbance (OD) | Activity (U/ml) |
|---|---|---|
| 1 | 0.019 | 0.0893 |
| 2 | 0.011 | 0.0517 |
| 3 | 0.725 | 3.4075 |
| 4 | 1.295 | 6.0865 |
| 5 | 1.884 | 8.8548 |
| 6 | 2.554 | 12.003 |
| 7 | 1.034 | 4.859 |
| 8 | 0.211 | 0.9917 |
| 9 | 0.026 | 0.122 |
| 10 | 0.012 | 0.564 |
Table 6. Analysis of ion exchange chromatography for activity of broccoli peroxidase.
| Sample | Enzyme activity (U/ml) | Protein content (mg/ml) | Specific activity (U/mg) | Purification fold | Percentage yield (%) |
|---|---|---|---|---|---|
| Crude sample | 13.17 | 1.998 | 6.592 | 1 | 100 |
| Ammonium sulfate Precipitation | 60.06 | 2.615 | 22.96 | 3.48 | 22.80 |
| Dialysis | 53.204 | 1.2 | 44.33 | 6.72 | 9.42 |
| Gel filtration Chromatography | 26.818 | 0.189 | 141.89 | 21.52 | 3.391 |
| Ion exchange Chromatography | 12.003 | 0.025 | 480.12 | 72.83 | 1.5 |
Table 7. Summary of broccoli (Brassica oleracea L. var. italica) peroxidase purification.
8.6 Characterization of peroxidase
Effect of time: The effect of time on broccoli peroxidase is showed in Table 8. Maximum activity was obtained after 6 minute of incubation of about 14.053 which started decline after 6 minute, after that no change in activity was noticed (Table 8).
| Serial.No. | Reaction Time (min) | Absorbance (OD) | Enzyme Activity (U/ml) |
|---|---|---|---|
| 1 | 3 | 2.153 | 10.119 |
| 2 | 6 | 2.99 | 14.053 |
| 3 | 9 | 2.86 | 13.442 |
| 4 | 12 | 2.783 | 13.0801 |
| 5 | 15 | 2.563 | 12.0461 |
Table 8. Effect of time on broccoli peroxidase activity.
Effect of pH: The purified broccoli peroxidase, assayed at different pH ranging from 4 to 9, exhibited higher activity over a broad pH range (pH 4 to 7), with maximum activity around pH 6. Broccoli peroxidase was stable in the pH range of 4.5 ̶ 7.5 (Table 9).
| Serial No. | Buffer | Change in pH | Absorbance (OD) | Enzyme Activity (U/ml) |
|---|---|---|---|---|
| 1 | Na-acetate buffer | 4 | 1.965 | 9.235 |
| 2 | Na-acetate buffer | 5 | 2.395 | 11.256 |
| 3 | Na-phosphate buffer | 6 | 2.554 | 12.003* |
| 4 | Na-phosphate buffer | 7 | 2.404 | 11.298 |
| 5 | Tris-HCl buffer |
8 | 1.526 | 7.1722 |
| 6 | Tris-HCl buffer |
9 | 0.345 | 1.6215 |
Table 9. Effect of pH stability on broccoli peroxidase activity.
Effect of temperature: Broccoli peroxidase was assayed at optimum pH in different temperatures, ranging between 30 to 80°C for 6 minute. The enzyme showed maximum activity at 30°C and remained active at 50°C (Table 10).
| Serial No. | Temperature (°C) | Absorbance (OD) | Enzyme Activity (U/ml) |
|---|---|---|---|
| 1 | 30 | 4.498 | 21.1406 |
| 2 | 40 | 1.883 | 8.8501 |
| 3 | 50 | 0.955 | 4.4885 |
| 4 | 60 | 0.134 | 0.629 |
| 5 | 70 | 0.124 | 0.582 |
| 6 | 80 | 0.098 | 0.4606 |
Table 10. Effect of temperature on broccoli peroxidase activity.
8.7 Effect of substrate
Effect of metals ions: The effect of metal ions on broccoli peroxidase activity is presented in Table 11 and Figure 1. The enzyme activity was enhanced by K+, Ca2+, Na+, Mg2+ and Zn2+ and no change in broccoli peroxidase activity was noticed with divalent cations such as Mg2+ , Zn2+and monovalent ions Na+, K+reported that peroxidase activated upto 50 mM (Table 12), while higher concentration inhibited the enzyme in a concentration dependent manner. During incubation for 6 minuteMg2+ and Zn2+ change the color of buffer substrate solution along with enzyme solution from brown to milky but activity remain same.
| Serial No. | Change in Substrate (Guaiacol) (mM) | Absorbance (OD) | Enzyme Activity (U/ml) |
|---|---|---|---|
| 1 | 0.2 | 1.466 | 6.890 |
| 2 | 0.4 | 2.481 | ÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡ÃâàÃÆââ¬Å¡Ãâà11.67 |
| 3 | 0.6 | 5.096 | 23.95 |
| 4 | 0.8 | 5.678 | 26.68 |
| 5 | 1 | 5.89 | 27.683 |
| 6 | 1.2 | 6.144 | 28.87 |
Table 11. Effect of substrate (Guaiacol) on the activity of broccoli peroxidase.
| Serial No. | Metal Ions | Absorbance (OD) |
Enzyme Activity (U/ml) |
|---|---|---|---|
| 1 | potassium chloride | 4.039 | 18.93 |
| 2 | sodium chloride | 5.305 | 24.93 |
| 3 | calcium chloride | 5.422 | 25.48 |
| 4 | zinc chloride | 4.869 | 22.884 |
| 5 | magnesium chloride | 4.443 | 20.88 |
Table 12. Effect of metal ions on broccoli enzyme activity.
9. Discussion
Results of this study revealed that broccoli is a good source of peroxidase with various isozyme forms. Peroxidase purified from the stem of broccoli has various industrial as well as pharmaceutical applications.
Peroxidase activity in the crude extract of broccoli stem using H2O2, guaiacol and 0.1 M phosphate buffer (pH 7) was 13.178 U/ml which have similarity with the work of (41) who followed the same method of extraction and at neutral pH have enzyme activity of about 23.6 U/ml. as the activity of peroxidase increases it provide defense mechanism to plants.
Peroxidase activity was increased through ammonium sulfate precipitation and at 80% precipitation, peroxidase showed maximum activity of 60.06 U/ml and after dialysis the activity was reduced to 53.20 U/ml. Cauliflower (Brassica oleracea L. var. botrytis) belongs to the broccoli (Brassica oleracea l. Var. Italica) family showed maximum activity of about 69.56 U/ml during ammonium sulfate precipitation and 59.44 U/ml after dialysis which is closed to broccoli PODs activity following unit definition which describe one unit of enzyme activity per micromole or microgram of substrate. Ammonium sulfate precipitation leads to partial purification of enzyme which is further purified through dialysis [27].
The purification fold for purified broccoli stem peroxidase after gel filtration and ion exchange chromatography was 72.83 with 1.5% yield. The protein fold was higher with that [41, 42] work who gained 30.6 fold protein with 2.6% yield after purification, while it was lower than red cabbage (Brassica oleracea var. capitata f. rubra), turnip roots (Brassica L.) belongs to broccoli family and kmatsuna (Brassica rapa) who gained 120.6 fold protein with 2.9% yield, 69.13 fold protein with 9% yield and 100 fold protein with 8% yield respectively [19, 21, 43].
Broccoli peroxidase was characterized with various parameters such as time, pH stability, thermostability, substrate specificity and effect of various metal ions. Maximum enzyme activity was obtained after 6 min of incubation which remained stable with pH range of 4 to 7 and had optimum pH 6, the enzyme purified from the turnip roots [21, 34], cauliflower [28], red cabbage [19] showed pH range from 4 to 6 with optimum pH of 5.5 to 6.
Broccoli peroxidase showed maximum activity at 30°C and activity was remained stable upto 50°C than activity started decline and at 80°C the activity was approximately nil. The work has similarity with the work of 21, 28, 19. Their purified enzyme was stable at 30°C and activity remained stable upto 60°C which was too low at 80°C.
The Km and Vmax values of bPOD were 0.35 m.mol/ml and 33 U/ml for guaiacol substrate respectively, by using Lineweaver-Burk graph and similarly using Michaelis Menten graph values were 0.34 m.mol/ml and 30 U/ml for both Km and Vmax respectively. From the previous study Km and Vmax value of red cabbage (Brassica oleracea var. capitata f. rubra) were 0.042 mM and 1.46 U/ml, respectively [19].
Broccoli peroxidase was characterized with various parameters such as time, pH stability, thermostability, substrate specificity and effect of various metal ions. Maximum enzyme activity was obtained after 6 min of incubation which remained stable with pH range of 4 to 7 and had optimum pH 6, the enzyme purified from the turnip roots [21, 34], cauliflower [28], red cabbage [19] showed pH range from 4 to 6 with optimum pH of 5.5 to 6.
Broccoli peroxidase showed maximum activity at 30°C and activity was remained stable upto 50°C than activity started decline and at 80°C the activity was approximately nil. The work has similarity with the work of 21, 28, 19. Their purified enzyme was stable at 30°C and activity remained stable upto 60°C which was too low at 80°C.
The Km and Vmax values of bPOD were 0.35 m.mol/ml and 33 U/ml for guaiacol substrate respectively, by using Lineweaver-Burk graph and similarly using Michaelis Menten graph values were 0.34 m.mol/ml and 30 U/ml for both Km and Vmax respectively. From the previous study Km and Vmax value of red cabbage (Brassica oleracea var. capitata f. rubra) were 0.042 mM and 1.46 U/ml, respectively [19].
Effect of various metal ions was observed on peroxidase such as chloride of Na+, K+, Ca2+, Mg2+ and Zn2+. Enzyme activity was not affected with these monovalent and divalent metal ions which showed high stability of peroxidase. The work correlated with the work of Abdurrahman and Mohammad v [44] who purified peroxidase from date palm leave and have same results for metal ion stability on peroxidase, however, they showed Cd+ inhibitory effect on peroxidase.
10. Conclusion
Plants have been used for purification of enzymes to meet present day industrial demand. In present study a peroxidase enzyme was purified from broccoli stems. The purified enzyme was characterized with time, pH stability, metal ions, thermostability, and substrate kinetics was also determined using guaiacol as substrate. The properties of this enzyme recommend that peroxidase could be auspicious tool for various applications in different analytical determination as well as in treatment of industrial waste.
Acknowledgment
Thanks to the beekeepers that gave us the samples, especially Novo Mel Enterprise. ACHF would like to thank FAPESP 2015/06215-4 for support.
References
- Chanwun T, Muhamad N, Chirapongsatonkul N, et al. (2013). Hevea brasiliensis cell suspension peroxidase: purification, characterization and application for dye decolorization. AMB Express 3: 14.
- Bansal N, Kanwar SS. (2013). Peroxidase (s) in environment protection. Scientific World J 2013: 714639.
- Préstamo G, Manzano P. (1993). Peroxidases of selected fruits and vegetables and the possible use of ascorbic acid as an antioxidant. HortScience 28: 48-50.
- Azevedo AM, Martins VC, Prazeres DM, et al. (2003). Horseradish peroxidase: a valuable tool in biotechnology. Biotechnol Annu Rev 9: 199-247.
- Matheis G, Whitaker JR. (1984). Modification of proteins by polyphenol oxidase and peroxidase and their products. J Food Biochem 8: 137162.
- Deepa S, Arumughan C. (2002). Purification and characterization of soluble peroxidase from oil palm (Elaeis guineensis Jacq.) leaf. Phytochemistry 61: 503511.
- Vetal MD, Rathod VK. (2015). Three phase partitioning a novel technique for purification of peroxidase from orange peels (Citrus sinenses). Food Bioproducts Pro 94: 284-289.
- Kalyn R, Ozdemir N, Ozdemir H. (2015). Purification and characterization of red beet (beta vulgaris) peroxidase. BCAMBL 1: 037-049.
- Tzika ED, Sotiroudis TG, Papadimitriou V, et al. (2009). Partial purification and characterization of peroxidase from olives (Olea europaea cv. Koroneiki). Eur Food Res Technol 228: 487-495.
- Altın S, Tohma H, Gülçin İ, et al. (2017). Purification, characterization, and inhibition sensitivity of peroxidase from wheat (Triticum aestivum ssp. vulgare). Int J Food Prop 1-11.
- Leon JC, Alpeeva I, Chubar T, et al. (2002). Purification and substrate specificity of peroxidase from sweet potato tubers. Plant Sci 163: 1011-1019.
- Guida V, Cantarella M, Chambery A, et al. (2014). Purification and characterization of novel cationic peroxidases from Asparagus acutifolius L. with biotechnological applications. Mol Biotechnol 56: 738-746.
- Rodríguez-López JN, Espín JC, del Amor F, et al. (2000). Purification and kinetic characterization of an anionic peroxidase from melon (Cucumis melo L.) cultivated under different salinity conditions. J Agric Food Chem 48: 1537-1541.
- Chisari M, Barbagallo RN, Spagna G. (2007). Characterization of polyphenol oxidase and peroxidase and influence on browning of cold stored strawberry fruit. J Agric Food Chem 55: 3469-3476.
- Zia MA, Kousar M, Ahmed I, et al. (2011). Comparative study of peroxidase purification from apple and orange seeds. Afr J Food Biotechnol 10: 6300-6303.
- Pandey VP, Singh S, Singh R, et al. (2012). Purification and characterization of peroxidase from papaya (Carica papaya) fruit. Appl Biochem Biotechnol 167: 367-376.
- Márquez O, Waliszewski KN, Oliart RM, et al. (2008). Purification and characterization of cell wall-bound peroxidase from vanilla bean. LWT-Food Scitechnol 41:1372-1379.
- Koksal E. (2011). Peroxidase from leaves of spinach (Spinacia oleracea): Partial purification and some biochemical properties. Int J Clin Pharmacol 7: 135-139
- Somtürk B, Kalın R, Özdemir N. (2014). Purification of Peroxidase from Red Cabbage (Brassica oleracea var. capitata f. rubra) by Affinity Chromatography. Appl Biochem 173: 1815-1828.
- Kharatmol PP, Pandit AB. (2012). Extraction, partial purification and characterization of acidic peroxidase from cabbage leaves (Brasicca olearacea var. capitata). J Bioch Technol 4.
- Kalin R, Atasever A, Özdemir H (2014) Single-step purification of peroxidase by 4-aminobenzohydrazide from Turkish blackradish and Turnip roots. Food Chem 150: 335-340.
- Galende PP, Cuadrado NH, Arellano JB, et al. (2015). Purification and structural stability of white Spanish broom (Cytisus multiflorus) peroxidase. Int J Biol Macromol 72: 718-723.
- Hu Y, Wu J, Luo P, et al. (2012). Purification and partial characterization of peroxidase from lettuce stems. Afr J Biotechnol 11: 2752-2756.
- Goyal P, Chugh LK. (2014). Partial purification and characterization of peroxidase from pearl millet (Pennisetum glaucum [L.] R. Br.) grains. J Food Biochem 38: 150-158.
- Basha SA, Prasada RUJ. (2017). Purification and characterization of peroxidase from sprouted green gram (Vigna radiata) roots and removal of phenol and p‐chlorophenol by immobilized peroxidase. J Sci Food Agric 97:3249-3260.
- Lavery CB, MacInnis MC, MacDonald MJ, et al. (2010). Purification of peroxidase from horseradish (Armoracia rusticana) roots. J Agri Food Chemi 58: 84718476.
- Köksal E, Gülçin I. (2008). Purification and characterization of peroxidase from cauliflower (Brassica oleracea L. var. botrytis) buds. Protein Pept Lett 15: 320326.
- Khatun S, Ashraduzzaman M, Karim MR, et al. (2012). Purification and characterization of peroxidase from Moringa oleifera L. leaves. BioResources 7: 3237-3251.
- Chang-peng Y, Ju-zhen L, Jing-jie G, et al. (2008) Partial Purification and Characteristic Study of Peroxidase from Banana (Musa spp.). J Anhui Agri Sci 36: 16148-16150.
- Rojas‐Reyes JO, Robles‐Olvera V, Carvajal‐Zarrabal O, et al. (2014). Purification and characterization of peroxidase from avocado (Persea americana Mill, cv. Hass). J Sci Food Agri 94: 1844-1853.
- Aghelan Z, Shariat SZS. (2015). Partial purification and biochemical characterization of peroxidase from rosemary (Rosmarinus officinalis L.) leaves. Adv biome Res 4.
- Koksal E, Bursal E, Aggul AG, et al. (2012). Purification and characterization of peroxidase from sweet gourd (Cucurbita Moschata Lam. Poiret). Int J Food Prop 15: 1110-1119.
- Spadiut O, Rossetti L, Dietzsch C, et al. (2012). Purification of a recombinant plant peroxidase produced in Pichia pastoris by a simple 2-step strategy. Protein Expr Purif 86: 89-97.
- Duarte-Vázquez MA, García-Almendárez B, Regalado C, et al. (2000). Purification and Partial Characterization of Three Turnip (Brassica n apus L. var. esculenta DC) Peroxidases. J Agri Food Chem 48: 1574-1579.
- Zia MA, Rehman K, Saeed MK, et al. (2001). Partial purification of peroxidase from tomato. Sci 1: 404-406.
- Lowry OH, Rosebrough NJ, Farr AL, et al. (1951). Protein measurement with the Folin phenol reagent. J Biochem 193: 265-275.
- Green AA, Hughes WL. (1955). Protein fractionation on the basis of solubility in aqueous solutions of salts and organic solvents. Methods Enzymol 1: 67-90.
- Crespo JG, Böddeker KW. (2013). Membrane processes in separation and purification. Sci Busi Media 272.
- Ó’Fágáin C, Cummins PM, O’Connor BF. (2011). Gel-filtration chromatography. Methods Mol Biol 681: 25-33.
- Jungbauer A, Hahn R. (2009). Ion-exchange chromatography. Methods Enzymol 463: 349-371.
- Thongsook T, Barrett DM. (2005). Purification and partial characterization of broccoli (Brassica oleracea Var. Italica) peroxidases. J Agric Food Chem 53: 3206-3214.
- Thongsook T, Barrett DM. (2005). Heat inactivation and reactivation of broccoli peroxidase. J Agric Food Chem 53: 3215-3222.
- Ishikawa T, Takeda T, Shigeoka S. (1996). Purification and characterization of cytosolic ascorbate peroxidase from komatsuna (Brassica rapa). Plant Sci 120: 11-18.
- Abdurrahman MA, Mohammad AI. (2011). Purification and characterization of membrane-bound peroxidase from date palm leaves (Phoenix dactylifera L.). Saudi J Biol Sci 18: 293-298.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences