Purification and Biological Activities of Novel Antibacterial Peptides from Musca domestica
Junmei Qu, Pingjie Chen, Xiaochu Qu, Wenping Li, Baoguo Tang, Tingru Huang
Academy of Animal Science and Technology,Hunan Agricultural University,Changsha 410128 ,China
Institute of Animal Science,Guangdong Academy of Agricultural Science,510640,China
Hunan Huada Feed Limited Company,Changsha 410128 ,China
Abstract
Novel antibacterial peptides were produced in induced Musca domestica larvae. Four biological activity peaks were obtained by a series of purification processes and an electrospray ionization mass spectrum. The molecular weights of these peptides are about 1KD.
Key words
Musca domestica; Antibacterial peptides; Biological activities; Electrospray ionization mass spectrum.
1. Introduction
Antibiotics have played an important role in therapy of infection diseases since 1929 when Amercian Fleming doctor discovered Pencillin. However,abuse of the antibiotics of large quantity usage increases multi-drug resistant proteins of bacteria,which results in that drug resistance bacterial strain of qualities are continuously increasing and the drug resistance mechanism is continuously perfecting. In order to antagonize the drug resistance of bacteria,studies are focusing on developing new drugs. In recent years,people find antibacterial peptides which resist the drug resistance bacterial infection disease. Antibacterial peptides are natural antibacterial drugs of organism. Hopefully,antibacterial peptides will become to a new kind of more tremendous potential drug. Today several hundreds of antibacterial peptides have been discovered from mammals,insects,amphibians,microorganisms,plants,etc. A large number of peptides produced in the host defense system had been isolated and their functions were characterized [1-4]. In spite of the diversity in size,amino acid composition and their primary structure,most of host defense peptides shared the features of being positively charged under physiological conditions and being able to adopt an amphipthaic structure upon association with lipid bilayers. It is an efficient,broad-spectrum antibacterial protein.
It was well known that Musca domestica closely contacts with pathogen,it has a very strong ability to antagonize the pathogen. Musca domestica is believed to produce the powerful antibacterial peptides. In the article,we made Musca domestica to produce biological activities of antibacterial peptides by pinprick inducing,and studied its biological activities.
2. Materials and methods
2.1 Materials and bacterial strain
The Musca domestica larvae were provided by Guangdong Health Prevention Station. Other equipments and strains are API200 LC/MS/MS (the United States ABI compay),the electronics microscope (the United States),TGL-16 centrifugal machine (China),Sephadex G-50,CM-SepharoseTM (Fast Flow) (AB company),P.multocida,Escherichia coli,Staphylocous,etc.
2.2 Methods
2.2.1 Purification of antibacterial peptides
Musca domestica larvae of three days ages were induced to produce antibacterial peptides by harm infection,antibacterial peptides of qualities reached highest peak after 24 hours.
Took weight 100 g of the larvae sample to mix into 100 ml 0.5mol/ml NH4AC-HAC buffer PH5.03 and with 0.2% Mercaptoethamol and PMSF 35ug/ml,made them to equal mix,became into pulp by triturating. Then the pulp were centrifuged at 12000rpm for 30mins,twice,collected; overlayer liquid were put into boiling water for 10 mins,mixed at the same time,quickly laid it to ice to cool,again centrifuged at 4000rpm for 30mins,collected overlay liquid are stored under -20 °C [5,6].
First,the liquid sample was subject to Gel chromatography cartridge column pre-equilibrate with 0.5mol/ml NH4AC-HAC buffer liquid PH5.03.The supernatant loaded on Sephadex G-50 gel chromatography column (90 cm × 1.4 cm). Fractions containing antibacterial peptides were pooled and subject to preparative resin. The fractions were eluted with a linear gradient of buffer,at a flow rate of 0.5ml/min,automatic part collections,10min/tube,measured density of its light at wavelength 280nms,vacuum dried,the eluate were used to detect antibacteial activity in radial diffusion assay,collected biological activities peaks; stored under -20 °C.
Then,by ion exchange chromatography,collected eluate was ran through CM- SepharoseTM (Fast Flow) (20 cm × 0.8 cm) ion exchange filtration column,used 300ml 0.5 mol/ml NH4AC-HAC buffer liquid to rinse at a flow rate of 0.2 ml/min collected biological activities parks,vacuum dried,purification was stored -20°C.
2.2.2 Measurement antibacterial activities
E.coli was cultivated overnight in tryptic soy broth (TSB: DIFCO,USA) and washed twice with autoclave 10mM sodium phosphate buffer (PH7.4) [5,7,8]. Underlay agar were made with 10ml of 1% (w/v) Type I (low electro-endosmosis) agarose (A6013: sigma) in a citrate phosphate buffer (9mM sodium phosphate,1mM sodium citrate,PH7.4) containing 0.03% TSB and the washed E.coli (4×106CFU). Four-millimeter diameter holes were punched in the set agarose and filled with 5ul of test sample. After allowing 3h for diffusion of the sample into the underlay gels a 10ml nutrient–rich overlay gel containing 6% TSB and 1% agarose was poured. After the plates had been incubated overnight at 37°C to allow surviving bacterial to grow,the diameters of the clearing zones were measured to the nearest 0.1mm and expressed in activity units (1mm=10 units). Alternatively to assess the dose-depended antibacterial activity of purified antibacterial peptides a stock solution was prepared at 200ug/ml in 0.01% acetic acid and serially diluted to 3.2ug/ml. 5ul of each dilution was then tested for antibacterial activity together with antibiotics in the radial diffusion assay as described above. Mean values of activity units obtained from experiment repeated at least three times.
2.2.3 MIC assay
The antibacterial activity of the peptides was examined in sterile 96-wellpalte (Nunc F96) microtiter plate,in final volume of 100ul as follows: Aliquots (50ul) of suspension bacteria at a concentration of 106 colony-forming units/ml LB medium were added to 50ul water or 66% pooled normal human serum in PBS,containing the peptides in 2-fold serial dilution. Growth inhibition was determined by incubation for 18-20h,at 37°C. Antibacterial activity is expressed as the minimal inhibitory of growth was observed after 81-20h of incubation. The bacteria strain were E.coli K88 and P.mulotcida [5,6,8] .
2.2.4 The molecular weight measurement assay
Purified peptides was subjected to LC-MS/MS in pre-treatment column (Water) pre-equilibrated with solvent C and chromatography column (Water) pre-equilibrated with solvent B,and eluted with solvent B [9,10]. Solvent A: 0.1% formyl acid solution,solvent B: 0.1% formyl acid in acetonitrile,solvent C: 0.1% formyl acid in water. First,the purified peptides was run through LC-MS/MS which have column of ten valves (Water 5mm×300um),then the eluted was subjected to pre–equilibrate pillar reversed-phase column (Water 150mm×75um),at a flow rate of 250nl/min,gradient:5%-60% B in 5-65min,95% B in 65-75min,A equilibrated chromatography column in 75-85min.
The eluted fraction of CaPLC was run through MS and MS/MS to be analyzed under positive ion analysis mode. Fountain head temperature: 80°C,wimble bore of voltage: 60V,and spray bore of voltage: 3000V,analysis of voltage: 2700V. By the Automated Data Dependent Acquisition (DDA) of mode,MS analysis changed to MS/MS analysis.
2.2.5 The electron microscope study of bacteria
Aliquots (50ul) of suspension bacteria at a concentration of 106 colony-forming units/ml LB medium were added to 50ul water or 66% pooled normal human serum in PBS,containing the peptides in 2-fold serial dilution [8]. Growth inhibition was determined by incubation for 18-20h,at 37°C. The suspension liquid were rinsed three times with PBS (100mM phosphate buffer/0.5M NaCl PH7.3) by centrifugation for 10min at 4000r/min,and resuspended in PBS. Precipitate were resuspended in 30ul PBS,suspension were fixed with 2.5% Glutaraldephyde,at 4°C for 4hrs. Repeated over steps,take out bacteria suspension on copperplate,under the electronic microscope observed [9].
2.2.6 Haemolytic of Animal Red Blood Cells
Haemolytic activity of the peptides were tested against animal red blood (e.g. pig,duck,goose,hen,rabbit,mice) [8,11]. Fresh RBC with EDTA were rinsed three times with PBS (35mM phosphate buffer/0.15M NaCl PH7.3) by centrifugation for 10min at 1500rpm,and resuspended in PBS. Peptides dissolved in PBS were added to 50ul of a solution of the stock RBC in PBS to reach a final volume of 100ul,final erythrocyte concentration 5% v/v. The suspension were incubated under agitation for 1-2h at 37°C,under nude eyes,we observed whether RBC were encountered haemolysis or agglutination.
3. Results
3.1 Antibacterial and haemolytic activity
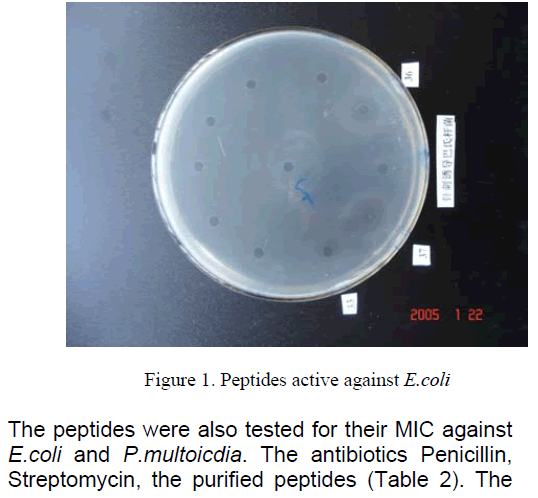
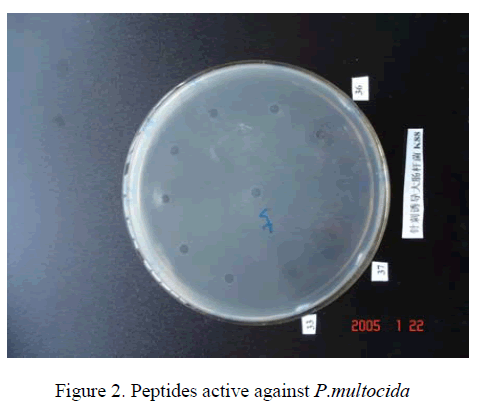
We compared the antibacterial activity of peptides,Streptomycin,Penicillin,Erythromycin,and Tetracycline. The peptides had similar equivalent antibacterial activity against E.Coli K88 and P.multocida [8]. The antibacterial activities units were respectively (Table 1). However,Streptomycin,Penicillin,Erythromycin,Tetracycline which they were active against E.Coli K88 less than peptides,and they were not active against P.multocida.
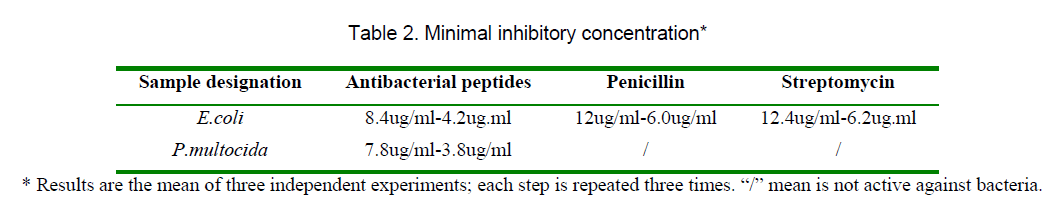
The peptides were also tested for their MIC against E.coli and P.multoicdia. The antibiotics Penicillin,Streptomycin,the purified peptides (Table 2). The data showed that the peptides antibacterial activities more sensitive than antibiotics.
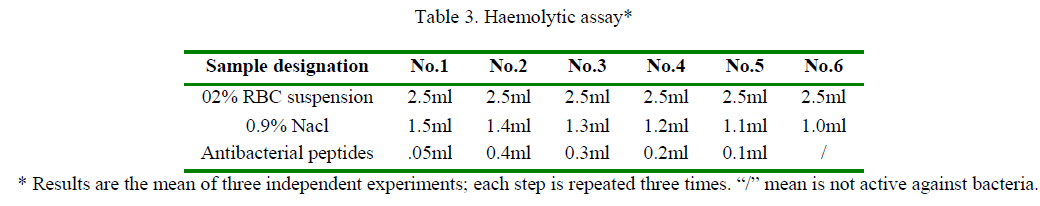
The haemolytic activities of the peptides were activated against the highly catalytically susceptible animal erythrocytes. Dose-response linearity for the haemolytic activity of the peptides is shown in (Table 3). The haemolytic activity of peptides served as test. Result showed no significant haemolytic activity up to the different concentration (Table 3). And also the RBC did not come out agglutination reaction,it is indicated that antibacterial peptides could become to an injection preparation.
3.2 Electron microscope study of bacteria analysis
The effect of the antibacterial peptides on the morphology of treat E.coli and P.multocida were visualized using transmission electron microscope. The peptides cause total lysis of the bacterial at the MIC (data not shown). However,when the peptides were utilized at concentration corresponding 80% of their MIC,some differences in the morphology of the treated bacteria were observed,depending upon the peptides used. The peptides caused the most damage to the cell wall and membranes (Figure 3 ).
Figure 3. Scanning electron microscopic of bacteria. (A) Scanning electron microscopic of Ecoli K88 treated with Musca domestica antibacterial peptides; (B) Scanning electron microscopic of P.multocid treated with Musca domestica antibacterial peptides; (C) Scanning electron microscopic of untreated Ecoli K88; (D) Scanning electron microscopic of untreated P.multocid.
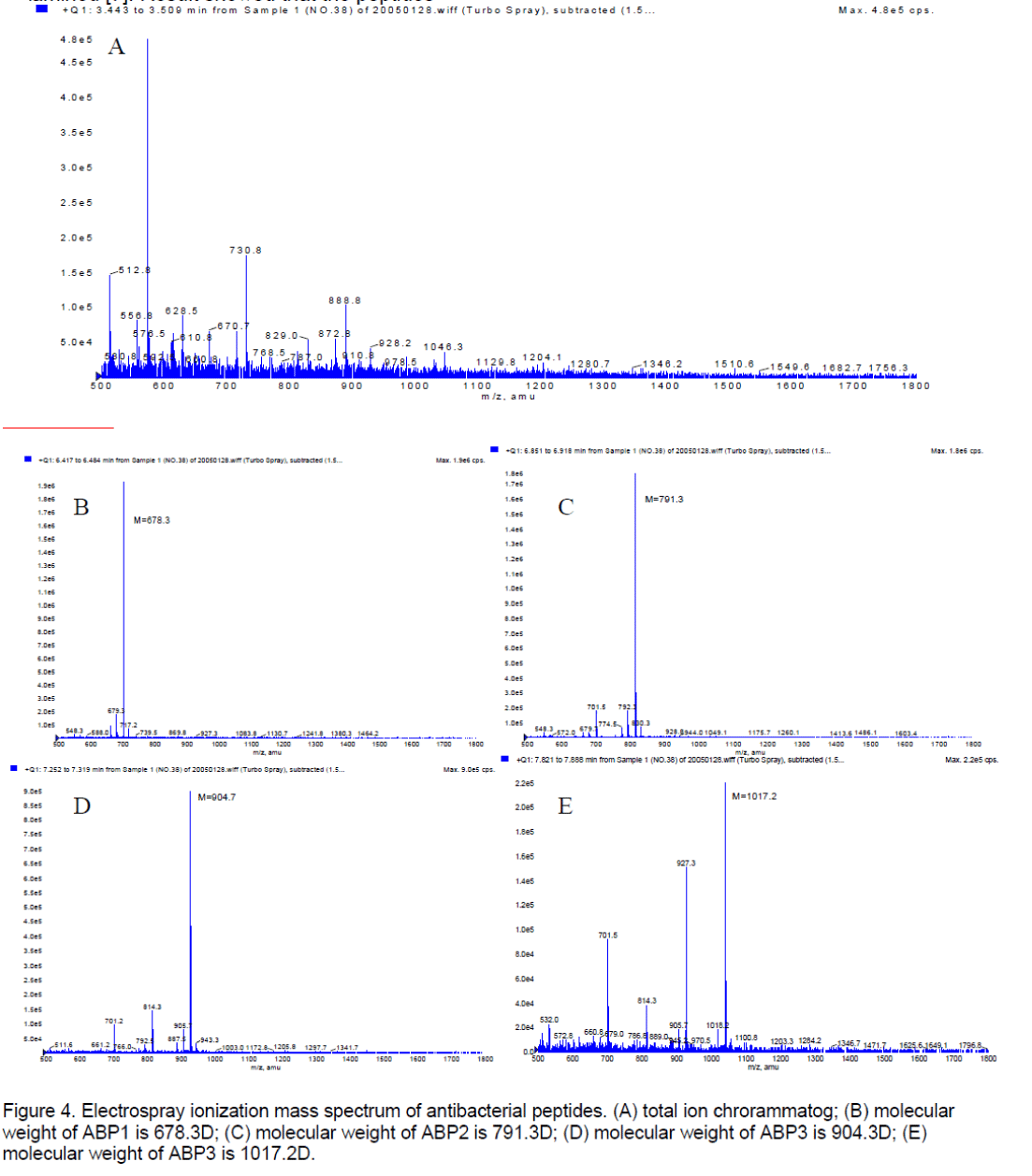
3.3 The molecular weight measurement
The purified sample was run through LC-MS/MS to be examined [7]. Result showed that the peptides contained four materials,their molecular weight were respectively (Figure 4 ). It was suspected that the molecular weight of peptides is 1017.2D.
4. Discussion
The cationic antibacterial peptides are ubiquitous multifunctional component of the innate immune systems of vertebrates and invertebrates. They are effective against a broad spectrum of microbe including Gram-positive and Gram-negative bacteria,fungus,virus,and protozoa. Recent advance in the field of innate immunity in invertebrates containing insect,have been revealed the important of antibacterial peptides in their defence system.
We have described the purified of the antibacterial peptides from the hemolymph of Musca domestica,we adopted buffer liquid extraction of hemolymph protein with 0.5mol/L NH4AC-HAC as the first step in purification become most antibacterial peptides have been shown to be cantionic and thus soluble in buffer solution. This procedure afforded substantially improved starting material for subsequent chromatography.
The fractions of lymphocytes extracts by chromatography resulted in the four groups’ fractions C1,C2,C3,C4,containing cationic antibacterial peptides,with minimal inhibitory concentrations against 7.6 ug/ml - 3.8 ug/ml and 8.4 ug/ml - 4.2 ug/ml,respectively. Since the antibacterial factors have not been purified to homogeneity the minimal inhibitory concentrations,refer to total protein concentrations and might be affected by possible synergistic activities between the different component in group C4 has apparent molecular weight similar to that of Cecropins from Musca domestica larvae,as well as an analogous gel chromatographic and ion exchange chromatographic behaviors. Groups C1,C2,C3 that do not seem to match any of the antibacterial peptides proteins and were previously identified in Musca domestica. However the hypothesis that the protein from Musca domestica lymphocyte is not antibacterial peptides is not supported,which is needed to learn. It would obviously be necessary to purify this protein to homogeneity and characterized it.
It has long been established lymphocytes the blood of invertebrates contains the number of innate defense factors that mediate rapid response to infection. In particular,the cytoplasmic granules of know to contain various antibacterial peptides. Nowadays,several hundreds of antibacterial peptides have been found in blood cell or serum of insect. These are insect defense derived from N-terminus of Stomoxy callcitrans,α-helical peptides of C-terminus isolated from Apis mellifera and Bombus pascuorum. To the best of our knowledge the investigation reported in the current paper provides the first evidence the lymphocyte of Musca domestica protein were derived from contain leucocytes as previous extensive research conducted in our antibacterial peptides can be found in lymphocyte from immunological naïve insect.
We obtained that the peptides have been shown to exhibit a broad spectrum of activity bacteria without haemolytic activity. In the study,the peptides are active against E.coli K88 and P.multocid. The minimal inhibitory concentrations (MIC) of peptides are 3.8ug/ml and 4.2ug/ml. Even though Okada et al. [12] showed that Sarcotoxin I was active against E.coli of resistant Streptomycin,its MIC is 3ug/ml,and Hara et al. [13] showed that Moricin was active against E.coli JM109,its MIC was 0.9Um. Cudic et al. [14] showed that antibacterial peptides of diptericin were active against E.coli D22 at a concentration of 4uM. Asthana et al. [15] showed that antibacterial peptides of Melittins was active against E.coli DH5a,B. sbutilis and S.aureus,respectively; theirs MICs were 3.9±0.6 ug/ml,2.0±0.2 ug/ml and 3.6±0.5 ug/ml. Navon-Venezia et al. [16] showed that the susceptibility of bacterial to dernaseptin S4 derivatives (e.g. K4K20-S4,K4-S4(1-16),K4-S4(1-13)) by measuring the peptides MICs against 66 clinical isolated including the multiding-resistant gram-negative bacterial P.aeruginosa and E.coli as well as the gram-positive bacterium S.areus. The results of K4K20-S4 were respective 2ug/ml,2ug/ml,8ug/ml; Results of K4-S4(1-16) were all 4ug/ml; However,Results of K4-S4(1-13) were all 8ug/ml. We obtained peptides of MIC was similar to K4K20-S4,K4-S4(1-16),K4-S4(1-13) and Melittins of MIC,however,higher than Sarcotoxin’s and Moricin’s MIC. The result was affected by many factors including bacteria strain,experiment conditions and antibacterial peptides of themselves,etc. The antibacterial peptides of activities were more than antibiotics. Because antibacterial peptides have several obvious advantages over know antibiotics: (1) The antibacterial material is a native material in organism,it doesn’t harm to the creature. (2) Inhibition of bacterial growth induced by diastereomers is associated with total lysis of bacterial wall,as shown of bacterial microscopy. Therefore,bacteria might not easily to develop resistance to drugs that trigger such a destructive mechanism. (3) Antibacterial peptides has the ability to perturb the cell wall of bacteria a concentration lower than their MIC,as seen by electron microscopy. The simultaneous administration of clinically used antibiotics. which have no activity due to their inability to penetrate cell wall,together with antibacterial peptides may present a solution to this resistance mechanism of bacteria.
We obtained Antibacterial peptides have no haemolytic activity. Even though Y.H.Sung. et al. [17] showed that haemolytic activity of KSL,KSL2,and KSL7 were measured. All tested peptides did not show hemolytic activity up to 500ug/ml,whereas cytotoxic peptides,melittin,caused the lysis of whole erythrocytes below 10ug/ml. This result suggested that the addition of lysis residues might not affect haemolytic activity. However below 500ug/ml,it was not sure that the addition of lysis residues did not affect haemolytic activity. Antibacterial peptides have no haemolytic activity due to bind and permeate negatively charged but not zwitterionic phospholipid vesicles are characreristic of native antibacterial peptides [18-20].
To understand the molecular no haemolytic activity and retention of antibacterial activity of the antibacterial peptides of Musca domestica analogs and to evaluate the structural and functional changes associated with the mutation in this motif,membrane permeability,secondary structure,localization onto the membrane with different lipid composition,and self-association in an aqueous environment were studied with peptides,which has been attributed to the fact that the bacterial surface contains lipopolysaccharides (in Gram-negative bacteria) and polysaccharides (teichoic acids ,in Gram-positive bacteria),and their inner membranes contain PG,all of which are negatively charge ,whereas normal eukaryotic cell such as erythrocytes predominantly express the zwitterionic phospholipid PC on their outer leaflet.
In conclusion,the results demonstrated a mechanistic dissection of the haemolytic and antibacterial activities of peptides. The results probably indicate the presence of different sequence elements,which control the haemolytic and antibacterial activity of peptides. The ability of peptides to efficiently permeate the negatively charged but nor zwitterionic membranes makes it a poor cell-selective molecule and renders it active against both prokaryotic (e.g. antibacterial activity) and eukaryotic cells (e.g. haemolytic activity). The results suggested that the peptides disturbed the helical assembly in an aqueous environment,the secondary structure,membrane permeability,and localization in zwitterionic membrane,which probably caused no haemolytic activity of peptides. However,alanine substitution did not alter much the amphipathic character of peptides,showed much less effect on its membrane permeability,secondary structure,and localization in negatively charged membrane and presumably therefore did not affect its antibacterial activity.
Antimicrobial peptides are potential lead molecules for designing novel drugs. However,one of the major issues is their selective lytic activity. The results presented here probably suggest that cell selectivity in some antimicrobial peptides can be introduced by modulating their secondary structures and/or assembly in an aqueous environment.
Acknowledgement
This work was supported by Guangdong Province Natural Science the Foundation (grant number 032019).The authors would like to thank Prof. Jianxin Chen from Huanan Africulture University for critical help in the project. The authors would also thank Prof. Zhenling Zeng and Prof. Fuan Liu for their helps in preparing this review.
References
- Maloy W.L.,Kari U.P. (1995) Structure-activity on magainins and other host defense peptides. Biopholymers,37: 105-122.
- Epand R.M.,Vogel H.J. (1999) Diversity of antimicrobial peptides and their mechanisms of action. Biophys Acta,1462: 11-28.
- Shai Y. (1999) Mechanism of the binding insection and destabilization of phospholipid bialyer membrans by alpha-helical antimicrobial and cell non-selectivemembrans-lytic peptides. Biochim Biophys Acta,1462: 55-70.
- Tossi A.,Sandri L.,Giangaspero A. (2000) Amphipathicalpha-helicalantimicrobial-peptides. Biopolymers,55: 4-30.
- An C.J.,Shi M.,Hao Y.J.,et al. (2003) Inducedment and activity analysis of antibacterial-related protein in housefly larvae. Acta Entomolgica Sinica,46(5): 545- 548.
- Bai M.,Zhou L. (2001) Purification of a kind of antimicrobial protein from Musca domestica larvae. Chin. J. Appl. Environ. Biol.,7(6): 568-571.
- Wade D.,Boman A.,Wahlin B.,et al. (1990) All-D Amino Acid-Containing Channel-Forming Antibiotic Peptides. Proc. Natl. Acad. Sci. USA,87: 4761-4765 .
- Oren Z.,Jiang H.,Ye C.S. (1997) A Repertoire of Novel Antibacterial peptides Diastereoeric Peptides with Selective Cytolutic Activity. JBC. 23: 14643-14649.
- Cao R.,Zhang L.J.,Nie S.,et al. (2005) Analysis of Mose Live Plasma Membrane Protein by Multidimensional Liquid Chromatography-Tandem Mass Spectrometry. Chinese Journal of Biochemistry and Molecular Biology,21: 134-142.
- Gong X.,Shi Y.H.,Le G.W. (2004) Purification of antimicrobial peptides MDL-1 from Musca domestcia larva and its effect on Esherichia coli ultrastructure. Acta Entomolgica,47(1): 8-13.
- Cheron M.,Cybulsk B.,Mazerski J.,et al. (1998) Quantitative structure-activity relationship in amphotericin B derivatives. Biochemical Pharmacology,37: 827-836.
- Okada M.,Natori S. (1985) Primary structure of sarcotoxin I,an antibacterial protein induced in the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J Biol Chem.,260(12): 7174-7177.
- Hara S.,Yamakawa M. (1995) Moricin,a novel type of antibacterial peptide isolated from the silkworm,Bombyx mori. J Biol Chem.,270(50): 29923-29927.
- Cudic M.,Bulet P.,Hoffmann R.,et al. (1999) Chemical synthesis,antibacterial activity and conformation of diptericin,an 82-mer peptide originally isolated from insects. Eur J Biochem.,266(2): 549-558.
- Asthana N.,Yadav S.P.,Ghosh J.K. (2004) Dissection of antibacterial and toxic activity of melittin: a leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J Biol Chem.,279(53): 55042-55050.
- Navon-Venezia S.,Feder R.,Gaidukov L.,et al. (2002) Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob Agents Chemother,46(3): 689-694.
- Sung Y.H.,Shin J.,Shin J.,et al. (2001) Solution structure of p21(Waf1/Cip1/Sdi1) C-terminal domain bound to Cdk4. J Biomol Struct Dyn.,19(3): 419-427.
- Gazit E.,Lee W.J.,Brey P.T.,et al. (1994) Mode of action of the antibacterial cecropin B2: a spectrofluorometric study. Biochemistry,33: 10681-10692.
- Pouny Y.,Rapaport D.,Mor A.,et al. (1992) Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry,31: 12416-12423.
- Strahilevitz J.,Mor A.,Nicolas P.,et al. (1994) Spectrum of antimicrobial activity and assembly of dermaseptin-b and its precursor form in phospholipid membranes Y. Biochemistry,33: 10951-10960.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences