Nutritional Evaluation of Fermented Poultry Feather Meal in the Formulated Diets of Fingerlings of Catla catla (Hamilton)
Kausik Mondal
Kausik Mondal*
Department of Zoology,Sidho-Kanho-Birsha University,Purulia-723104,India
- Corresponding Author:
- Kausik Mondal
Department of Zoology
Sidho-Kanho-Birsha University,Purulia-723104,India
Tel: +919434510521
E-mail: kausik.mondal2007@gmail.com
Abstract
Attempts were made to recycle poultry feathers in the formulation of diet for fish. Hydrolyzed poultry feather meal (PFM) was fermented with the help of a microbial suspension of keratinolytic bacteria. The completion of fermentation was determined by pH, total organic carbon and total nitrogen content of the fermented product. Four experimental diets were prepared with fermented PFM replacing 25, 50, 75 and 100% of the fishmeal (FM). The reference diet was prepared with fishmeal as the major source of protein. All the diets were isonitrogenous with 30% protein. A 90 days trial was conducted with fingerlings of Indian major carp Catla catla under laboratory conditions. The results reveled that all the experimental diets were as effective as the reference diets. Deposition of crude protein in the whole body was also significantly higher in the diet with 30% PFM and 10% FM, as compared to the other experimental diets. It was concluded from the study that the diet with 30 % PFM and 10 % FM, replacing 75% of FM if hydrolyzed PFM was processed appropriately through fermentation.
Keywords
Organic wastes; Carp; Fermentation; Recycling.
1. Introduction
Fishmeal is one of the primary proteins used in fish diet. There has been a considerable research effort towards utilization of less expensive renewable ingredients in fish diet formulations to alleviate problems related to shortage of quality fishmeal [1]. In order to sustain aquafeed industry,a great part of nutritional research has been focused on the search for alternative proteins. One of the most promising alternatives is poultry by-product meal (PBM). Huge quantities of different types of poultry wastes are generated daily in the retail markets mainly from the dressing of poultry birds [2]. Careless disposal of these wastes results in pollution of environment and loss of enormous quantities of nutrients contained in the wastes. Poultry-feather meal has been included up to 10% level in the concentrates for dairy cattle [3]. PBM consists of ground rendered clean parts of the carcass of slaughtered poultry such as necks,feet,undeveloped eggs and intestines,exclusive of feathers,except in the amounts as might occur unavoidably in good processing practices [4]. By-products of the poultry processing industry are high in protein and contain favorable profiles of indispensable amino acids (IAA) for fish production [5]. Poultry feathers are one of the commonly available by-products which is rich in protein. It has also been used as protein source in the diets for rainbow trout (Oncorhynchus mykiss) [6],coho salmon (0. kisutch) [7],chinook salmon (0. tshawytscha) [8] and gilthead sea bream Sparus aurata [9].
Hydrolyzed PFM has been used previously as protein resource in fish diet formulations [2],but hydrolyzed PFM results in considerable loss of the nutrients. In the present study an attempt was made to evaluate if PFM could be fermented and used to replace fishmeal (FM) in the preparation of formulated fish diet. The process of fermentation improves nutritional quality of animal product also. Mondal et al. [10] observed that fermnted fish offal was nutritionally superior to untreated fish offal meal or fishmeal. Collection of these wastes from the urban and suburban markets,treating them suitably to preserve their nutrients and finally recycling them into fish diet production can have a significant benefit in terms of abatement of pollution of environment as well as reduction in the recurring cost of fish production.
The objective of this study was to determine the extent to which PFM could replace fishmeal in carp (Catla catla) diet while maintaining nutritional quality almost identical to the diet based on fishmeal and thus be an ideal cost effective renewable alternative.
2. Materials and Methods
2.1 Hydrolysis,Fermentation and Diet Preparation
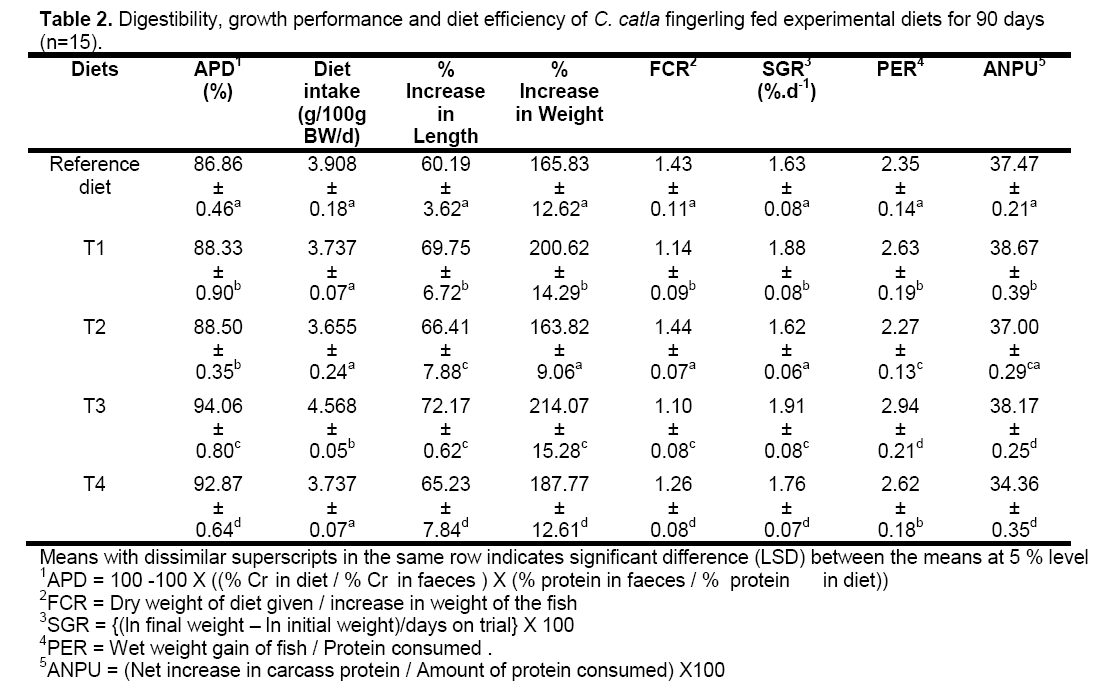
Poultry feathers were collected from retail markets. The feathers were hydrolyzed by cooking under pressure at 130°C for 2.5 h. After cooking,the material was dried at about 60°C in a thermostatic oven and ground to pass through a 200 pm screen [2]. Then Poultry feathers were converted into poultry feather meal. For solid state fermentation,the Poultry feather meal (PFM),mustard oil cake and rice bran were mixed at proportion mentioned in Table 1. The mixture was added to a solution of microbial suspension (109 cell mL–1) (keratinolytic bacteria: Bacillus cereus [11] and was fermented anaerobically in an anaerobic fermentation chamber under ambient temperature (27–30°C) for 4 to 6 days,depending on the proportion of PFM. Degradation of poultry feathers sample was determined following the method of Nagal and Jain [11]. Based on a preliminary evaluation,40% PFM was determined as the maximum level for fermentation by the microbial suspension. Samples of fermentation mixture were taken from each level of trial fermentation and were analyzed for aroma,physical condition,total nitrogen and organic carbon percentage every 2 days till the end of fermentation [12]. The fermented mixture was mixed with fishmeal (FM),mineral and vitamin mixture to prepare four experimental diets: 1) T1 diet containing 10% PFM fermented mixture and 30% fishmeal (FM),2) T2 diet containing 20% PFM fermented mixture and 20% FM,3) T3 diet containing 30% PFM fermented mixture and 10% FM and 4) T4 diet containing only 40% PFM fermented mixture. The reference diet was prepared with 40% fishmeal (FM) as the major source of protein,without any fermentation mixture (Table 1). The mixtures were ground and pelleted using a hand pelletizer fitted with a 2-mm dia to prepare the final experimental diets (Table 1). To test the protein digestibility of the diet,1% chromic oxide (Cr2O3) was included in each diet separately as non-absorbed reference substance,blended,and pelleted with 0.5% carboxymethyl cellulose as a binder. This was sun dried before using in the feeding trial.
2.2 Experimental Design
The diets were formulated in such a way that these contained not less than 30% crude protein. The experiment was conducted in flow-through circular fibre-glass tanks. Fingerlings of Catla catla (mean initial length 6.48 ± 0.12 cm and mean initial weight 3.98 ± 0.15 g) were obtained from a local fish farm and were acclimatized to the laboratory conditions for a week prior to start of the experiment. During acclimatization the fish were fed,ad libitum,with a mixture of rice bran and mustard oil cake. The acclimatized fingerlings were randomly distributed at the rate of 20 fish per tank for the digestibility and growth trial. Deep tube-well (400 feet) water stored in an overhead tank was used as test medium for all the tests. The tanks were laid out in a completely randomized block design [13] so that there were three replicates for each of the four experimental diets. The fish were fed a ration at 5% of their body weight per day for the entire experimental period of 90 days. The quantity of the diet given was readjusted every 15 days after weighing the fish.
The ration was provided at 08:00 hours and the fish were allowed to eat for 6 h. Left over diets were collected by siphoning after 6 hours of feeding,oven-dried,weighed and stored at –20°C. The leaching rate of nutrients was estimated by placing weighed diets in tank without fish for 6 h and then recollecting,drying and re-weighing the diets. The average leaching rate was used to calibrate the amount of uneaten diets. Faecal samples were collected from each tank continuously at 3 to 4 h interval for a period of 17 h after the removal of uneaten diets by careful siphoning method and following the ‘‘immediate pipetting’’ method outlined by Spyridakis et al.[14],from three replicates of each dietary treatment. To minimize nutrient leaching,only fresh and intact faeces were collected and dried to a constant weight at 60°C in an oven and weighed before preserving at –20°C. Apparent protein digestibility (APD) of the diet was calculated from the proportion of Cr and protein in the diet and faeces following the methods described by Ellestad et al. [15].
2.3 Chemical Analyses and Data Collection
Proximate analysis of diet ingredients,experimental diets and faecal samples was performed following the AOAC [16] procedures as follows: moisture was determined by oven drying at 1050C for 24 h; crude protein (Nitrogen X 6.25) was determined by micro Kjeldahl digestion ; lipid was determined by extracting the residue with 40-600C petroleum ether for 7-8 h in a Soxhlet apparatus,crude fibre was determined as loss on ignition of dried lipid-free residues after digestion with 1.25% H2SO4 and 1.25% NaOH and ash was determined by ignition at 5500C in a Muffle furnace to a constant weight. Nitrogen-free extract (NFE) was calculated by taking the sum of values for crude protein,crude lipid,moisture and ash and subtracting this from 100 [17]. Cr in the diet and faecal samples were determined by acid digestion and analyses in flame atomic absorption spectrophotometer (Varian Spectra AA240) following the methods described by Saha and Gilbreath [18].
2.4 Growth Trial
All fish from each tank were sampled at the end of 90 day trial; length and weight of the fish were recorded and five sampled fish from each tank were subjected to biochemical analyses to determine moisture,crude protein,lipid and ash content of the fish. Percent increase in length and weight,specific growth rate (SGR),feed conversion ratio (FCR),protein efficiency ratio (PER) and apparent net protein utilization (ANPU %) were calculated using standard methods [19]. Water quality parameters were determined by the procedures of APHA [20].
2.5 Statistical Analyses
The nature of distribution of the observations of each response variable from both the trials was verified by Kolmogorov-Smirnov (K-S) and Shapiro-Wilks (S-W) tests to ensure a Gaussian distribution. Since all data were found normally distributed they were subjected to Single factor ANOVA,without any further transformation,followed by least significant difference (LSD) test to compare mean between the treatments [13,21].
3. Results
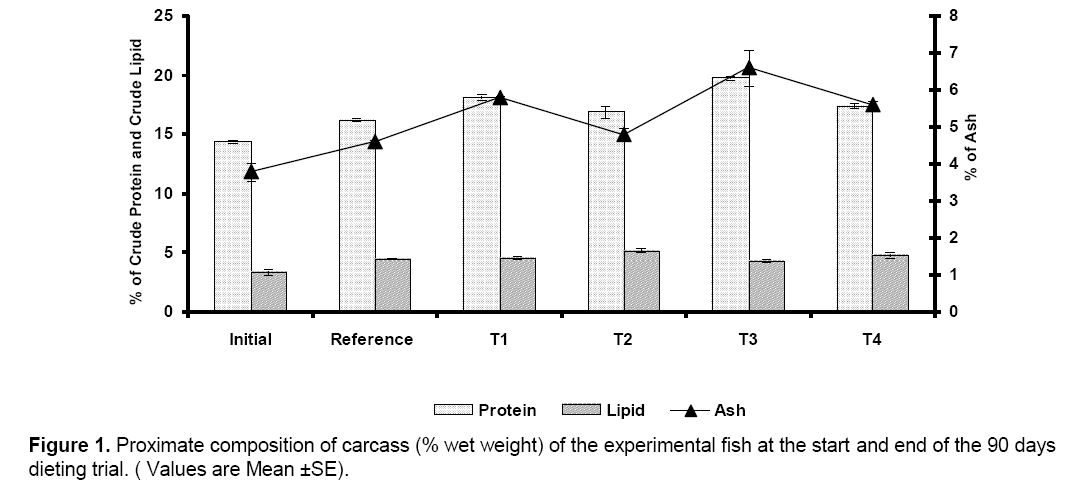
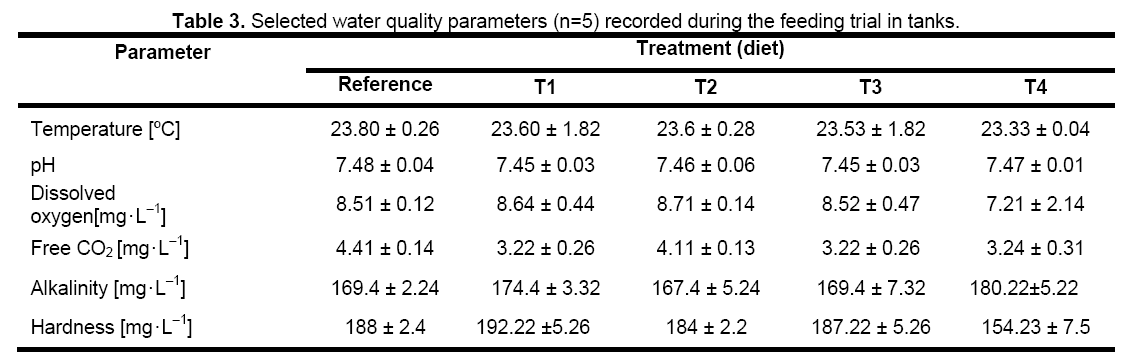
Completion of fermentation of hydrolyzed PFM mixture was indicated by the generation of a characteristic pleasant sweet aroma,which appeared on the 6th day. This was further confirmed from pH,total nitrogen,and organic carbon content; gradually change of the percentage of total nitrogen and organic carbon occured till the end of fermentation,pH of the fermented mixture gradually decreased from an initial value of 8.7–8.0 to 4.2–4.4. The growth response and performance data of carp fingerlings fed diets containing various inclusions of fermented mixture of PFM are presented in Table 2. The survival rate of the fingerlings during growth trial in the tanks ranged from 90% to 95% and showed no significant variation between the dietary treatments. There was no difference in the initial weights and lengths of fish stocked but the growth performances differed significantly (P<0.05) in terms of weight gain and specific growth rate (SGR). Growth responses of carp fingerlings fed the reference diet and T2 diets (20% fermented PFM mixture and 20% FM) were not significantly different but were different from fingerlings fed T1 (10% fermented PFM mixture and 30% FM),T3 diet (30% fermented PFM mixture and 10% FM) and T4 diet (40% fermented PFM mixture) (Table 2). Fingerlings reared in tanks with T3 diet showed significantly higher growth (increase in length,weight,and specific growth rate) than the T1,T2,T4 and reference diet (Table 2). Apparent protein digestibility (APD),Voluntary diet intake rate,food conversion ratio (FCR),Protein Efficiency Ratio (PER),and Average Net Protein Utilization (ANPU) also showed similar trends i.e.,T3 was significantly higher than the T1,T2,T4 and reference diet (Table 2). Proximate composition of the carcass at the end of the trial also showed significantly higher value of crude protein and lipid in T3 as compared to T1,T2,T4 and reference diet (Figure 1). Temperature,pH,dissolved oxygen,free carbon dioxide,total alkalinity and hardness of water recorded during the trial showed values in optimum range required for rearing carp fingerlings (Table 3).
4. Discussion
The present study indicates that diets supplemented by fermented PFM are accepted well by fingerlings of Catla catla. The diet intake rate of the fingerlings of C. catla observed in the present investigation (3.655 to 4.568 g per 100 g BW per day) is higher than the rate observed by Mondal et al. [22]. Poultry feather meal previously was considered to be an inferior source of protein for animal because of its poor digestibility and biased essential amino acid profile [23]. Feathers were highly insoluble because it contains keratin,which contains a high percentage of the amino acid cystine [2]. Autoclaving reduces the cystine from about 10 to 3.5%,thereby making the feathers more soluble and digestible [3]. The quality of the product mainly depends on the efficiency of the hydrolysis and fermentation. In the present investigation protein digestibility varied from 88.33 to 94.06 % and the highest digestibility found in T3 (30% fermented PFM and 10% FM) diet. Using practical diets,Hasan et al. [2] observed 83.15% protein digestibility of the reference diet for the fingerlings of Labeo rohita. Maximum value of protein digestibility (85.20%) was obtained by Hasan et al. [2] when fishmeal was replaced (25%) by hydrolyzed feather meal. However,after hydrolysis and fermentation of feathers it becomes highly digestible. High protein digestibility is often correlated with high indispensable amino acid availability [24]. The present study shows that hydrolyzed poultry feather meal after fermentation can be used at 30% of dietary level (i.e.,75% of fishmeal replacement) for C. catla fingerlings without compromising growth and diet utilization.
The results of the present study reveal that growth of fingerlings of C. catla grew better on diets supplemented by fermented PFM (T3) as compared with the reference diet and other experimental diets. This diet (T3) contained the highest level of crude lipid (10.80 %) and low level of crude fibre (2.80%). This could be explained as protein sparing effect of the higher level of lipid in the diet containing fermented PFM. Diets containing fermented fish offal meal exhibited protein sparing effects in the diet of L. rohita [10]. Lipid as a non-protein energy source allows protein sparing by effectively reducing organic matter and nitrogen losses. Protein sparing effects of dietary lipids have been demonstrated for common carp [25],and grass carp [26]. Lipid as a non-protein energy source allows protein sparing by effectively reducing organic matter and nitrogen losses. But high level of dietary lipid may lead to deposition of fat in the body of fish and depression of activities of lipogenic enzymes [27,28]. Fish fingerlings are equipped with lipase enzyme necessary for digesting lipid which are the most important sources of energy and essential fatty acids for the fish for stress resistance,securing high membrane fluidity for rapid cellular divisions and growth [29,30]. The capacity of fish fingerlings to digest dietary lipid is therefore of great importance for optimal nutrition in development [31,32].
Fermentation by bacterial mass is a most effective way of recovering nutrients from organic wastes. Bertsch and Coello [33] used a strain of keratinolytic bacteria to ferment poultry feather and obtained poultry feather meal with 71 % crude protein and improved bioavailability of some amino acids. The present results indicated that considerable proportion of crude protein and lipid could be recovered from PFM when it was fermented with suitable microbial solution. Bioavailability of the essential amino acids to fish is not uniform and there is marked difference in metabolism of a particular amino acid between species of fish [34,35]. From the better growth of C. catla fed diets containing fermented PFM it is assumed that such mixture renders higher amount of bioavailable amino acids in the formulated diet.
The outcome of the study is that fermented PFM mixture can be effectively used as a diet ingredient to replace fishmeal partially in the diet for fingerlings of C. catla. Hundred percent replacement of fishmeal is possible when 40% PFM is fermented along with MOC (30 %) and RB (28%) in diet T4 but higher level of dietary crude fibre (4.80%) may lead to reduction of the growth of fish than other experimental diets. Therefore,seventy five percent replacement of fishmeal is possible when 30% PFM is fermented along with MOC (30 %) and RB (28%) and is used as ingredient in the formulated diet. It is concluded from the present study that fermentation is a viable option to recycle poultry feather wastes through fish diet formulation. It served dual purpose of controlling organic pollution and serving as an alternative source to fishmeal in fish diet formulations.
Acknowledgements
The author is thankful to the Head,Department of Zoology,Sidho-Kanho-Birsha University for providing necessary facilities for this research.
References
- Yang Y.,Xie S.,Cui Y.,Lei W.,Zhu X.,Yang Y. and Yu Y. (2004) Effect of replacement of dietary fish meal by meat and bone meal and poultry by-product meal on growth and feed utilization of gibel carp,Carassius auratus gibelio. Aquac. Nutr.,10: 289-294.
- Hasan M.R.,Haq M.S.,Das P.M. and Mowlah G. (1997) Evaluation of poultry feather meal as a dietary protein source for Indian major carp Labeo rohita fry. Aquaculture,151: 47-54.
- Gohl B. (1981) Tropical Feeds. Feed Information Summaries and Nutritive Values. FAO Animal Production and Health Series 12. p. 528 FAO,Rome.
- Cruz-Su´arez L. E.,Nieto-L´opez,M.,Guajardo- Barbosa C.,Tapia-Salazar M.,Scholz U. and Ricque-Marie D. (2007) Replacement of Fish Meal with Poultry By-Product Meal in Practical Diets for Litopenaeus vannamei,and Digestibility of the Tested Ingredients and Diets. Aquaculture,272 : 466-476.
- Tacon A.G.J. (1993) Feed formulation and on-farm feed management. In M.B. New,A.G.J. Tacon and I. Csavas,eds. Farm-made aquafeeds. Proceedings of the FAO/AADCP Regional Expert Consultation on Farm-Made Aquafeeds. Bangkok,FAO-RAPA/AADCP,pp. 61-74.
- Tiews K.,Koops H.,Gropp J. and Beck H. (1979) Compilation of fish meal-free diets obtained in rainbow trout (Salmo gairdneri) feeding experiments at Hamburg (1970-1977/78). In: J.E. Halver and K. Tiews (Editors),Finfish Nutrition and Fish Feed Technology. Vol. II,Heenemann,Berlin,pp. 219-228.
- Higgs D.A.,Markert J.R.,Macquarrie D.W.,McBride J.R.,Dosanjh B.S.,Nichols C. and Hoskins G. (1979) Development of practical dry diets for coho salmon,Oncorhynchus kisutch,using poultry by-product meal,feather meal,soybean meal and rapeseed meal as major protein sources. In: J.E. Halver and K. Tiews (Editors),Fintish Nutrition and Fish Feed Technology,Vol. II,Heenemann,Berlin,pp. 191-218.
- Fowler L.G. (1990) Feather meal as a dietary protein source during Parr-smolt transformation in fall chinook salmon. Aquaculture,89: 301-314.
- Nengas I.,Alexis M.N. and Davies S.J. (1999) High inclusion levels of poultry meals and related byproducts in diets for gilthead seabream,Sparus aurata L. Aquaculture,179: 13-23.
- Mondal K.,Kaviraj A. and Mukhopadhyay P.K. (2008) Evaluation of fermented fish-offal in the formulated diet of the freshwater catfish Heteropneustes fossilis. Aquac. Res.,39:1443-1449.
- Nagal S. and Jain P. C. (2010) Feather degradation by strains of Bacillus isolated from decomposing feather. Brazil J Microbiol. 41: 196-200.
- Mondal K.,Kaviraj A.,Mukhopadhyay P.K.,Datta M. and Sengupta C. (2007) Evaluation of fermented fish-offal in formulated diet of the Indian major carp,rohu,Labeo rohita (Hamilton). Acta Ichthyol. Piscat.,37: 99-105.
- Gomez K.A. and Gomez A. A. (1984) Statistical Procedures for Agricultural Research. 2nd ed.,John Wiley and Sons,New York.
- Spyridakis P.,Metallier R.,Gabaudan J. and Riaza A.,(1989) Studies on nutrient digestibility in European sea bass (Dicendrarchus labrax). Methodological aspects concerning faeces collection. Aquaculture,77: 61-70.
- Ellestad L.E.,Angel R. and Soares J.H. Jr. (2002) Intestinal phytase II: a comparison of activity and in vivo phytate hydrolysis in three teleost species with differing digestive strategies. Fish Physiol. and Biochem.,26: 259-273.
- AOAC (Association of Official Analytical Chemists) (1990) In: Helrich,W. (Ed.) Official Methods of Analysis of the Association of Official Analytical Chemists. Vol. 1,15th ed. Assoc. of Official Analyt. Chem.,Washington,DC,USA. p.1134.
- Maynard L.,Loosil J.,Hintz H. and Warner R. (1979) In: Zappa,C.R. (Ed.) Animal Nutrition. 7th edn. McGraw-Hill,New York,NY. pp. 13-14.
- Saha D.C. and Gilbreath R.L. (1991) Analytical recovery of chromium in diet and faeces determined by colorimetry abd atomic absorption spectrophotometry. J. Sci. Food. Agr.,55: 433-446.
- Castell J.D. and Tiews K. (1980) Report of the EIFAC,IUNS and ICES Working Group on the standarisation of methodology in fish nutrition research,EIFAC Technical Paper 36,Hamburg,Federal Republic of Germany,21-23 March.
- APHA (American Public Health Association) (1995) Standard methods for the examination of water and wastewater,American Public Health Association,American Water Works Association and Water Pollution Control Federation,Washington,DC,U.S.A.
- Johnson R. A. and Wichern D. W. (1992) Applied Multivariate Statistical Analysis. Prentice Hall of India,New Delhi. pp. 241-284.
- Mondal K.,Kaviraj A. and Mukhopadhyay P. K. (2011) Partial replacement of fishmeal by fermented fish-offal meal in the formulation of diet for Indian minor carp Labeo bata. J. Apl. Aqa.,23: 41-50.
- Roley D.D.,Roley S.E.,Hardy R.W. and Brannon E.L. (1977) Feather meal and candida yeast as substitute for fishmeal in the diets of chinook salmon fry (Oncorhynchus tshawytscha). Annual Report of the College of Fisheries,University of Washington,Seattle,WA,pp. 70.
- Wilson R.P. (2002) Amino acids and proteins,In: Halver,J.E.,Hardy,R.W. (Eds.),Fish Nutrition,third edition. Elsevier Science (USA),New York,pp. 143-179.
- Manjappa K.,Keshavanath P. and Gangadhara B. (2002) Growth performance of common carp (Cyprinus carpio) fed varying lipid levels through low protein diet,with a note on carcass composition and digestive enzyme activity. Acta. Ichthyol. Piscat.,32: 145-155.
- Du Z.-Y.,Liu Y.-J.,Tian L.-X.,Wang J.-T.,Wang Y. and Liang G.-Y. (2005) Effect of dietary lipid level on growth,feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella). Aquac. Nutr.,11: 139-146.
- Arnesen P.,Krogdahl A. and Kristiansen I.O. (1993) Lipogenic enzyme activities in liver of Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. B Comp. Biochem.,105: 541–546.
- Alvarez M.J.,López-Bote C.J.,Diez A.,Corraze G.,Arzel J.,Dias J.,Kaushik S.J. and Bautista J.M. (1998) Dietary fish oil and digestible protein modify susceptibility to lipid peroxidation in the muscle of rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchus labrax). Br. J. Nutr.,80: 281-289.
- Watanabe T.,(1982) Lipid nutrition in fish. Comp. Biochem. Physiol. B.,73: 3-15.
- Murray H.M.,Gallant J.W.,Perez-Casanova J.C.,Johnson S.C. and Douglas S.E. (2003) Ontogeny of lipase expression in winter flounder. J. Fish. Biol,62: 816-833.
- Bolasina S.,Perez A. and Yamashita Y. (2006) Digestive enzymes activity during ontogenetic development and effect of starvation in Japanese flounder,Paralichthys olivaceus. Aquaculture,252: 503-515.
- Kaviraj A.,Mondal K.,Mukhopadhyay P.K. and Turchini G.M. (2012) Impact of Fermented Mulberry Leaf and Fish Offal in Diet Formulation of Indian Major Carp (Labeo rohita). Proc. Zool. Soc.,DOI 10.1007/s12595-012-0052-1.
- Bertsch A. and Coello N. (2005) A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Biores. Technol.,96: 1703-1708.
- Conceição L.E.C.,Grasdalen H. and Rønnestad I. (2003) Amino acid requirements of fish larvae and post-larvae: new tools and recent findings. Aquaculture,227: 221-232.
- Saavedra M.,Beltran M.,Pousão-Ferreira P.,Dinis M.T.,Blasco J. and Conceição L.E.C. (2007) Evaluation of bioavailability of individual amino acids in Diplodus puntazzo larvae: Towards the ideal dietary amino acid profile. Aquaculture,263: 192-198.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences