Medicinal Uses and Molecular Identification of Two Momordica charantia Varieties ÃÆâÃâââ¬Ãâââ¬Å a review
Ananya Paul, Sarmistha Sen Raychaudhuri
Ananya Paul and Sarmistha Sen Raychaudhuri*
Plant Tissue Culture and Molecular Biology Laboratory, Department of Biophysics, Molecular Biology and Bioinformatics, University of Calcutta, 92, Acharya Prafulla Chandra Road, Calcutta 700009, India
- *Corresponding Author:
- Tel: +91(0) 332-3508386 (Ext: 324)
Fax: +91(0) 332-8661573
E-mail: sarmistha_rc@rediffmail.
Abstract
Momordica charantia is a tendril bearing medicinally important vine. Medicinal properties of the plant include antimicrobial, antihelminthic, anticancerous, antimutagenic, antitumourous, abortifacient, antifertility, antidiabetic. Amongst the various medicinal properties, antidiabetic property of M. charantia is of utmost importance to human beings and animals. Mixture of steroidal saponins known as charantins, insulin-like peptides and alkaloids are the hypoglycemic constituents of M. charantia and these constituents are concentrated in fruits of Momordica charantia. Momordica charantia can be considered as an alternative therapy for lowering blood glucose levels in patients with diabetes. Although Momordica charantia has hypoglycemic effects, but available scientific data is not sufficient to recommend its use for treating diabetes, in the absence of careful supervision and monitoring. Investigation of the traditional uses of Momordica charantia in India revealed that it is one of the most important plant for ethnobotanical practices. Ethnobotanical uses of this plant in India suggest that it is capable of lowering blood glucose level in diabetic patients. Furthermore, RAPD markers have been used to analyze the genetic diversity among 12 different accessions of M. charantia, collected from different districts of West Bengal. The clustering pattern based on RAPD markers was not in accordance with the grouping based on morphological characters. The presence of SCAR markers in the two varieties of M. charantia namely var. muricata and var. charantia has been determined so that nutritional and medicinal properties could be exploited judiciously.
Keywords
Antidiabetic; hypoglycaemic; phytochemic-als; RAPD.
1. Introduction
Momordica charantia L. commonly known as bittergourd is an economically important medicinal plant belonging to the family cucurbitaceae. Two varieties of this plant are cultivated in India 1. M. charantia var. charantia with large fruits which are fusiform in shape and 2. M. charantia var. muricata, which are identified by small, round fruit [1]. The immature fruits are eaten as vegetables and are a good source of vitamin C, vitamin A and phosphorus and iron [2, 3]. The bitter flavour of both the varieties is due to the alkaloid momordicine produced in fruits and leaves.
Fruits and seeds of bittergourd possess medicinal properties such as anti-HIV, anti-ulcer, anti-inflammatory, anti-leukemic, antimicrobial, antitumor and last but not the least the important antidiabetic property [4].
The present investigation is being aimed in studying the medicinal and, ethnobotanical properties of Momordica charantia with specific importance to antidiabetic property of the plant. Furthermore estimation of genetic diversity among 12 different accessions of M. charantia growing in different districts of West Bengal, using RAPD markers has also been perfomed. A SCAR marker has been developed by the present investigators to distinguish the two varieties of M. charantia.
2. Phytochemicals related to anti-diabetic activity
It is evident from literature reviewed that diabetes is a killer disease that affects human subjects of different ages according to its type and the recurrence is specially noted in Indian population [5]. Mixture of steroidal saponins known as charantins, insulin-like peptides and alkaloids are the hypoglycemic chemicals of Momordica charantia [6] and these chemicals are concentrated in fruits of Momordica charantia, therefore fruit of M. charantia has shown most effective hypoglycemic property (Table 1) [7].
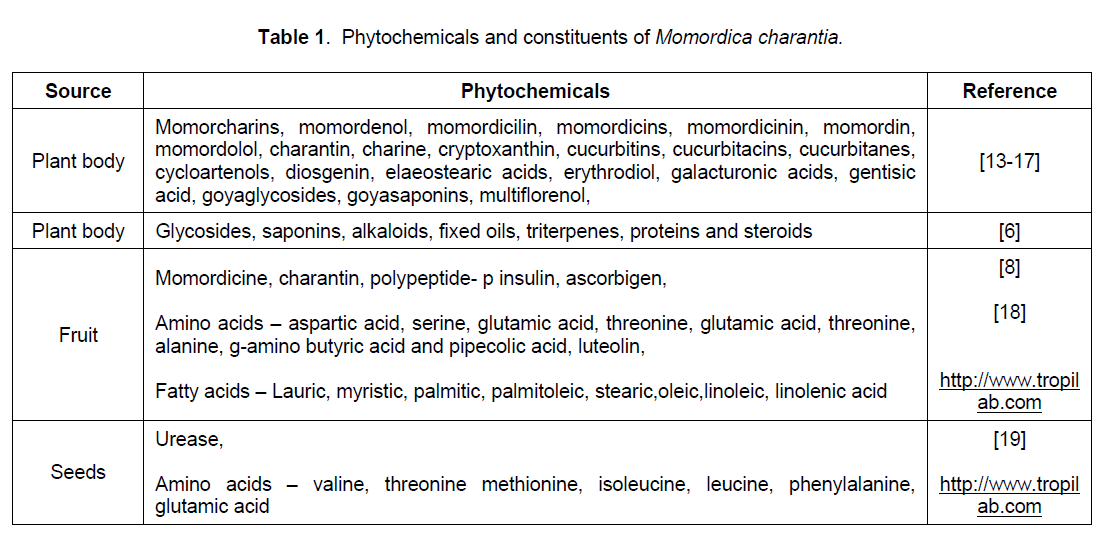
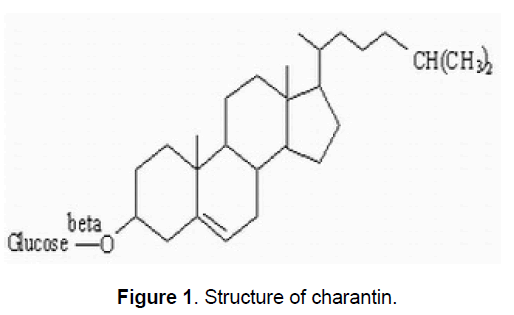
2.1 Steroids, Charantin
Charantin (Figure 1) is one of the hypoglycemic compounds, which can be isolated from Momordica charantia fruit. It is a mixture of two compounds (1:1) sitosteryl glucoside (C35H60O6) and stigmasteryl glucoside (C35H58O6), both of which are steroidal saponins. Lolitkar and Rao [8] have shown charantin when taken taken either orally or intravenously in rabbits, it produces hypoglycemic effects.
2.2 Protein, P-insulin
Protein P- insulin is a polypeptide with molecular weight of about 11,000 Dalton and consists of 166 amino acids. Clinical study study revealed that the polypeptide-p-ZnCl2 produced blood sugar lowering effect. Khanna and Mohan [9] reported that besides the fruits, p insulin was also found in seeds and tissue cultures of Momordica charantia.
2.3 Alkaloids
According to [10,11], seeds of bittergourd contain pyrimidine nucleoside vicine. Vicine has been found to induce hypoglycemia in rats, when administered intraperitoneally.
3. Medicinal uses
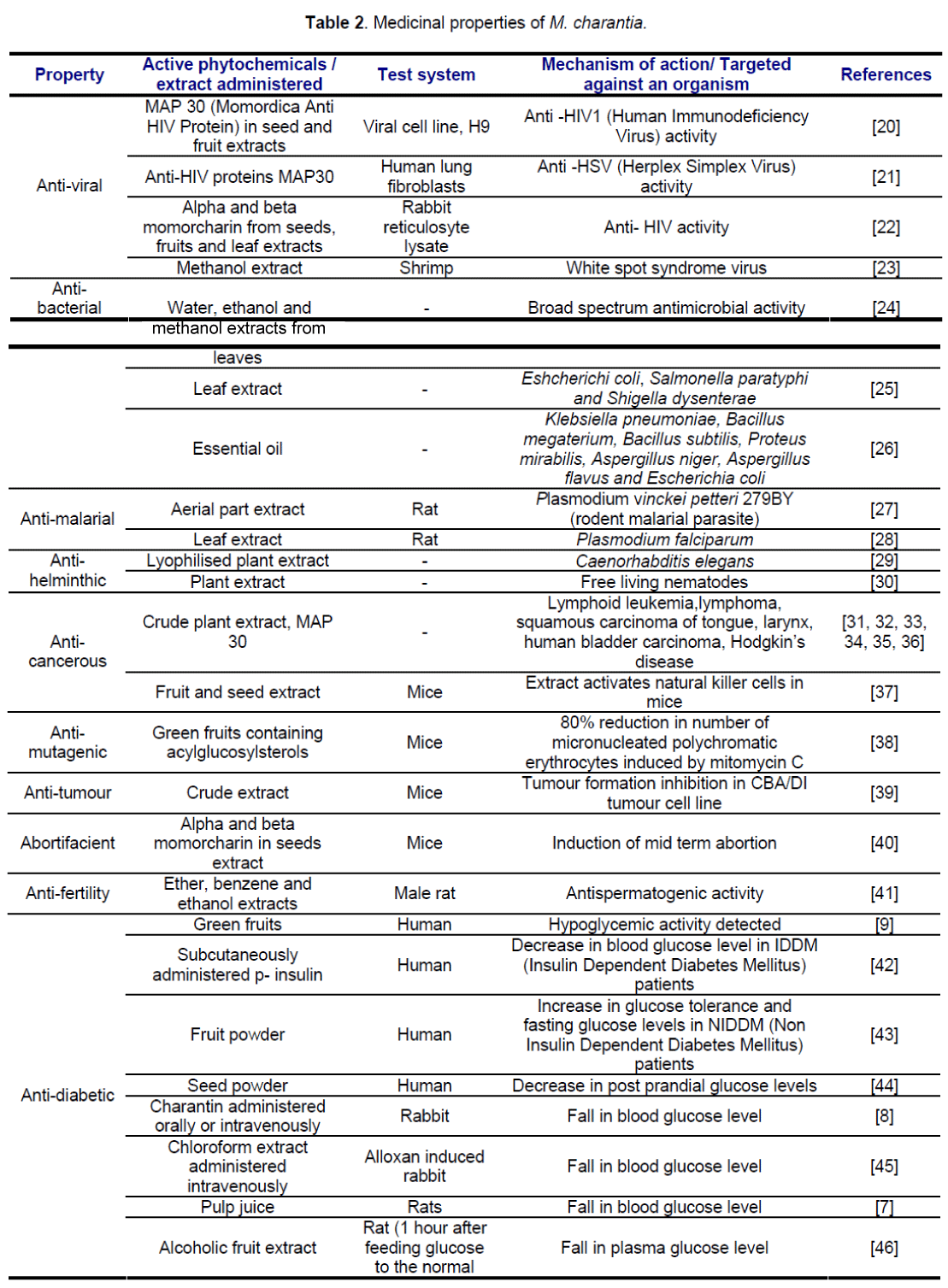
Medicinal properties include antimicrobial, antihelminthic, anti-cancerous, anti-mutagenic, antitumourous, abortifacient, anti-fertility, anti-diabetic. Medicinal properties of M. Charantia are summarized in Table 2.
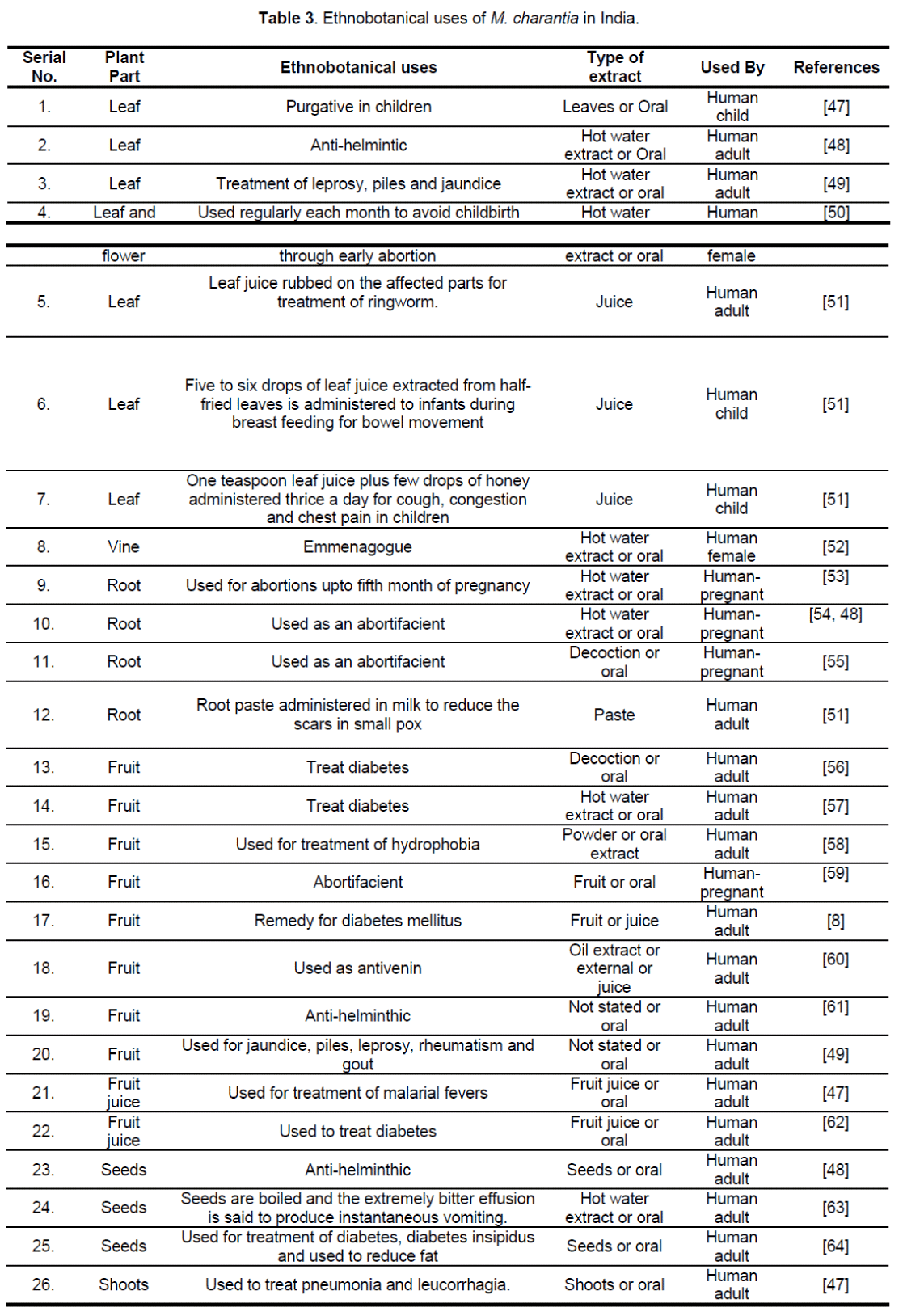
4. Ethnobotanical uses
Chakravarty [12], documented about the ethnobotanical uses of the plant in India. Since both the varieties grow abundantly throughout India, M. charantia is a very important so far as ethnomedical practices are concerned. Ethnobotanical uses of M. charantia in India are enumerated in Table 3.
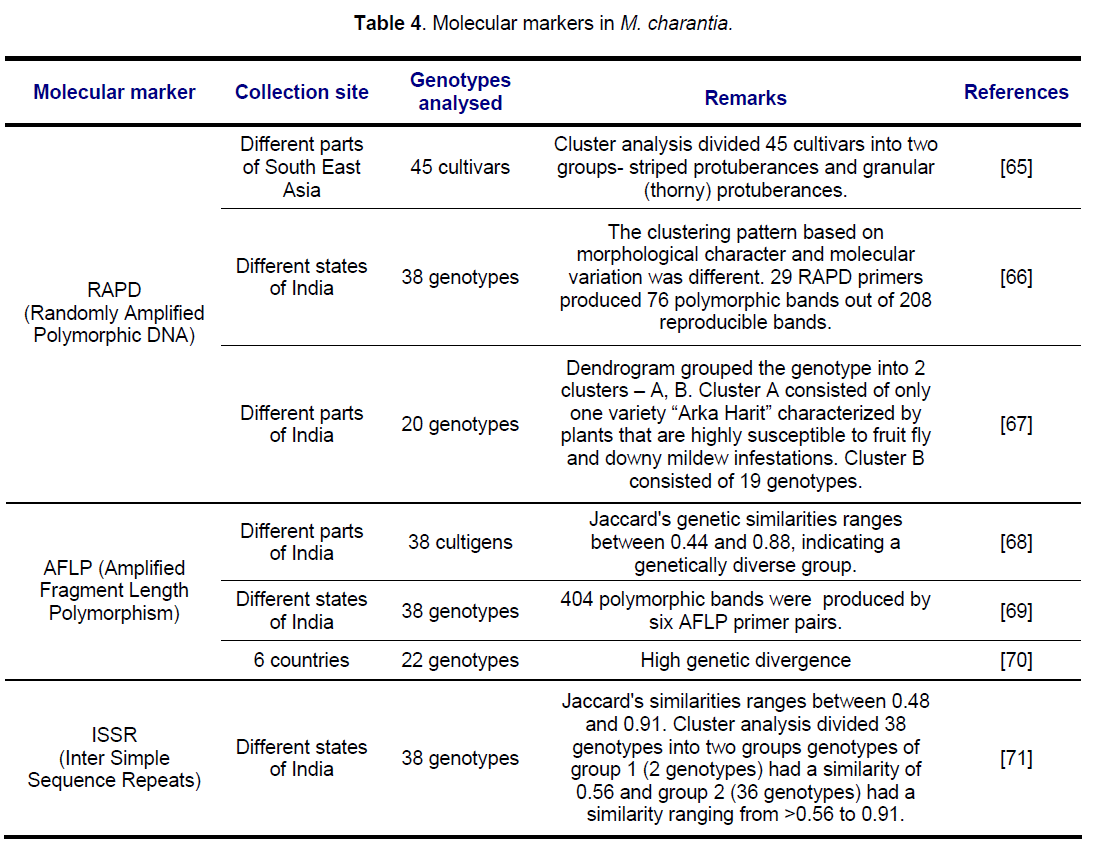
5. Molecular markers in M. charantia
Genetic diversity among populations can be determined using molecular markers. Different types of molecular markers which has been used to assess the genetic diversity of M. charantia are mentioned be in Table 4.
6. APD Analysis
Two varieties of this plant are cultivated in India. One is M. charantia var. charantia with large fruits which are fusiform in shape and other is M. charantia var. muricata, which is identified by small, round fruits. The present investigation has been carried out to determine genetic diversity using RAPD marker and developing a SCAR marker to distinguish between the varieties of M. charantia as stated above.
RAPD markers have been used extensively in bittergourd to classify accessions identify cultivars and analyze genetic diversity. Changyuan et al. (2005) have employed RAPD markers in order to detect genetic relationship in 45 bittergourd cultivars, collected from different parts of South East Asia. Dendrogram based on UPGMA cluster analysis divided 45 cultivars into two groups- striped protuberances and granular (thorny) protuberances. Striped protuberances type included two groups, which came from South-eastern Asia and China. Granular protuberances type included two groups, which came from Hongkong and China mainland.
Dey et al. (2006) have analyzed the diversity of 38 genotypes of M. charantia, collected from different parts of India, both at morphological (agronomic traits) and molecular (RAPD) level, RAPD analysis revealed 76 polymorphic bands out of total 208 bands. The clustering pattern based on yield related traits and molecular variation was different.
Rathod et al. (2008) have used morphological characters along with RAPD markers to access genetic diversity and relationships among 20 genotypes of bittergourd collected from different parts of India. 69 polymorphic bands were obtained out of total 143 bands. Dendrogram grouped the genotype into 2 clusters – A, B. Cluster A consisted of only one variety “Arka Harit” characterized by plants that are highly susceptible to fruit fly and downy mildew infestations. Cluster B consisted of 19 genotypes.
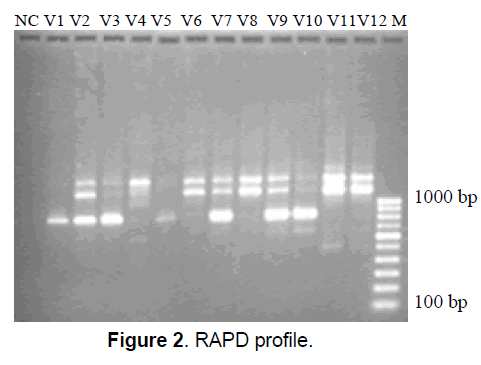
In our laboratory, RAPD markers have been used to analyze the genetic diversity among 12 different accessions of M. charantia, collected from different districts of West Bengal. Genetic variation patterns of 12 accessions (V1, V2, V3, V4, V5, V6 and V7 belonging to variety muricata and V8, V9, V10, V11, V12 belonging to variety charantia) of two bittergourd varieties were examined using 23 selected RAPD primers. Amongst 23 primers used, 17 primers produced clear and reproducible bands. Among 17 primers, 16 primers produced polymorphic bands and only 1 primer produced monomorphic band. The highest number of 5’GACCGCTTGT3’ (Figure 2) (NC-Negative Control). The similarity coefficients based on 81 RAPD markers ranged from 0.562 – 0.881. A dendrogram was constructed by cluster analysis to establish the affinity and relationship between the 12 accessions of Momordica charantia, using average linkage between the groups.
The dendrogram divided 12 accessions of M. charantia into 2 major clusters. It was observed that, varieties - V4, V6 and V7 belonging to variety muricata (based on morphology) grouped along with the varieties - V8, V9, V10, V11, V12 belonging to variety charantia (based on morphology) to form a single cluster. Another cluster comprised of the varieties - V1, V2, V3, V5 belonging to variety muricata. Thus, the clustering pattern based on RAPD markers was not in accordance with the grouping based on morphological characters. The similarity coefficients based on 81 RAPD markers ranged from 0.562 – 0.881. A dendrogram was constructed by cluster analysis to establish the affinity and relationship between the 12 accessions of Momordica charantia, using average linkage between the groups. The dendrogram divided 12 accessions of M. charantia into 2 major clusters. It was observed that, varieties - V4, V6 and V7 belonging to variety muricata (based on morphology) grouped along with the varieties - V8, V9, V10, V11, V12 belonging to variety charantia (based on morphology) to form a single cluster. Another cluster comprised of the varieties - V1, V2, V3, V5 belonging to variety muricata. Thus, the clustering pattern based on RAPD markers was not in accordance with the grouping based on morphological characters.
Due to the sensitivity of RAPD technique to PCR conditions, there is less reproducibility of RAPD results. This problem was solved by Paran and Michelmore (1993) who first documented the use of SCAR (Sequence Characterised Amplified Regions) marker in lettuce, where the marker was related to downey mildew resistance genes. SCAR markers can be developed by producing primers from unique polymorphic RAPD band. The primer is then allowed to amplify the genomic DNA of any particular genotype and not of other genotypes. Thus, PCR conditions of RAPD technique can be made more reliable by using SCAR markers.
Thus, we can take the help of SCAR markers in studying the genetic relatedness amongst intra and inter varieties and species of plants. Reproducible SCAR markers for studying inter and intra specific genetic diversity have already been successfully obtained from RAPD fragments in Lactuca, Triticum, and Agrostis [72,73,74].
In the present investigation, one polymorphic band was identified using RAPD primer 5’CAAACGTCGG3’. A SCAR marker was developed from the RAPD data [75]. This marker will be useful for future studies to identify the polymorphisms (6) was observed with primer no. 5, molecular differences between the two varieties used in agricultural practices.
7. Conclusion
Two varieties of M. charantia namely var. muricata and var. charantia, grown in India are used for various medicinal properties like antimicrobial, antihelminthic, anticancerous, antimutagenic, antitumourous, abortifacient, antifertility, antidiabetic. In vitro and in vivo uses of the compounds isolated from extracts of M. charantia have been documented in this review with retrospective as well as recent publications. Ethnobotanical uses of this plant in India suggest that it is capable of lowering blood glucose level in diabetic patients. Thus, numerous medicinal and ethnobotanical uses of nearly all parts of the plant indicate a long association of the plant with people, especially in India. In the present study several plants were collected from different districts of West Bengal and subjected to DNA amplification. A polymorphic band was identified and used for developing a SCAR marker. Molecular markers such as RAPD and SCAR could be used to explore the genes associated with medicinal properties of M. charantia.
References
- Chakravarty H.L. (1990) Cucurbits of India and their role in the development of vegetable crops. In Biology and Utilization of Cucurbitaceae (Bates D.M., RW Robinson R.W., Jeffrey C.), Cornell University Press, Ithaca, NY, pp. 325-334.
- Sultana R.S., Bari Miah M.A. (2003) In vitro Propagation of Karalla (Momordica charantea Linn.) from nodal segment and shoot tip. Journal of Biological Sciences, 3: 1134-1139.
- Paul A., Mitter K., Sen Raychaudhuri S. (2009) Effect of Polyamines in in vitro Somatic Embryogenesis in Momordica charantia L. Plant Cell Tissue and Organ Culture, 97: 303-311.
- Taylor L. (2002) Technical Data Report for Bitter melon (Momordica charantia). In Herbal Secrets of the Rainforest 2nd ed, Sage Press Inc.
- Patel P.M., Patel K.N., Patel N.M., Goyal R.K. (2006) Development of HPTLC method for estimation of charantin in herbal formulations. Pharmacognosy Magazine, 2: 224-226.
- Raman A., Lau C. (1996) Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine, 2: 349–362.
- Ali L., Khan A.K., Mamun M.I., Mosihuzzaman M., Nahar N., Nur-e- Alam M., Rokeya B. (1993) Studies on hypoglycemic effects of fruit pulp, seed, and whole plant of Momordica charantia on normal and diabetic model rats. Planta Medica, 59: 408–412.
- Lolitkar M.M., Rao M.R.R. (1966) Pharmacology of a hypoglycaemic principle isolated from the fruits of Momordica charantia. Linn. Indian Journal of Pharmacy, 28: 129-133.
- Khanna P., Mohan S. (1973) Isolation and identification of diosgenin and sterols from fruits and in vitro cultures of Momordica charantia Linn. Indian Journal of Experimental Biology, 11: 58-60.
- Dutta P.K., Chakravarty A.K., Chowdhury U.S., Pakrashi S.C. (1981) Vicine, a favism-inducing toxin from Momordica charantia Linn. Indian Journal of Chemistry, 20B: 669-671.
- Barron D., Kaouadji M., Mariotte A.M. (1982) Etude comparative de deux cucurbitacees a usage medicinal. Planta Medica, 46: 184-186.
- Chakravarty H.L. (1959) Monograph on Indian Cucurbitaceae. In Records of Botanical Survey of India, pp. 86–99.
- Husain J., Tickle I.J., Wood S.P. (1994) Crystal structure of momordin, a type I ribosome inactivating protein from the seeds of Momordica charantia. FEBS Letters, 342: 154–158.
- Xie H. Huang S., Deng H., Wu Z., Ji A. (1998) Study on chemical components of Momordica charantia, 21: 458–459.
- Yuan Y.R., He Y.N., Xiong J.P., Xia Z.X. (1999) Three-dimensional structure of beta-momorcharin at 2.55 A resolution. Acta Crystallographica Section DBiological Crystallography, 55: 1144–1151.
- Parkash A., Ng T.B., Tso W.W. (2002) Purification and characterization of charantin, a napin-like ribosome-inactivating peptide from bittergourd (Momordica charantia) seeds. Journal of Peptide Research, 59: 197–202.
- Murakami T, Emoto A, Matsuda H, Yoshikawa M (2001) Medicinal foodstuffs. Part XXI. Structures of new cucurbitane-type triterpene glycosides, goyaglycosides-a, -b, -c, -d, -e, -f, -g, and -h, and new oleanane-type triterpene saponins, goyasaponins I, II, and III, from the fresh fruit of Japanese Momordica charantia L. Chemical & Pharmaceutical Bulletin (Tokyo), 49: 54–63.
- Yuwai KE, Rao KS, Kaluwin C, Jones GP, Rivett DE (1991) Chemical composition of Momordica charantia L. fruits. Journal of Agricultural and Food Chemistry, 39: 1762-1763.
- Orlovskaya T.V., Chelombitko V.A. (2007) Amino acid composition of Momordica charantia seeds and pericarp. In Chemistry of natural compounds, pp. 43.
- Lee-Huang S., Huang P.L.., Nara P.L., Chen H.C., Kung H.F., Huang P., Huang H.I., Huang P.L. (1990) MAP 30: a new inhibitor of HIV-1 infection and replication. FEBS Letters, 272: 12–18.
- Bourinbaiar A.S., Lee-Huang S. (1996) The activity of plant-derived antiretroviral proteins MAP30 and GAP31 against herpes simplex virus in vitro. Biochemistry and Biophysics Research Communication, 219: 923–929.
- Au T.K., Collins R.A., Lam T.L., Ng T.B., Fong W.P., Wan D.C. (2000) The plant ribosome inactivating proteins luffin and saporin are potent inhibitors of HIV- 1 integrase. FEBS Letters, 471: 169–172.
- Balasubramanian G., Sarathi M., Kumar S.R., Hameed A.S.S. (2007) Screening the antiviral activity of Indian medicinal plants against white spot syndrome virus in shrimp. Aquaculture, 263: 15–19.
- Khan M.R., Omoloso A.D. (1998) Momordica charantia and Allium sativum: broad-spectrum antibacterial activity. Korean Journal of Pharmacognosy, 29: 155–158.
- Omoregbe R.E., Ikuebe O.M., Ihimire I.G. (1996) Antimicrobial activity of some medicinal plants extracts on Escherichia coli, Salmonella paratyphi and Shigella dysenteriae. African Journal of Medical Science, 25: 373–375.
- Ajaji I.A. , Jonathan S.G., Adewuyi A., Oderinde R.A. (2008) Antimicrobial Screening of the Essential Oil of Some Herbal Plants from Western Nigeria. World Applied Sciences Journal, 3: 79-81.
- Munoz V., Sauvain M., Bourdy G., Callapa J., Rojas I., Vargas L., Tae A., Deharo E. (2000) The search for natural bioactive compounds through a multidisciplinary approach in Bolivia .Part II. Antimalarial activity of some plants used by Mosetene indians. Journal of Ethnopharmacology, 69: 139–155.
- Gbeassor M., Kedjagni A.Y., Koumaglo K., De Souza C., Agbo K. , Aklikokou K., Amegbo K.A. (2006) In vitro antimalarial activity of six medicinal plants. Phytotherapy Research, 4: 115-117.
- Beloin N., Gbeassorb M., Akpaganab K., Hudsonc J., Soussab K.D., Koumaglob K., Arnasona J.T. (2005) Ethnomedicinal uses of Momordica charantia (Cucurbitaceae) in Togo and relation to its phytochemistry and biological activity. Journal of Ethnopharmacology, 96: 49–55.
- Das P., Sinhababu S.P., Dam T. (2006) Screening of antihelminthic effects of Indian plant extracts: a preliminary report screening of antihelminthic effects of Indian plant extracts: a preliminary report. Journal of alternative and complementary medicine, 12: 299-301.
- Licastro F., Franceschi C., Barbieri L., Stirpe F. (1980) Toxicity of Momordica charantia lectin and inhibitor for human normal and leukaemic lymphocytes. Virchows Archives of B Cell Pathology Including Molecular Pathology, 33: 257–265.
- Ng T.B., Liu W.K., Sze S.F., Yeung H.W. (1994) Action of alphamomorcharin, a ribosome inactivating protein, on cultured tumor cell lines. General Pharmacology, 25: 75–77.
- Battelli M.G., Polito L., Bolognesi A., Lafleur L., Fradet Y., Stirpe F. (1996) Toxicity of ribosomeinactivating proteins-containing immunotoxins to a human bladder carcinoma cell line. International Journal of Cancer, 68: 485–490.
- Ganguly C., De S., Das S. (2000) Prevention of carcinogen-induced mouse skin papilloma by whole fruit aqueous extract of Momordica charantia. European Journal of Cancer Prevention, 9: 283–288.
- Sun Y., Huang P.L., Li J.J., Huang Y.Q., Zhang L., Huang P.L., Lee-Huang S. (2001) Anti-HIV agent MAP30 modulates the expression profile of viral and cellular genes for proliferation and apoptosis in AIDSrelated lymphoma cells infected with Kaposi’s sarcomaassociated virus. Biochemical and Biophysical Research Communication, 287: 983–994.
- Basch E., Gabardi S., Ulbricht C. (2003) Bitter melon (Momordica charantia): a review of efficacy and safety. American Journal of Health and Systemic Pharmacology, 65: 356–359.
- Cunnick J.E., Sakamoto K., Chapes S.K., Fortner G.W., Takemoto D.J. (1990) Induction of tumor cytotoxic immune cells using a protein from the bitter melon (Momordica charantia). Cellular Immunology, 126: 278–289.
- Guevara A.P., Lim-Sylianco C., Dayrit F., Finch P. (1990) Antimutagens from Momordica charantia. Mutation Research, 230: 121-126.
- Jilka C., Strifler B., Fortner G.W., Hays E.F., Takemoto D.J. (1983) In Vivo Antitumor Activity of the Bitter Melon (Momordica charantia). Cancer Research, 43: 5151-5155.
- Yeung H.W., Li W.W., Chan W.Y., Law L.K., Ng T.B. (1986) Abortifacient proteins from the seeds of the bitter gourd Momordica charantia (Family Cucurbitaceae). International Journal of Protein and Peptide Research, 28: 518-524.
- Naseem M.Z., Patil S.R., Patil. (1998) Antispermatogenic and androgenic activities of Momordica charantia (Karela) in albino rats. Journal of Ethnopharmacology, 61: 9-16.
- Khanna P., Jain S.C., Pangria A., Dixit V.P. (1981) Hypoglycaemic activity of polypeptide p from the plant source. Journal of Natural Products, 44: 648-655.
- Akhtar, M.S. (1982) Trial of Momordica charantia Linn. (karela) powder in patients with maturity onset diabetes. Journal of Pakistan Medical Association, 32: 106-107.
- Grover J.K., Gupta, S.R. (1990) Hypoglycaemic activity of seeds of Momordica charantia. European Journal of Pharmacology, 183: 1026-1027.
- Tiangda C., Mekmanee R., Praphapraditchote K., Ungsurungsie M., Paovalo C. (1987) The hypoglycaemic activity of Momordica charantia Linn. in normal and alloxan-induced diabetic rabbits. Journal of the National Research Council, 19: 1-11.
- Sarkar S., Pranao M., Marita R., (1996) Demonstration of the hypoglycaemic action of M. charantia in a validated animal model of diabetes. Pharmacological Research, 33: 1-4.
- Reddy M.B., Reddy K.R., Reddy M.N. (1989) A survey of plant crude drugs of anantapur district, andhra pradesh, India. International Journal of Crude Drug Research, 27(3): 145-155.
- Sharma L.D., Bahga H.S., Srivastava P.S. (1971) In vitro anthelmintic screening of indigenous medicinal plants against haemonchus contortus (rudolphi,1803) cobbold, 1898 of sheep and goats. Indian Journal of Animal Research, 51: 33-38.
- Kedar P., Chakrabarti C.H. (1982) Effects of bittergourd (Momordica charantia) seed & glibenclamide in streptozotocin induced diabetes mellitus. Indian Journal of Experimental Biology, 20: 232-235.
- Saksena S.K. (1971) Study of antifertility activity of the leaves of Momordica (karela). Indian Journal of Physiology and Pharmacology, 15: 79-80.
- Joseph J.K., Antony V.T. (2008) Ethnobotanical investigations in the genus Momordica L. in the Southern Western Ghats of India. Genetic Resources and Crop Evolution, 55: 713–721.
- Morton J.F. (1967) The balsam pear-an edible, medicinal and toxic plant. Economic Botany, 21: 57-68.
- Oommachan M., Khan S.S. (1981) Plants in aid of family planning program. Sci. life, 1: 64-66.
- Jamwal K.S., Anand K.K. (1962) Preliminary screening of some reputed abortifacient indigenous plants. Indian Journal of Pharmacy, 2: 218-220.
- Kamboj V.P. (1988) A review of indian medicinal plants with interceptive activity. Indian Journal of Medical Research, 4: 336-355.
- Khan M.A., Singh V.K. (1996) A folklore survey of some plants of Bhopal district forests, Madhya Pradesh, India, described as antidiabetics. Fitoterapia, 67: 416-421.
- Singh N., Tyagi S.D., Agarwal S.C. (1989) Effects of long term feeding of acetone extract of Momordica charantia (whole fruit powder) on alloxan diabetic albino rats. Indian Journal of Physiology and Pharmacology, 33: 97-100.
- Nagaraju N., Rao K.N. (1990) A survey of plant crude drugs of rayalaseema, Andhra Pradesh, India. Journal of Ethnopharmacology, 29: 137-158.
- Quisumbing E. (1951) Medicinal plants of the Philippines: Tech bull 16, Rep Philippines,Dept agr nat resources, Manilla.
- Selvanayahgam Z.E., Gnanevendhan S.G., Balakrishna K., Rao R.B. (1994) Antisnake venom botanicals from ethnomedicine. Journal of Herbs, Spices and Medicinal Plants, 2: 45-100.
- Ayensu E.S. (1978) Medicinal plants of West Africa. Inc. Algonac, Michigan, USA.
- Vedavathyk S., Rao D.N. (1995) Herbal folk medicine of tirumala and tirupati region of Chittoor district, Andhra Pradesh. Fitoterapia, 66: 167-171.
- Koelz W.N. (1979) Notes on the ethnobotany of Lahul, a province of the Punjab Q J. Crude Drug Research, 17: 1-56.
- Rajurkar N.S., Pardeshi B.M. (1997) Analysis of some herbal plants from india used in the control of diabetes mellitus by NAA and AAS techniques. Applied Radiation of Isotopes, 48: 1059-1062.
- Changyuan Z., Ni S., Kailin H. (2005) RAPD analysis in Genetic relationship among varieties of balsam pear. Molecular Plant Breeding, 3: 515-519.
- Dey S.S., Singh A.K., Chandel D., Behera T.K. (2006) Genetic diversity of bittergourd (Momordica charantia L.) genotypes revealed by RAPD markers and agronomic traits. Scientia Horticulturae, 109: 21-28.
- Rathod V., Narasegowda N.C., Papanna N., Simon L. (2008) Evaluation of genetic diversity and genome fingerprinting of bitter gourd genotypes (Momordica charantia L.) by morphological and RAPD markers. International Journal of Plant Breeding, 2: 79-84.
- Gaikwad A.B., Behera T.K., Singh A.K., Chandel D., Karihaloo J. L., Staub J.E. (2008) Amplified fragment length polymorphism analysis provides strategies for improvement of bitter gourd (Momordica charantia L.). Horticultural Science, 43: 127-133.
- Behera T.K., Gaikwad A.B., Singh A.K., Staub J.E. (2008) Relative efficiency of DNA markers (RAPD, ISSR and AFLP) in detecting genetic diversity of bitter gourd (Momordica charantia L.). Journal of the Science of Food and Agriculture, 88: 733–737.
- Kole C., Olukolu B., Kole P., Abbott A.G. (2010) Towards Phytomedomics with Bitter Melon (Momordica charantia L.) as a Model. In Plant & Animal Genomes XVIII Conference.
- Behera T.K., Singh A.K., Staub J.E. (2008) Comparative analysis of genetic diversity in Indian bittergourd (Momordica charantia L.) using RAPD and ISSR markers for developing crop improvement strategies. Scientia Horticulturae, 115: 209-217.
- Paran I., Michelmore R.W. (1993) Development of reliable PCR based markers linked to downey mildew resistance genes in lettuce. Theoretical and Applied Genetics, 85: 985-993.
- Hernandez P., Martin A., Dorado G. (1999) Development of SCARs by direct sequencing of RAPD products: a practical tool for the introgression and marker-assisted selection of wheat. Molecular Breeding, 5: 245–253.
- Elizabeth A.S., Michael D.C., Geunhwa J. (2003) Development of speciesspecific SCAR markers in Bentgrass. Crop Science, 43: 345–349.
- Paul A. (2009) In vitro somatic embryogenesis and identification of different varieties of Momordica charantia L. using molecular markers. Thesis (unpublished).

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences