Utilization of the extract of pulp of carob for Lactic Acid Production by Streptococcus thermophilus
Hariri A, Ouis N, Bouhadi D
Bouhadi D1,*, Raho B2, Hariri A1, Benattouche Z1, Ould Yerou K1, Sahnouni F3
1 Bioconversion Laboratory, Microbiology Engineering and Health Safety, faculty of Science the Nature and Life, University of Mascara, Algeria
2 Molecular Microbiology Laboratory, Health and Proteomics, University of Sidi Bel-Abbès, Algeria
3 Environmental Monitoring Network, faculty of Science, University of Oran, Algeria
Received date: January 14, 2016 Accepted date: February 08, 2016 Published date: February 14, 2016
Citation: Bouhadi D, Raho B, Hariri A, et al., Utilization of The Extract of Pulp of Carob for Lactic Acid Production by Streptococcus thermophilus. Electronic J Biol, 12:2
Abstract
The main aim of this present study was the isolation and characterization of lactic acid bacteria from carob pods. A total of 6 isolates of lactic acid bacteria were screened and was identified from carob pods as Lactobacillus curvatus, Streptococcus diacetylactis, Streptococcus feacalis, Streptococcus mitis and Streptococcus thermophilus. This identification was based on phenotypic characters and the API system. Lactobacillus curvatus was shown to produce a high amount of acidity, why it is used for studying the kinetics of growth and acidification of carob pods syrup. The carob pods syrup which has been the subject of our work has a high water content 85%, very rich of total sugars 22 g/L and ash 0.8% and the protein fraction is considerable 0.25%. This study was designed not only to evaluate the potential of carob extract for lactic acid production but also to determine the effect of alternative addition of Sodium Acetate and Tween 80 on lactic acid fermentation. The results clearly indicated that the highest amount of biomass and lactic acid were obtained with the carob enriched with sodium Acetate and Tween 80.
Keywords
Carob extract; Lactic acid production; Streptococcus thermophilus; Optimization
1. Introduction
Lactic acid, also known as milk acid, is the most widely occurring carboxylic acid in nature [1]. It is considered to be one of the most useful chemicals, used in the food industry as a preservative, acidulant, and flavorings, in the textile and pharmaceutical industries, and in the chemical industry as a raw material for the production of lactate ester, propylene glycol, 2,3-pentanedione, propanoic acid, acrylic acid, and acetaldehyde [2]. In the cosmetic industry, lactic acid is used in the manufacture of hygiene and esthetic products, owing to its moisturizing, antimicrobial and rejuvenating effects on the skin, as well as of oral hygiene products [3]. It can be obtained either by the action of fermentative microorganisms or chemical synthesis [4]. Approximately 90% of the total lactic acid produced worldwide is by bacterial fermentation and the rest is produced synthetically by hydrolysis of lactonitrile [5]. Chemical synthesis from petrochemical resources always results in a racemic mixture of D/L-lactic acid, which is a major disadvantage of this approach, while microbial lactic acid fermentation offers an advantage in terms of utilization of renewable carbohydrate biomass, low production temperature, low energy consumption, and the production of optically high pure lactic acid [6].
The biotechnological production of lactic acid has received a significant amount of interest recently, since it offers an alternative way to prevent environmental pollution caused by the petrochemical industry and the limited supply of petrochemical resources [7]. In recent years research effort has focused on looking for new and effective nutritional sources and new progressive fermentation techniques enabling the achievement of both high substrate conversion rates and high production yields [8]. A number of different substrates have been used for the biotechnological production of lactic acid, including glucose, sucrose, lactose, maltose, mannose, xylose and galactose [9]. Raw material cost is one of the major factors in economic production of organic acids. Biological production offers significant advantages over chemical synthesis due to the fact that biological production can use cheap raw materials which mainly include plants [10]. Till date various agro industrial wastes such as whey, molasses, starch waste, beet, cane sugar, cassava, bagasse, apple pomace, soybean, potato residue, pine apple waste, wheat bran, kiwi fruit peel, and citric pulp have been used for the production of organic acids [11].
The carob tree (Ceratonia siliqua L.) belonging to the family Cesalpiniaceae sub family of the family Leguminoseae, is widely used in the Mediterranean regions cultivated for ornamental and industrial purposes. It produces edible pods used as a fodder for breeding cattle; it has also a long history of application as a source of health products [12]. Carob pulp is high (40-56%) in total sugar content (mainly sucrose, glucose, fructose and maltose) [13], which can be low cost substrate for the production of lactic acid. The aim of the present study was to optimize lactic acid production by Streptococcus thermophilus grown in Carob extract.
3. Materials and Methods
3.1 Microorganism
Streptococcus thermophilus strains were obtained from GIPLAIT Dairy unit of Mascara city (North West of Algeria). It is an important starter for the dairy industry. It is used in combination with Lactobacillus delbrueckii subsp. bulgaricus for the production of yogurt. It is also used for the manufacture of cheeses in which high cooking temperatures are applied [14]. The strain of Streptoccus thermophilus is homofermentative and produces lactic acid from glucose fermentation [15].
3.2 Sample collection and extraction procedure
Samples of carob were collected during April- May, in 2014; from Mostaganem city North West of Algeria. Carob are washed and destoned and grounded. 2.5 liters of hot water were added to 1 kg of carob, homogenized and through a cloth. The extract obtained was centrifuged at 15.000 rpm for 10 minutes. The collected supernatant was used as culture medium. The extract is fixed in a pH between 6-6.5 and sterilized during 20 minutes at 120°C.
3.3 Growth experiments
Batch cultures of Streptococcus thermophilus were performed using 250 ml of the carob extract in Erlenmeyer flasks. To study the influence of different factors on bacterial growth and lactic acid production. The effect of the initial pH of the medium was studied at 41°C with pH 5.0, 5.3, 5.6, 5.8, 6.2, 6.6, 6.9 and 7.2, the pH being adjusted with 5M NaOH. The effect of temperature was studied at 36, 39, 41, 43, 45 and 48°C.
3.4 Analytical methods
Total sugars concentrations were analyzed by the Dubois method using phenol and sulphuric acid [16]. Lactic acid concentrations were evaluated by acidity titration with NaOH [17]. The measurement of the optical density (OD) at 620 nm was used to the biomass determination.
3.5 Kinetic of growth
We take off every two hours until 24 hours 10 ml of medium of fermentation. We make a reading in a spectrophotometer to a length of wave 620 nm.
3.6 Data processing
The calculation of the kinetic parameters of fermentations μmax (h-1), Qsmax (g/g.h) and QALmax (g/g.h) has been performed using KALEIDAGRAPH software (Release 4.0.3 for windows, Synergy Software).
4. Results and Discussion
4.1 Effect of temperature on growth of Streptococcus thermophilus and lactic acid production
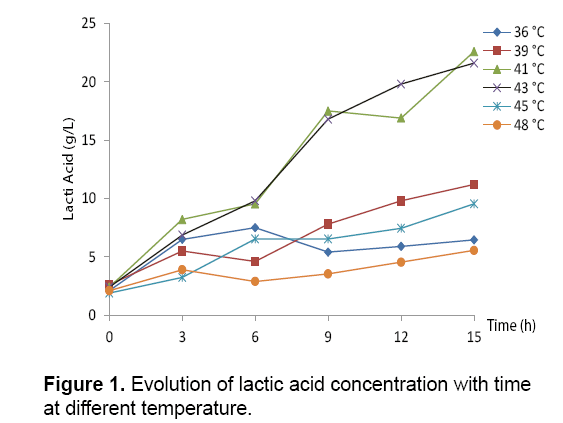
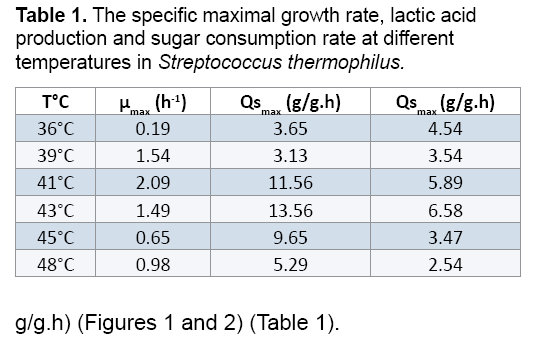
Table 1 and Figures 1 and 2 show the effects of temperature on the growth of Streptococcus thermophilus and lactic acid production. Streptococcus thermophilus was grown in M17 at 36, 39, 41, 43, 45 and 48°C, while the pH was kept constant at 6.5. As shown in the tab.1 and Figures 1 and 2 the optimum temperature for lactic acid production was 41°C, yielding 22.5 g/L of lactic acid at 15 h of fermentation. At 36, 43, 45, 48°C, lactic acid production was 6.45, 11.2, 21.6, 5.55 g/L respectively (Figure 1).
The follow up study of the biomass production of Streptococcus thermophilus by measuring the optical density at 620 nm, incubated at a temperature of 43°C, started at an initial OD of 0.31 after 20 hours fermentation, showed that biomass reached a maximum value of 4.56. This increase in biomass is stimulated by the consumption of an amount of sugar 36.54 g/L. However, the biomass production of Streptococcus thermophilus, incubated at temperatures of 35, 40, 43, 45 and 48°C gave 2.55, 2.67, 3.29 and 3.03 respectively (Figure 2).
On the basis of the obtained results, we can conclude that the maximum of biomass production of Streptococcus thermophilus is 2.1. Several authors like [18,19] showed that a strain is acidifying if the pH reduction equal at least 0.5 units within four hours [20]. Reported that the optimal temperature for the growth of Streptococcus thermophilus is 42°C. The maximal growth rate and the lactic acid production obtained after 15 hours of incubation at 36°C are 0.19 h-1 and 3.65 g/g.h, respectively. The values of μmax and Qsmax decrease when the temperature increase to 41°C (μmax=13.56 h-1 and ALQmax=6.58 g/g.h) (Figures 1 and 2) (Table 1).
4.2 Effects of initial pH on lactic acid and biomass production and sugar consumption by Streptococcus thermophiles
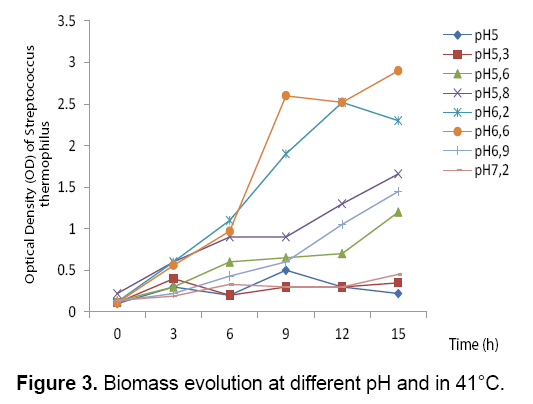
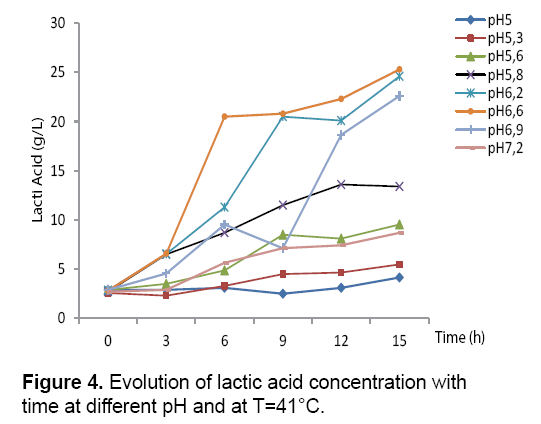
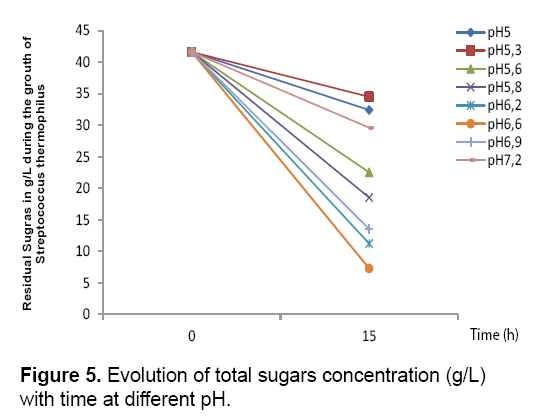
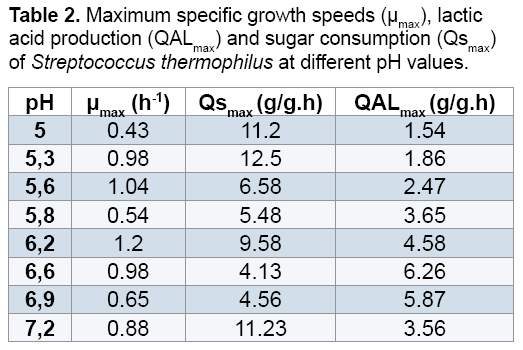
The results obtained (Figure 3 and 4) showed that the bacterial biomass and lactic acid production and the sugar consumption by Streptococcus thermophilus are inhibited in the culture medium at a pH value of 5, 5.3 and 5.8. However, there is a considerable increase in the biomass and lactic acid levels with the elevation of the pH value. Homofermentative Streptococcus ferment sugars by the Embden- Meyerhoff-Parnas (EMP) pathway to pyruvate, which is converted into lactic acid and ATP. During the exponential phase of growth, bacteria require a lot of ATP to ensure the synthesis and production, but it this requirement decreases in the stationary phase.
At pH 6.6, Streptococcus thermophilus culture gave the best yield of the lactic acid and biomass productions 25.3 g/L and 2.9 respectively. This explains the optimum fermentation conditions when we add the carob extract to the culture medium.
On the basis on the obtained results, we can conclude that the lactic acid production inhibits the growth of lactic acid bacteria. However, lactic acid bacteria maintain its alkaline intracellular pH than the extracellular medium, since the cell membrane is impermeable to extracellular protons (lactate molecule) and cells secrete quickly dissociated lactic acid in the extracellular medium. The difference in pH values between the cytoplasm and the extracellular medium causes a pH gradient (ΔpH), which permit the protons excretion to the external medium. At the end of the exponential phase of growth, the amount of available ATP becomes insufficient. The pH gradient disappears and the hexokinasic activity decreases which inhibit the bacterial growth [21] (Figures 3-5) (Table 2).
The comparison of the kinetic profiles revealed that the sugar consumption rate varies with different pH. For example, the sugar consumption rates are higher in pH 6.5 than to pH6.2, the values range from 9.58 g/g.h (at pH 5.2 at 4.65) to 11.23 g/g.h for the other pH (6.9 and 7.2).
4.3 Optimization of the culture medium parameters
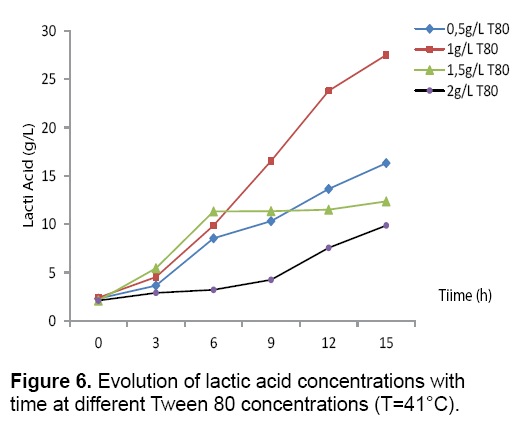
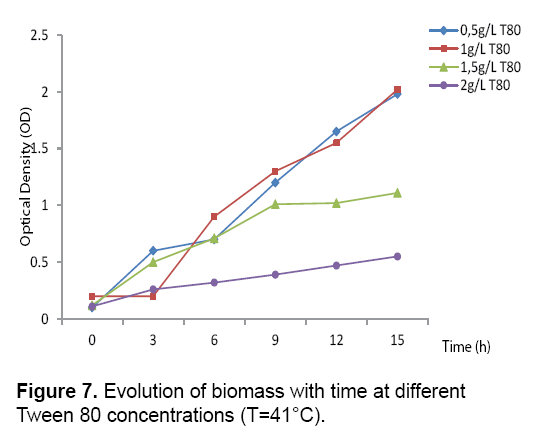
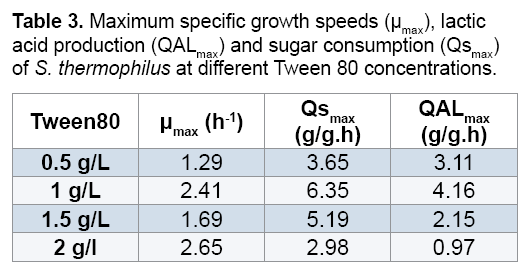
Effect of Tween 80: The effect of Tween 80 on the production of the bacterial biomass and lactic acid by Streptococcus thermophilus, cultivated on a culture medium based on carob extract was studied. The obtained results Figures 6 and 7 revealed that the culture of Streptococcus thermophilus in the presence of a concentration of 0.5 g/L of Tween 80 increase significantly the lactic acid production with a rate of 16.32 g/L. After addition of 1 g/L Tween 80 and incubation of 15 hours a high level of lactic acid 27.5 g/L is obtained, but the 2 g/L of Tween 80 showed a negative effect on the biomass and lactic acid production. According to the literature, the addition of Tween 80 allows the excretion of lactic acid in the culture supernatant after the creation of pores in the cell membrane [22].
Reported that the addition of Tween 80 showed a positive effect on the biomass and lactic acid production [23]. Suggested that the addition of Tween 80 in the culture medium stimulated considerably the transfer of carbohydrates through the cell membrane and consequently the increasing of the lactic acid production (Figures 6 and 7) (Table 3). The addition of 1g/L of was found to be most interesting to the lactic acid production which was 4.16 g/g.h for QAL max and 2.41 h-1 for μmax (Table 3).
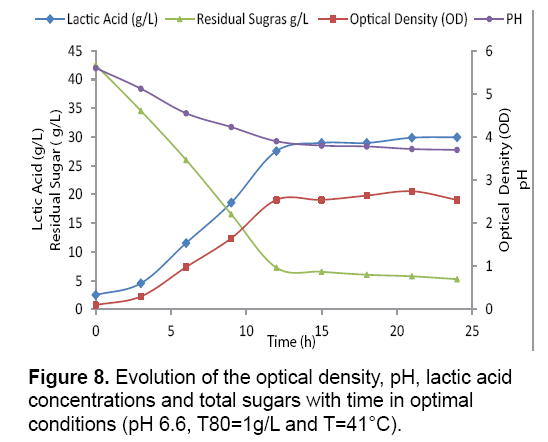
Lactic acid production in the optimal conditions: The Figure 8 shows the patterns of growth (OD620), pH, carbohydrate consumption and lactate production in the optimized medium. The Streptococcus thermophilus strain grows immediately without latency phase and reached the stationary phase after 14 hours of culture in the optimized environment. According to the kinetic profiles, the maximum biomass plate coincides with the consumption of the majority of the carbon substrate (sugars=7.25 g/L to 15 hours of fermentation). It also appears that the growth and lactic acid production are inhibited when the pH becomes 3.9 in the optimized medium. This is due to the strong acidity resulting from the accumulation of lactic acid [24,25], concluded that inhibition may be due to carbon source exhaustion.
But in our work, it is not the case, as the concentration of sugars consumed equal 37.29 g/L, so it remains in the optimized culture medium 5.25 g/L of the sugars not consumed by the bacteria (Figure 8).
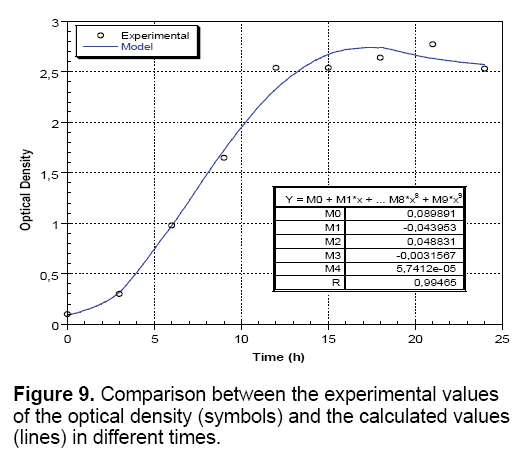
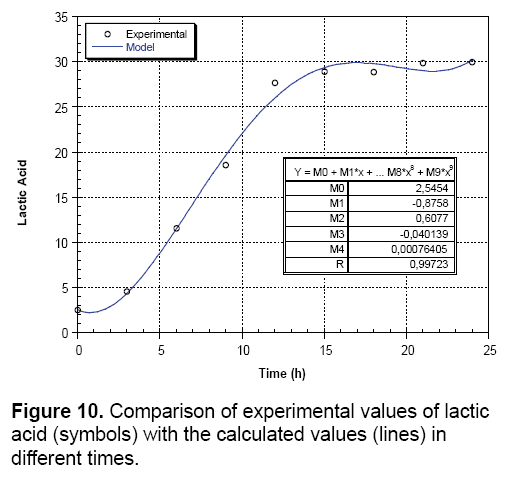
Growth kinetics and lactic acid production: In order to understand and interpret properly biological phenomena and specifically the growth kinetics and production of lactic acid, the obtained experimental results are expressed with a mathematical model using "Kaleidagraph" software, which can indicate the growth or acidification variables by equations depending on time and comprising a variable number of parameters with no biological significance.
Growth kinetics: The growth kinetics of Streptococcus thermophilus (Figure 9) showed that the obtained curves adjusted by the optimization program and test concentrations are very close, which manifest a square error well minimized by the optimization function with models describing although the dynamic behavior of microorganisms. Therefore, the adjustment of the bacterial growth is expressed by the following quartic function: Y=0.089- 0.043+0.048x2-0.003x3+5.741x4. With regard to the percentage of explanation of the model (R), we can see in the figure that we have obtained very good values of R=0.994. This means that the polynomial models identified from experiments allow predicting the variable values of responses in the explored area (Figure 9).
Kinetics of lactic acid production: The kinetics of the production of lactic acid illustrated in Figure 10. Shows that the adjustment has stimulated the production during all phases of growth with a very high correlation coefficient R (R=0.997). This explains that the experimental points are in very good correlation with those calculated. Therefore, the amount of lactic acid is expressed by the cubic equation below: Y=2.545-0.875x+0.607x2-0.0401x3+0.00076x4 (Figure 10).
5. Conclusion
This study has shown the potential use of Streptococuus thermophilus for the bioconversion of Carob extract into lactic acid. Under optimized conditions, the best result for lactic acid production (29.95 g/L) was obtained after 15 hours of fermentation in optimal conditions, 41°C, pH of 6,6 and 1% of Tween80. Carob extract was proven to be an economically feasible and promising raw material for industrial production of lactic acid.
References
- RenJ. (2011).Biodegradable Poly (Lactic Acid): Synthesis, Modification, Processing and Applications. Springer Science &Business Media. 4.
- Varadarajan S, Miller DJ. (1999).Catalytic Upgrading of Fermentation-Derived Organic Acids.15:845-854.
- Castillo Martinez FA, Balciunas EM, Salgado JM, et al. (2013).Lactic acid properties, applications and production: A review. Trends Food Sci Technol.30:70-83.
- Wee YJ, Kim JN, Ryu HW. (2006).Biotechnological Production of Lactic Acid and Its Recent Applications.Food Technol. Biotechnol. 44:163–172.
- Patil SS, Kadam SR, Patil SS, et al. (2006).Production of lactic acid and fructose from media with cane sugar using mutant of Lactobacillus delbrueckiiNCIM 2365. LettApplMicrobiol.43:53-7.
- Juodeikiene G, Vidmantiene D, Basinskiene L, et al. (2015).Green metrics for sustainability of biobased lactic acid from starchy biomass vs. chemical synthesis.Catalysis Today.239: 11-16.
- Sharma R, Sanodiya BS, Thakur GS, et al.(2013). Characterization of Lactic Acid Bacteria from Raw Milk Samples of Cow, Goat, Sheep, Camel and Buffalo with Special Elucidation to Lactic Acid Production.British Microbiology Research Journal.3: 743-752.
- Kurbanoglu EB, Kurbanoglu NI. (2003). Utilization for lactic acid production with a new acid hydrolysis of ram horn waste.FEMS MicrobiolLett.225:29-34.
- De Lima CJB, Coelho LF, da Silva GP, Alvarez G, Contiero J. (2010). L(+) Lactic Acid Production by New LactobacillusrhamnosusB 103.J Microbial Biochem Technol.2: 064-069.
- Vashishth A, Ganguli A, Tehri N. (2014). Organic acids production from Lactococcuslactis and Leuconostocmesenteroides using a novel citrus and potato waste medium.J InnovBiol1:175-180.
- Suresh K, Jayasuni JS, Gokul CN, Anu V. (2013). Citric acid production from agronomic waste using Aspergillusniger isolated from decayed fruit.Journal of Chemical Biological and Physical Sciences.3:1572-1576.
- Hasib A, EL Batal H. (2014). Optimization of Production of Carob Pulp Syrup from Different Populations of Moroccan Carob(Ceratoniasiliqua L.).International Journal of Emerging Technology and Advanced Engineering. 4: 855-863.
- Batlle I, Tous J. (1997). Carob tree: Ceratoniasiliqua L. - Promoting the conservation and use of underutilized and neglected crops.International Plant Genetic Resources.
- PastinkMI., TeusinkB, HolsP, et al. (2009).Genome-Scale Model of Streptococcus thermophilus LMG18311 for Metabolic Comparison of Lactic Acid Bacteria. Appl Environ Microbiol.75: 3627-3633.
- Zisu B, Shah NP. (2003). Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophilus 1275.J. Dairy Sci. 86: 3405-15.
- Dubois M, Gilles KA, Hamilaton JK, Rebers PA, Smith F.(1956). Colorimetric Method for Determination of Sugars and Related Substances. Anal Chem.28: 350-356.
- Audigie CL, Fagerella J, Zonszain F. (1984). Manupulation d’analyse biochimique. Edition Tec et Doc. Lavoisier. 270.
- Chamba JF, Prost F. (1989). Mesure de l’activité acidifiante des bactéries lactiques thermophiles pour la fabrication des fromages à pâtes cuites. 417-431.
- Chamba JF. (1990). Pas de piston pour les bactéries lactiques thermophiles. Revue laitièrefrançaise.492: 47-50.
- RadkeMitchelL C, Sandine WE. (1986).Influence of temperature on associative growth of Streptococcus thermophilusand Lactobacillus bulgaricus. J. Dairy. Sci, 2558-2568.
- Racine M, Dumoht J, Morin. (1991). Production de polysaccharides microbiens sur des milieux glucidiques en surplus au Québec.Centre de recherche, de développement et de transfert technologique en agriculture.1-27.
- Chauhan K, Trivedi U, Patel KC. (2005). Statistical screening of medium components by Plackett-Burman design for lactic acid production by Lactobacillus sp. KCP01 using date juice. BioressourceTechnology. 2005.
- Naveena BJ, Altaf M, Bhadriah K, Reddy G. (2005). Selection of medium components by Plackett-Burman design for production of L(+) lactic acid by Lactobacillus amylophilusGV6 in SSF using wheat bran. Bioresource Technology.96:485-490.
- Djilali B, Bouziane A, Ahmed H, Kada I, Nawal O.(2012). Study of the Behaviour of Lactobacillus delbrueckiiSubsp. bulgaricusin Date Syrupin Batch Fermentation with Controlled pH.J.Biotechnol Biomaterial. 2:129.
- Amrane A. (2001).Lactic acid production during the associated and the deceleration growth phases of Lactobacillus helviticuscultivated in various conditions and media.Physiology, metabolism. Lait. 91-103.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences