Induced Breeding, Embryonic and Larval Development of Comet Gold Fish (Carassius auratus)
S.M. Bazlur Rahaman, Zakaria Mahmud, Faysal Ahmed, Alokesh Kumar Ghosh,Wasim Sabbir
S.M. Bazlur Rahaman, Zakaria Mahmud, Faysal Ahmed, Alokesh Kumar Ghosh* and Wasim Sabbir
Fisheries and Marine Resource Technology Discipline, Khulna University, Khulna-9208, Bangladesh.
Abstract
The present study was aimed to perform induced breeding and to observe embryonic and larval development stages of Comet gold fish (Carassius auratus) in mature males and females by administering hormone of double dose in female and a single dose in male by intramuscular injection of ovaprim at a dosage of 0.5, 0.7, 1.0 and 1.2 ml/kg body weight. Spawning was observed six hours after the injection at ambient temperature (18-22oC). Fertilization rate of eggs were found 50.34, 51.47, 47.30 and 48.24 % respectively with the response of 0.5, 0.7, 1.0 and 1.2 ml/kg of ovaprim. Hatching rates were found 44.35, 43.79, 39.83 and 36.00 % respectively with the same doses. The fertilized eggs were adhesive, translucent and spherical in shape with diameter ranging between 0.9- 1.0 mm and were whitish in color. The hatchlings were transparent and measured 1.3- 1.7 mm of total length with a large oval head, a well defined yolk sac and a short tail. At approximately seven weeks the larvae attained an average total length of 15.2 mm and the fry had acquired some scales, visible pigmentation was general, and the body essentially resembled the adult. Due to good response to synthetic hormone (ovaprim), considerable fertilization and hatching rate, short embryonic period and fast larval development it is possible to conduct breeding program of this species commercially and is suitable for commercial culture.
Keywords
Ovaprim; fertilization rate; embryonic development.
1. Introduction
Ornamental fish keeping is one of the most popular hobbies in the world today. The largest importer of Ornamental fish is the USA followed by Europe and Japan. The emerging markets are China and South Africa. Over US $ 500 million worth of ornamental fish are imported into the USA each year. The major part of this export trade is based on wild collection. In Bangladesh the trade of ornamental fish is confined to its own territory till now. Moreover, it costs a lot of money each year importing ornamental fish to meet the requirement of the country. But domestic production of ornamental fish can save these money and apparently can be regarded as a very potential mean of export earnings. Artificial propagation of fish is done by induced breeding technique. Induced breeding of endemic major carps has been established as a dependable source of fish seeds since the mid 1960’s [1] in hatcheries for production of fry or fingerlings which contributes significantly to the overall aquaculture production of Bangladesh. Usually small fishes, less than 20 cm long, are kept in aquariums and prized for their beauty and rarity is called ornamental fish. In order to sustain the growth it is absolutely necessary to shift the focus from capture to culture based development. Organized trade in ornamental fish depends on assured and adequate supply of demand, which is possible only by mass breeding. India's share in ornamental fish trade is estimated to be Rs 158.23 lakh which is only 0.008% of the global trade. The earning potential of this sector has hardly been understood and the same is not being exploited in a technology driven manner. Considering the relatively simple technique involved, this activity has the potential to create substantial job opportunities, besides helping export earnings [2].
The comet or comet-tailed goldfish is one of the most common varieties of fancy goldfish in the world. It is similar to the common goldfish, except slightly smaller and slimmer, and is mainly distinguished by its long deeply forked tail. This goldfish variety is an excellent choice for beginners. They are an easy fish to keep as they are not picky and will readily eat what is offered. They are basically delicate but very peaceful towards other occupants and hence well suited to aquarium setup. There is various color variation of comets but comet with yellow, orange, red, white, and red-and-white coloration are common. Goldfish, like all cyprinids, are egg-layers. They produce adhesive eggs that attach to aquatic vegetation. The eggs hatch within 48 to 72 hours, releasing fry large enough to be described as appearing like "an eyelash with two eyeballs" [3]. Within a week or so, the fry begin to look more like a goldfish in shape, although it can take as much as a year before they develop a mature goldfish color; until then they are a metallic brown like their wild ancestors. Breeding usually happens after a significant change in temperature, often in spring. Comets are prolific breeders and are bred commercially for sale to pet shops throughout the world and in Bangladesh as well. They are more active than most other goldfish breeds. They are not only bred in outdoor ponds; they frequently reproduce in large aquariums as well. They are naturally inclined to spawn in spring when the water gradually becomes warmer and warmer after the winter. However, this species is not listed on the IUCN (International Union for the Conservation of Nature and Natural Resources) Red List, and presumably there are no wild populations of this captive bred variety [4]. If proper management steps are not taken, this variety of gold fish may become extinct due to inbreeding depression or other problems. Since the goldfish is becoming increasingly important not only from the standpoint of the ornamental fish trade, and as an experimental test animal, but also because of its ability to adapt itself successfully to environmental conditions [5], this study was carried out to highlight some aspects of the early life history of comet gold fish (embryonic and larval stages) as well as induced breeding in context of Bangladeshi environment. In addition this study was a step to observe the influence of lowered water temperature (15- 20 0C) on eggs hatching as well as on larval growth of comet as temperature is the main environmental factor governing the development of fish eggs [6].
2. Materials and methods
Profile of the study area
The experiment was conducted in Fish Physiology Laboratory of Fisheries and Marine Resource Technology (FMRT) Discipline of Khulna University, Bangladesh. All preparation needed for induced breeding of ornamental fish were done in the laboratory. Aquarium set up, water supply facilities, working space etc were assured before the breeding program. The study period was from November 2009 to January 2010.
Preparation of rearing and spawning tank
For induced breeding glass aquarium was used for both rearing and spawning of fish. Two large size glass aquaria (4×1.5×1.5ft) were made and 8 small size glass aquaria (1.5×1.0×1.0ft) of the laboratory were used. The aquarium was supported with filter, stone, continuous air-pump etc. The broods were conditioned in the large tank and after injecting the brood set were separated in the small tank. After fertilizing the fertilized eggs were also kept in the small tank.
Experimental design
There were two broad parts of the activities in this experiment. The first part of the activity concerned with the collection of brood fish. The second part of the experiment was performed in the physiology laboratory of FMRT Discipline, Khulna University to carry out the induced breeding program, embryonic and larval stage development.
Brood fish selection, collection and conditioning
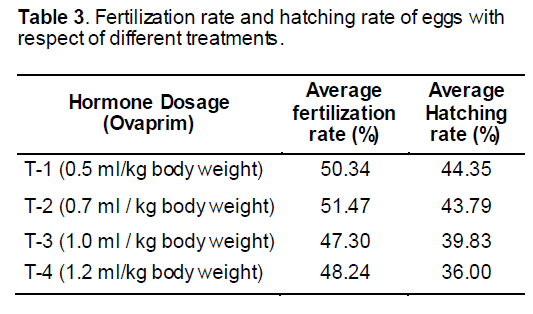
Mature healthy comet gold fish brooders (31.37-72.90 g), were selected by sexual dimorphism for breeding experiments. The brood fish were selected on the basis of size and color pattern. Female is usually easier to spot, as the belly of a mature female is generally plump, whereas male remains streamlined and more torpedo shaped. When males are ready for spawning, they develop breeding tubercles on the head and pectoral fins, principally along the bones of the fin rays. These are used during breeding, when the male nudges the female with its head and fins to induce her to spawn. Eighteen pairs (24 male and 12 female) brood fishes were collected from the local aquarium fish market and from the aquarium fish market of Dhaka. To increase the diversity among the parents and to select healthy brood fish, it was collected from the diversified sources. Avoiding inbreeding problem was also a major objective of selection of brood fish from diversified sources. The brood fishes were carried to the laboratory within 30 minutes and kept in the aquarium (4×1.5×1.5 ft). The brood carrying pack was submerged into the aquarium water for 10 minutes. Then the brood were unpacked and released into the well aerated aquarium (D.O: 4.5-5.5 mg/L, pH: 7.2-7.4; temperature: 15-18°C). The brood was kept 3-5 days here before the breeding program.
Induced breeding
The selected broods collected from the conditioning aquarium were kept on separate aquarium. Continuous air flow was provided in the tanks by aerator. The sex ratio of the spawners was kept at 2:1 for male and female. The breeding program was conducted by a synthetic hormone (ovaprim) administration. The selected brood was weighted in the electric balance and then the hormone was administered. The breeding set was released into separate aquarium after the hormonal administration.
Hormone dose optimization
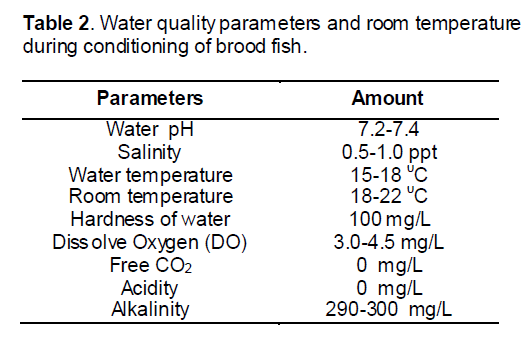
For induced breeding synthetic hormone (ovaprim) was injected to the spawners. The powdered ovaprim preserved in vial was bought from the market. Then distilled water was added (10 ml) to the vial to dissolve it and mixed it very well. The supernatant solution of hormone was then taken for the injection. Female was given two doses and male was given a single dose of injection. Male was injected at the time of second dose given to the female. The doses used in the two different sexes were listed in Table 1.
Stripping and fertilization
Stripping was done very suspiciously. At first female was stripped and eggs were collected in a bowl. In the average time, respective male was stripped and collected milt was mixed well with previously collected eggs. For better result of fertilization physiological saline was used and mixed well with a clean and sterile feather. The inseminated eggs were then transferred in to incubation jar providing with continuous water flow. Fertilized eggs hatched in this condition.
Determination of fertilization rate
After a certain period (1-2hrs) the eggs were examined to observe the fertilization rate. For this purpose 1cc of water sample from bottom of aquarium was taken in a Petri dish from the hatching tank and counted. Then the eggs were observed under a magnifying glass and fertilized eggs were counted with the help of a soft thin brush. The fertilized eggs were easily separated from the unfertilized eggs by the presence of transparent shell with gray/ black spot within the egg shell, while the unfertilized eggs were opaque.
The fertilization rate was determined following the formula:

Determination of hatching rate
To determine hatching rate the samples were collected from the hatching tank and the total number of fertilized eggs in the sample and number of hatchling were counted by visual observations. Then hatching rate was determined following the formula:

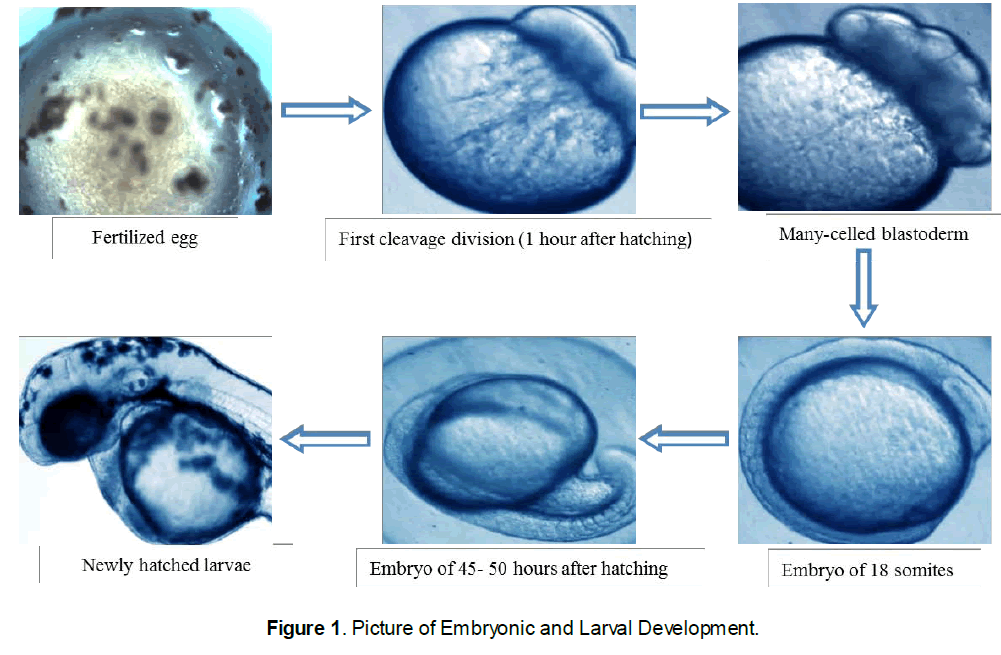
Embryonic and Larval stages observation
Samples of eggs before fertilization and at every 30-min interval were taken for further studies. In the present study, the developmental stages were divided into embryonic, larval and post larval development. The embryonic stage occurs inside the chorion and ends in hatching. The larval stage was characterized by nutritive contribution of the yolk sac and the stage ends when the larva becomes capable of exogenous feeding. The post larval stage begins immediately upon absorption of the yolk sac and was characterized by autonomous feeding. After, the yolk sac absorption, the larvae were fed boiled egg yolk twice daily with zooplankton (Artemia). Developmental time from post fertilization was rounded to the nearest minute until the morula stage and then to the nearest hour. The age of the larvae was denoted as hour after activation.
Descriptions of the developing stages were made by examining live specimens under Electron microscope and microphotographs of the developmental stages of eggs and larvae were taken. For clear observation, individuals were temporarily stained with methylene blue. The specimens were measured by placing them over a slide having 1.0 mm graph paper at the bottom. Five to ten specimens were used to describe each stage.
3. Results
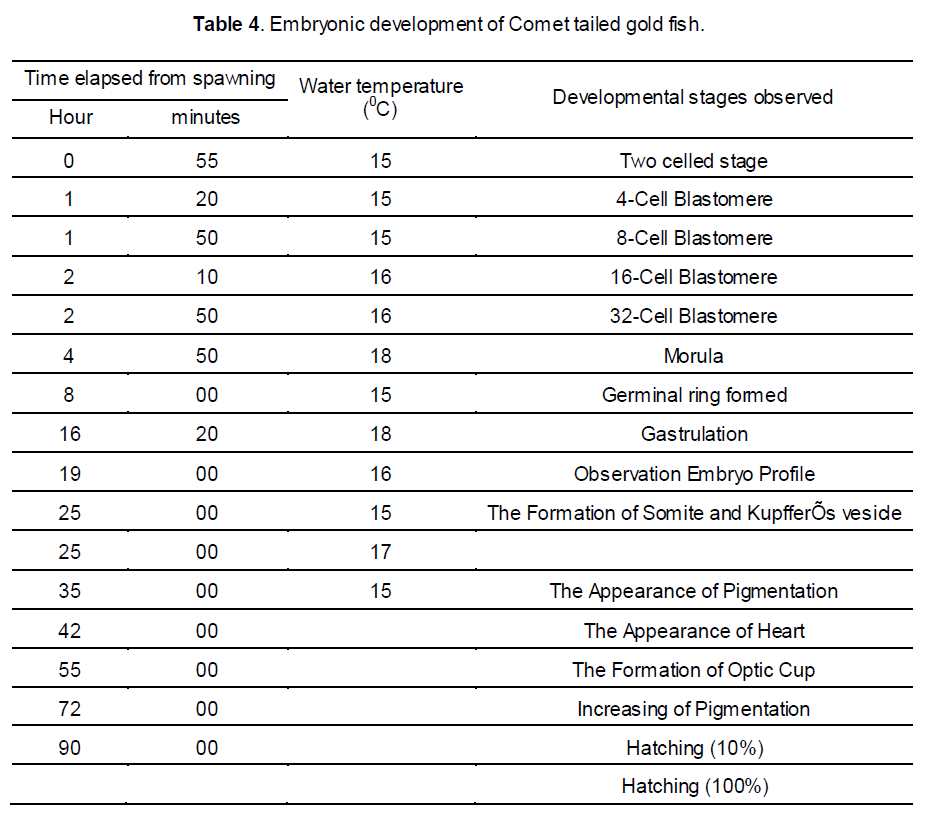
In the present study, spawning behavior observed after 1-8 hours of hormone injection and breeding was performed 5-6 hours after the 2nd dose of injection. After completing spawning, the fertilized eggs of comet gold fish were adhesive, demersal and spherical characteristics in nature. Looking of egg was being transparent, thick and sticky. Those eggs were deposited singly and highly adhesive throughout the incubation period. The yellowish white egg capsule was transparent, while the yolk was pale yellow or green and granular. The eggs of comet gold fish became translucent as development progressed. The diameter of the fertilized egg capsule ranged between 0.9 mm and 1.1 mm while the yolk sphere ranged from 0.5-0.9 mm. In the present study the eggs hatched 48 hrs at a water temperature of 15-180C. The water quality parameters that were maintained during the experiment are given in Table 2.
3.1 Fertilization rate and hatching rate
There were four treatments as T-1, T-2, T-3 and T-4 to determining the fertilization rate of egg. The fertilization rate of egg found by the recent study was 50.34, 51.47, 47.30 and 48.24 % in corresponding to 0.5, 0.7, 1.0 and 1.2 ml/ kg body weight of fish using ovaprim (Table 3). Hatching rate for fertilized eggs was obtained at 44.34, 43.79, 39.83 and 36.00% corresponding to 0.5, 0.7, 1.0 and 1.2 ml hormone administration per kg body weight of the brood (Table 3).
3.2 Embryonic development of comet gold fish
Characteristics of the egg: the spherical pale cream-colored globular eggs of 0.9 to 1.0 mm of the comet fish were found during the current study. These eggs were slightly flattened along the margin of attachment. When first laid the whole surface was adhesive, but this quality was lost as soon as they had become "water hardened" and attached to the aquarium walls and the preset substratum. Due to the adhesive nature of the egg, considerable debris adhered to the capsule of the egg.
Cleavage Stages: a few minutes after fertilization the blastodisc were contracted and a white dome was formed on the upper surface of the yolk. In approximately an hour the first cleavage occurred, forming two large and approximately equal blastomeres. Thirty minutes later the second cleavage appeared. Following this, cleavage becomes irregular and in four hours' time the blastoderm was composed of a mass of relatively small but distinct cells. After six hours of development the cells have became relatively smaller owing to rapid cell division, and the margin of the blastoderm has begun to extend slightly farther over the yolk.
3.3 Differentiation of the Embryo
Fifteen hours: The surface view gave no indication of the embryonic axis aside from a slightly opaque area of undifferentiated tissue passing interiorly along one radius from the margin of the blastopore.
Eighteen to nineteen hours: The axis of the fish embryo was visible as a narrow but rather high transparent ridge extending forward from the blastopore, nearly encircling the yolk, and bounded on either side by the lateral margins of the embryonic shield.
Twenty five hours: Although the blastopore had not yet closed, marked changes had taken place in the anterior axial region of the embryo. The brain was clearly visible and the optic evaginations were somewhat oval extending posteriorly from the primary cerebral lobe.
Thirty hours: The tail reached almost around to the anterior limit of the head, but the embryo were still adherent to the yolk sac at both ends. The notochord extends as a solid rod of cells from the auditory vesicle to the undifferentiated caudal mass.
Thirty five to forty hours: The embryonic body had increased in length, the tail process reaching almost to the anterior limit of the head. Both the head and tail were freed from the yolk surface. A narrow fin fold surrounds the tail and extends forward dorsally to the mid body region.
Fifty to fifty five hours: The embryo had now formed more than a complete circle around the circumference of the yolk. Its tail was lying either to the right or left of the head and was often bent at an angle across the front of the latter. The pectoral fins appeared as fleshy folds lying over the yolk sac at the level of the auditory vesicle.
Sixty five to seventy hours: The embryo had increased considerably in size occupying most of the perivitelline space. Rhythmic movements occured freely within the egg capsule.
Seventy five to eighty hours: Newly born larva of 1.3-1.7 mm was found. The larvae took a sessile form and did not show free movement.
Development of the larva and post larva eventually rupture the egg capsule. Total length was 1.3-1.7 mm No visible pigmentation was found. The yolk sac beard large scattered
Newly-hatched larva: The larva freed itself by violent lashing movements of the tail which melanophores more especially anteriorly. Swimming movements were somewhat restricted owing to the mass of yolk material. The larva shows a positive thigmotropism, adhering to the aquarium walls. The heart was differentiating into chambers although it is still almost vertical in position at the anterior end of the yolk sac.
11-13h old larva: After 11- 13 hrs of hatching the fin folds were seen continuously around the tail. The vent and gill rudiments were formed. Gut was straight to slightly curve in anterior portion. Air bladder was shallow, behind pectoral region, which develop into two chambers in the post larval stage. Pigmentation was more pronounced throughout the head and body. The larvae attained a very little free movement with the help of fins.
2-4 day’s old larva: The total length of the larvae was 2.5- 2.9 mm. The embryo showed a distinct reduction in the size of the yolk sac which had now become almost tubular due to its greater absorption anteriorly. Pigmentation resembled that of the recently hatched larva except for an increase in density especially in the eye, and the appearance of heavy masses of yellow pigment spots (xanthophores) along the dorsal musculature and over the head.
7-8 day’s old larva: Total length of the larvae was 4.4- 4.8 mm. The mouth has enlarged and the lower jaw moves rhythmically. The posterior end of the notochord had become bent upward slightly and rudiments of caudal fin rays were evident in the fin fold among the melanophores below the curved notochord.
15-18 day’s old larva: Total length became 5.8- 6.5 mm but the larva still did not resemble the parents. The caudal fin had forked into dorsal and ventral lobes supported by unbranched fin rays. The otoliths had become almost as large as the lens of the eye.
35 day’s old larva: The caudal fin showed little advancement over the 6.5 mm. stage, but the dorsal now beard nine true rays. The median fin fold was beginning to disappear dorsally between the dorsal and caudal fins and between the caudal and anal fins ventrally. The anal and pectoral fins had developed distinct rays. The pelvic fins had appeared as minute lateral fleshy protuberances from the body wall midway between the pectorals and anus.
4. Discussion
In many papers dealing with induced breeding of fish that was the aims of our study [7,8,9,10,11,12]. But only a limited number of papers are related about induced breeding of comet gold fish [8,9]. Experiments were conducted in December with temperature around 18-20°C. Specially induced breeding occurred with temperature around 28 0C [11]. Little showers of rain and weather are conducive for breeding [13]. In that factor experiments were conducted flow of water by aerator. In this study only a single dose of ovaprim were used for induced breeding within 6 hr while the control females were given two doses of extract. The hatching rate may be affected by various factors, such as temperature, rate of water flow, water quality etc. The optimum temperature range for gold fish is 15 °C to 28°C [14]. The hatching and fertilization rate are slightly affected by different doses of hormone. Finding of the present study indicates that both the hatching rate and the fertilization rate were increased in treatment 2 (T-2). The incubation period of eggs depends largely on water quality parameters such as salinity and temperature [15,16]. Khan and Hamid [17] found the hatching period to vary from forty-six to fifty-four hours at 29 °C. Innes [18] reports a hatching period of from four to fourteen days according to temperature. Fertilization and hatching rates also differ with the condition of brood. The diameter of the fertilized egg capsule found in this study ranged between 0.8 mm and 1.00 mm while the yolk sphere ranged from 0.5- 0.7 mm while Haniffa et al [12] found that the fertilized eggs were adhesive and transparent with diameter ranging between 0.9 mm and 1.10 mm. This slight variation can be caused by smaller size brood fish than the brood size selected for the study by [12].
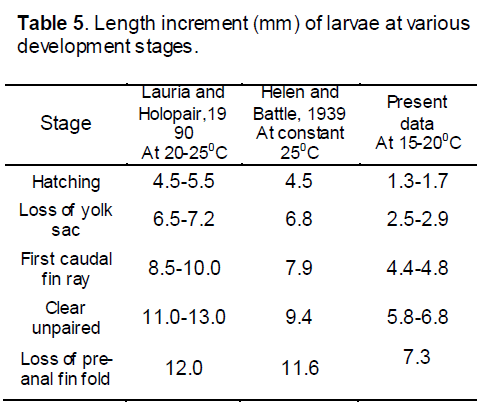
The comet goldfish is typical of the teleosts in its general development. The eggs which were very much adhesive in nature and were 0.9- 1.0 mm in diameter and hatch in approximately 90 hours at 15- 20°C (Helen and Battle [5] found eggs of 1.25 to 1.46 mm. in diameter and hatch in sixty five hours at 25 0C.) the difference in diameter of eggs may be due to the brood size and environmental variation. The first cleavage during our study took place an hour after fertilization, and in fifteen hours the blastoderm had completely encircled the yolk with the exception of a small spherical blastopore. In sixteen hours heart was found beating rhythmically. Further development to hatchling involved pigmentation by melanophores, enlargement of all the embryonic structures, and an elongation of the yolk sac posteriorly. At hatching the larva was 1.3- 1.7 mm in length, and restricted in movement by the weight of the yolk sac. By two to four days, a length of 2.5- 2.9 mm was attained, and the yolk sac had been reduced to a narrow tubular band (Hubbs [19], defined this stage as post-larva as the stage began immediately after absorption of the yolk sac that last as long as the structure and form are unlike that of fry). Yellow pigmentation had appeared. The air bladder was partially inflated. At seven to eight days (4.4- 4.8 mm stage), the yolk material had practically disappeared. Rudiments of caudal fin rays were evident then. The operculum practically covered the gills and the liver was present as a triangular mass at the anterior end of the body cavity. The 5.8- 6.5 mm stage (age 15 to 18 days) showed increased pigmentation and reduction of melanophores to blackish spheres in the head region. The air bladder had divided into two chambers. By 35 days (10.5 mm stage) the organism became more opaque. The pelvic fin buds have just appeared and fin rays are present in all the other fins. Pigmentation is concentrated above the lateral line. At approximately nine weeks a 15.2 mm of total length was attained and the fish has acquires scales, pigmentation is general, and the body essentially resembled the adult.
Changes in the pattern of the entire structure of an organ in relation to the environment are decisive for evaluating the developmental patterns of species [20]. The early development of fish is strongly affected by incubation temperature. Generally, lower temperature retards the rate of embryonic development of fish and higher temperature accelerates it [21]. During my study period the ambient temperature was low and fluctuating. This might retard the embryonic and larval development of comet fish during my study period. A comparative study is given bellow on the study of embryonic and larval development of gold fish and comet fish at different temperature.
During this study, we found a varied incubation period for eggs of comet. Although all eggs for the developmental study were collected from a single pair of parent, but the incubation period for all the eggs was not the same. The hatching of larvae started after 76± 3 hours of hatching and the process continued up to 90 hours after hatching. Furthermore, during my study, I found that the comet’s eggs were very adhesive with a special adhesive layer on it. A detailed analysis on the structure of the chorion is needed to discern how the eggs adhere and if they have any functional effect on the embryonic and larval development of this fish.
5. Conclusion
From the available references along with my present investigation on the induced breeding of the comet fish, it was observed that higher percentages of fertilization and hatching were achieved from a comparatively lower dosage of hormone. Steel plate for fertilization must be avoided because steel plate reduces the fertilization rate and creates spoilage of huge number of eggs. In this induced breeding process quality hormone should be used to ensure better production. Proper knowledge on the embryonic and larval development will help us to optimize the problem and led us to a sustainable management of comet. Further studies on embryological development of ornamental fishes will give an opportunity to learn their development stages in detail and this would be helpful for the commercial production of this elegant fish.
Acknowledgements
The authors would like to thank to Khulna University Research Cell and Fisheries and Marine Resource Technology Discipline for their help during the research trials and for financial support.
References
- Ali M.H. (1967) Induced breeding of major carps in ponds by pituitary hormone injection. Agriculture Information Survey, Dhaka.
- Food and Agricultural Organization (FAO) (2000) World status of ornamental fish, 3: 5-225.
- Lauria S., Piironen J., Holopainen I.J. (1987) Notes on egg development and larval and juvenile growth of crucian carp (Carassius carassius (L.)). Annales Zoologici Fenn, 24: 315-321.
- Andrews D.C. (2002) An Interpet Guide to Fancy Goldfish. Interpet Publishing IUCN 1-902389, p64.
- Helen I., Battle H.I. (1939) The embryology and larval development of the goldfish (Carassius auratus) from Lake Erie. Department of Zoology, University of Western Ontario.
- Gamal A.H., El-Greisy Z.A. (2008) Effect of Temperature on Hatching and Larval Development and Mucin Secretion in Common Carp, Cyprinus carpio (Linnaeus, 1758), National Institute of Oceanography and Fisheries, Anfoushy, Alexandria, Egypt. Global Veterinaria, 3(2): 80-90.
- Bhuiyan K., Kumar D., Haniffa M.A. (2006) Induced spawning of puntius gonionotus (bleeker). Journal of biological sci, 14: 121-125.
- Yamamoto K.Y., Nagahama Y., Yamazaki F. (1966) A method to induce artificial spawning of goldfish all through the year. Bulletin of Japanese Social Science of Fish, 32: 977-983.
- Fernando A. A., Phang V.P.E. (1985) Culture of the guppy, Poecilia reticulata, in Singapore. Aquaculture, 51: 49-63.
- Haniffa M.A., Sridhar S. (2002) Induced spawning of spotted murrel (Channa punctatus) and catfish (Heteropneustes fossilis) using human chorionic gonadotropin and synthetic hormone (ovaprim). Vertebrate archive, 72: 51-56.
- Rokade P., Ganeshwade R.M., Somwane S.R. (2004) A comparative account on the induced breeding of major carp Cirrhina mrigala by pituitary extract and ovaprim. Journal of Fish Biol, 27(2): 309-310.
- Haniffa M.A., Benziger P.S.A, Arockiaraj A.J, Nagarajan M., Siby P. (2006) Breeding Behaviour and Embryonic Development of Koi Carp (Cyprinus carpio), Journal of Bio-Sci, 14: 121-125.
- Kucharczyk D., Kujawa1 R., Mamcarz1 A., et al. (1995) Induced spawning in bream (Abramis brama L.) using pellets containing GnRH. Czech Journal of Animal Sci, 50(3): 89-95.
- Haque M.R. (1997) Management of an ideal carp hatchery in Quality Assurance in Induced breeding. Department of Fisheries, Jessore, Bangladesh, pp. 31-37.
- Ku C.M., Shehade Z.H., Milison K.K. (1973) A preliminary report on the development, growth and survival of laboratory reared larvae of the grey mullet, Mugil cephalus (L.). Journal of Fish Biol, 5: 459-470.
- Liao I.C. (1975) Experiments on the induced breeding of the grey mullet in Taiwan from 1963-1973. Aquaculture, 6: 31-58.
- Khan M., Hamid M. (1929) Early stages in the development of the goldfish. (Carassius auratus). Journal of the Bombay Natural History Society, Vol. XXXIII: 614- 617.
- Innes W.T. (1936) The complete aquarium book. Innes Publishing Company, Phila. 15th edition, pp. 212.
- Hubbs C.L. (1943) Terminology of early stages of fishes. Copeia 4, pp. 260.
- Balon E.K. (1999) Alternative ways how to become a definitive phenotype or a juvenile (and on some persisting linguistic offences). Environment and Biology of Fish, 56: 17-38.
- Saka S., Forat K., Kamaco H.O. (2001) The development of European sea bas (Dicentrarchus labrax L., 1758) eggs in relation to temperature. Turkish Journal of Veterinary and Animal Sci, 25: 139-147.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences