In vitro conservation under slow growth conditions of two rare plant species from Caryophyllaceae family
Rodica Catan, Elena Monica Mitoi, Florena Helepciuc, Irina Holobiuc
Institute of Biology,296 Spl. Independentei,060031,Bucharest,Romania.
- Corresponding Author:
- Rodica Catană
Institute of Biology
296 Spl. Independentei,060031
Bucharest,Romania.
Tel: +4(0)21 2239072
Fax: +4(0)21 2219071
E-mail: catanarodica@yahoo.com
Abstract
The purpose of this study was to evaluate the aspects of medium term conservation under slow growth conditions of two rare plant species from Caryophyllaceae family: Gypsophila petraea (Baumg.) Rchb. and Dianthus callizonus Schott et Kotschy. Different methods like reducing carbon source, reducing mineral concentration and temperatures (25°C and 10°C) were tested. The evaluated parameters were represented by the healthy shoots and rhizogenesis. The presence of hiperhidricity was observed. To check the genetic stability of the shoots maintained under slow growth conditions, the biochemical analysis were performed after 6 months. The physical factors (lack of nutrients and temperature) changed the enzymatic spectra, but the biochemical analysis did not show differences between the clones cultured on the same media variant. The 10°C and the reducing of micro- and macronutrients at 1/4 for G. petraea and at 1/10 for D. callizonus allowed the conservation in optimal parameters for more than 12 months.
Keywords
in vitro conservation; slow growth conditions; rare plants; genetic stability; enzymatic spectra.
1. Introduction
According to the World Conservation Union,over 8,000 plant species are threatened with extinction. The are several approaches that can help to save the plant species like: the documentation of global distribution and conservation status of the plant species,the protection of the areas,the education of people about the values of the biodiversity,the existence of national and international legislation etc [1].
In the last decades,the ex situ conservation methods have an important role in the conservation of threatened plants. The three main methods used of ex situ conservation are represented by: maintaining living plants in cultivation,in vitro conservation (short and medium-term conservation) and cryoconservation (long-term conservation).
A proper medium term conservation protocol involves those methods which allow the reduction of plant growth in order to increase the intervals between subcultures [2] and to minimize the use of plant growth regulators,osmolites and other growth retardants [3]. The maintenance of the viability and the genetic stability is the goal of any in vitro conservation approach.
Regarding the priority in conservation according to taxonomic categories,the rare plant species have the highest priority induced by their restrictive area [4]. Also,the endemic (restricted in distribution) to small sites or require specialized habitats are listed as in danger [1] and requires different conservation methods (in situ and/or ex situ).
G. petraea and D. callizonus are the two rare plant species studied. The distributional range of G. petraea (sub-endemic) is Bulgary and Romania [5]. D. callizonus is endemic in Romania (Piatra Craiului Massif) [6]. Both are included in the list of endemic plant species recorded within Carpathian alliances [7] and are cited like rare plant species in the red list of superior plant species from Romania [8].
The present paper deals with investigations related with in vitro conservation under slow growth conditions at two rare plant species: G. petraea and D. callizonus.
According to the authors knowledge this is first description into the field of in vitro conservation under slow growth conditions without using retardants or other osmolites and also in the field of the evaluation of the effects of slow growth conditions on the genetic stability at biochemical level.
2. Material and Methods
In vitro cultures of the two rare plant species were initiated starting from in vitro germinated seeds. The explants (0.5 cm shoots) from the same clone were multiplied by direct organogenesis based on the established protocols [9,10]. The experiment (10 replicates/treatment) was planned to evaluate the responses of the plant species under slow-growth conditions,using seven different media variants (Table 1) and two different temperatures (25°C and 10°C). The media variants used were based on MS formula [11] supplemented with B5 vitamins and 8g/l Plant Agar (Duchefa) without growth factors,retardant factors or other osmolites.
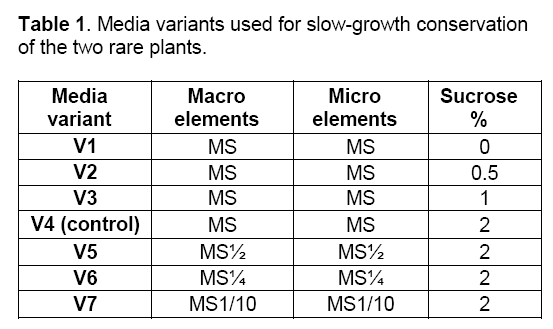
The methods tested in this experiment were represented by reducing the carbon source (V1-V3 media variants),reducing the mineral concentration (V5-V7 media variants) [12] (Table 1) and two different temperatures for storage 25º and 10ºC [13,14,15]. The V4 media variant is the control variant represented by 2% carbon source.
In order to evaluate the in vitro aspects of medium-term conservation,the health of shoots and rhyzogenesis were registered. The health of shoots is referred to the viable and green explants. The both parameters were calculated as percentage.
The in vitro cultures were maintained 12 months with 3 subculture passages for the cultures stored at 25ºC and 2 subculture passages for the cultures stored at 10ºC.
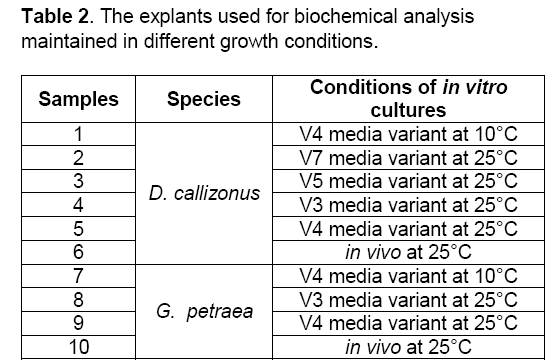
The biochemical analyses were performed after 6 months of in vitro culture under different slow growth conditions. The samples used for biochemical analysis are presented in Table 2.
The pattern of izoenzymes (peroxidases,esterases,glutamate oxaloacetate transferase and malate-dehidrogenase) was studied in the explants prelevated from in vivo and from plantlets maintained in different slow growth conditions. The extraction of soluble cytosolic proteins was performed by grinding the plantlets in 0.1 M phosphate buffer,pH 7 at 40C. After centrifugation at 15000 rpm for 10 min,the supernatant was used for electrophoretic analysis. Electrophoreses were carried out by the samples migration at 90/120V,5h,in a discontinuous system using a running gel 8% PAA (polyacrylamide) a stacking gel 4% PAA and a buffer Tris-Gly 0.05M,pH 8.3. After electrophoresis for locating esterase activity,it was used 0.2 % α- and β-naphtyl phosphate as substrate and 0.05% Fast Blue BB in 0.1M phosphate buffer,pH 6.5. The bands were stained in red. For peroxidase activity,0.08% benzidine in 0.5 M acetate buffer,pH 5 and hydrogen peroxide was used. 10 minutes incubation in 40% methanol and 10% trichloroacetic acid,followed by a stained with Coomasie Brilliant Blue for 12 hours were used for proteins. A mixture of PVP,EDTANa2,aspartic acid and cetoglutaric acid was used to view the glutamate oxaloacetate transferase. For malate-dehidrogenase it was used an incubation mix of NBT,PMS and MTT in a Tris buffer added with malic acid,MgCl2 and NAD.
The evaluation of the effects of slow growth conditions on the genetic stability at biochemical level was achieved by observing similarities and dissimilarities between the samples maintained 6 months in different in vitro conditions.
3. Results
Reducing carbon source and mineral concentrations
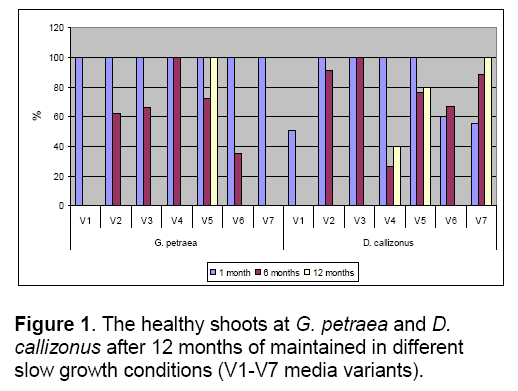
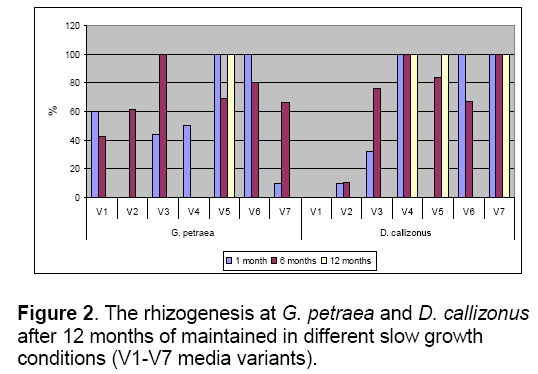
In the case of G. petraea species,reducing of carbon source concentration had effects on the morphological and biochemical aspects. The control media variant (V4) allowed the preservation with a good rate of shoots’ health (100%) for 12 months but with low rate of rhizogenesis (45%) (Figure 1,2). The lack of sucrose (V1 media variant) stopped the explants growth. After 60 days of in vitro cultures all the explants of both species degenerated on the media with no sucrose.
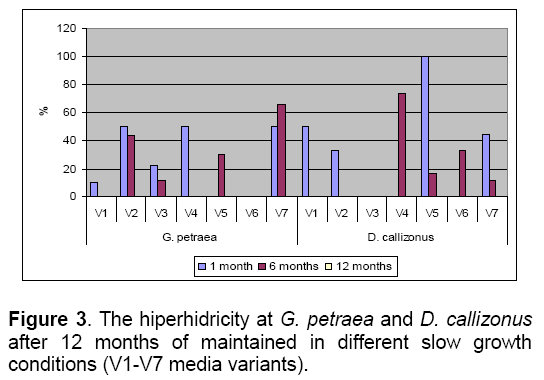
The hiperhidricity process is present in different percents in all explants maintained of the variants with reduced carbon source (V1 - V3) and mineral concentration (V5 - V7) compared with the control variant (Figure 3).
The V6 media variant (level of mineral concentration reduced at 1/2) allowed the conservation of the species with no hiperhidricity,best shoots viability and rhizogenesis (100%). Having good results concerning the evaluated parameters,the V6 media variant is the only one media variant from the variants tested which allowed the maintenance of medium term cultures for over 1 year (Figure 1).
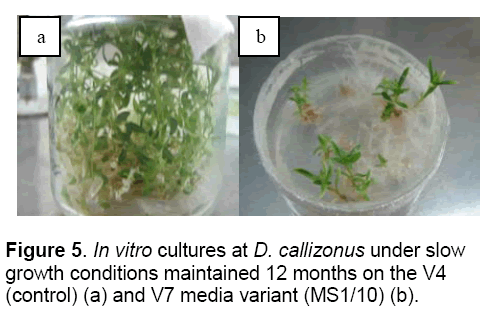
From the all media variants tested,only the V5 (nutrients reduced at 1/2) and V7 (nutrients reduced at 1/10) media variants allowed the medium term conservation of D. callizonus for more than 12 months (Figure 1,4,5).
Starting with the 6th month,the shoots maintained in these slow growth conditions were characterized by hiperhiricity in different percents (11-73% for D. callizonus and 11-65% for G. petraea). The rhizogenesis was less developed on the media with reduced carbon source (V1-V3) than on the media with reduced mineral concentration (V5-V7) for both species (Figure 2 ).
Different temperatures
In order to limit the growth of the two rare plant species analyzed,two different temperatures were used. Between different methods of in vitro conservation under slow growth conditions,the change of the storage temperature is the method most used.
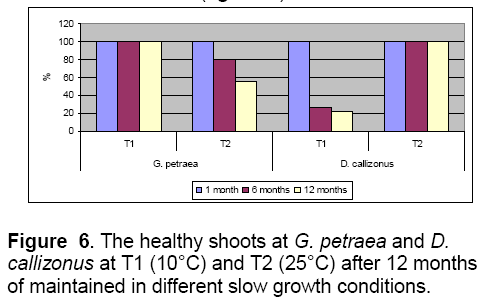
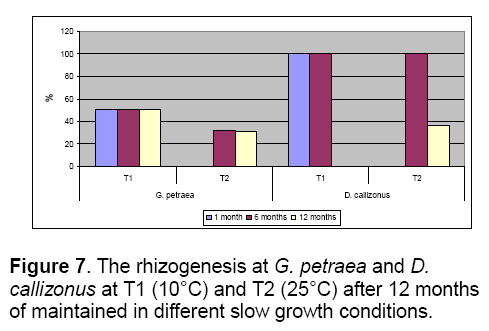
The temperature represented by 10°C allowed the maintenance of G. petraea more than 12 months in optimal parameters,with a good viability and rhizogenesis rates (Figure 6 ,7). The explants maintained for 12 months on the V4 (control) at 25°C were characterized by 100% of hyperhidricity compared with the explants maintained at 10°C (Figure 6 ).
Also,the 10°C temperature was proper for maintaining D. callizonus for more than 12 months with a good viability rate (100%) comparing with the explants maintained at 25°C (20%) (Figure 6 ).
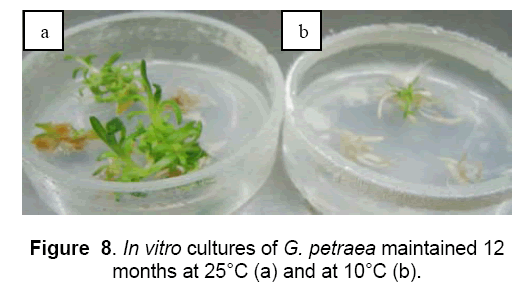
The shoots length of both species was reduced at half comparing with the control temperature (25°C) (Figure 8 ,9).
To check the genetic stability of the shoots under slow growth conditions,the biochemical analysis were performed. Although the DNA-based molecular techniques have become increasingly popular in the study of taxonomy and conservation of the endangered plant species,the enzymes spectra are still the basic method for check the genetic stability of the conserved plant species because are able to identify the somaclonal variability arising from the changes induced by in vitro culture method.
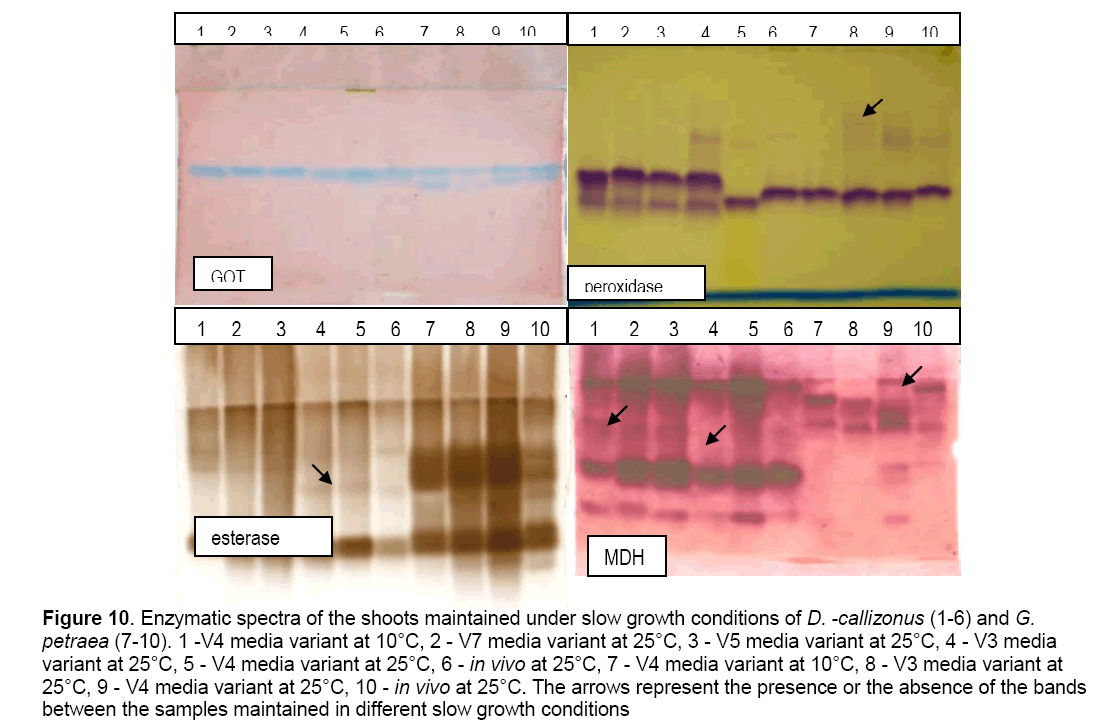
The enzymatic spectra (1.GOT,2. peroxidase,3. esterase,4. MDH) of the G. petraea and D. callizonus shoots maintained under slow growth conditions are showed in the Figure 10. The physical factors (lack of nutrients and temperature) had some influences on the enzymatic spectra. The biochemical analysis of the samples maintained on the same kind of treatment (reduced sucrose concentration or reduced mineral level) did not shown differences in the enzymatic spectra.
Figure 10: Enzymatic spectra of the shoots maintained under slow growth conditions of D. -callizonus (1-6) and G. petraea (7-10). 1 -V4 media variant at 10°C, 2 - V7 media variant at 25°C, 3 - V5 media variant at 25°C, 4 - V3 media variant at 25°C, 5 - V4 media variant at 25°C, 6 - in vivo at 25°C, 7 - V4 media variant at 10°C, 8 - V3 media variant at 25°C, 9 - V4 media variant at 25°C, 10 - in vivo at 25°C. The arrows represent the presence or the absence of the bands between the samples maintained in different slow growth conditions
The glutamate oxaloacetate transferase and malate-dehidrogenase patterns relived that there are no significant differences between samples maintained in different slow growth conditions used.
The peroxidase pattern,which is an inducible enzyme,showed that there are differences between the in vivo and in vitro plants in the case of G. petraea. The peroxidase expression depends on the culture condition,physiological,developmental stages [16,17] and oxidative stress [18,19].
The pattern of esterases showed minor differences between samples for both plant species analyzed. These differences were observed between samples maintained on the media variants characterized by different physical factors. In the case of D. callizonus differences were observed between samples maintained in different conditions like: sample 3 maintained on the media variant V5 characterized by reduced mineral level,sample 4 maintained on the V3 media variant characterized by reduced sucrose concentration and sample 6 (in vivo condition).
It is important to underline that no differences were observed between the samples maintained on the same media variant characterized by reduced mineral concentration (samples 2 and 3).
At G. petraea,in the pattern of esterases were observed differences between all the samples maintained in the all different conditions (in vivo,in vitro,low temperature) used.
Concerning the samples maintained at 10°C,minor differences and only in the intensity expression can be observed compared with the sample stored at 25°C (samples 1 and 5 - D. callizonus ; samples 7 and 9 - G. petraea) (Figure 10).
4. Discussions and Conclusions
In this paper we report the establishment of an efficient medium term preservation protocol based on slow growth conditions for two rare plant species from Caryophyllaceae family. It was showed that the slow growth procedures allow clonal plant material to be held in in vitro conditions with periodic sub-culturing depending on the species [20]. In this proposed species like Musa spp.,potato,sweet potato,cassava,yam,Allium spp. and temperate tree species are routinely preserved using these kinds of methods [21]. Concerning the storage at low temperature,the first report of successful in-vitro storage was of shoot tips of Vitis rupestris stored up to 290 days at 9°C [22].
There are some previous data regarding the germplasm conservation of Caryophyllaceae ornamentals using cryoconservation [23,24] and using media variant added with different mannitol concentration for some rare Dianthus species [25,26,27].
Our results concerning the biochemical analysis of the samples maintained on the same kind of treatment (reduced sucrose concentration or reduced mineral level) did not shown differences in the enzymatic spectra. Similar results were obtained at in vitro regenerants of Marsilea quadrifolia [28]. Also,in the case of the species Veronica multifida ssp. capsellicarpa maintained for 8 months in a medium- term protocol based on media variant supplemented with mannitol showed no differences between regenerants [29].
Figure 10. Enzymatic spectra of the shoots maintained under slow growth conditions of D. -callizonus (1-6) and G. petraea (7-10). 1 -V4 media variant at 10°C,2 - V7 media variant at 25°C,3 - V5 media variant at 25°C,4 - V3 media variant at 25°C,5 - V4 media variant at 25°C,6 - in vivo at 25°C,7 - V4 media variant at 10°C,8 - V3 media variant at 25°C,9 - V4 media variant at 25°C,10 - in vivo at 25°C. The arrows represent the presence or the absence of the bands between the samples maintained in different slow growth conditions
Our data concerning the in vitro conservation of rare plant species using slow growth conditions are in accordance with those reported by Botău et al.,2005. In this work a cheap protocol of slowing plants growth that uses in vitro cultures and low temperatures (16°C and 24°C) was established [30]. Also,slow growth conditions (reduced mineral and sucrose concentrations) were used to preserve 14 species of Dioscorea spp. more than 2 years with passages at 6-8 months [31]. Low temperature storage of in vitro cultures appears to be a highly promising method for storage of Rauvolfia serpentina Benth. ex Kurz [32] which was stored 15 months at 15°C. An efficient medium term preservation protocol based on reduced mineral concentration at 1/4 for Gypsophilla petraea (Baumg.) Reichenb and 1/10 for Dianthus callizonus Schott&Kotschy at 25°C was established. We showed that the storage at 10°C is a good way to maintain the analyzed species for more than 12 months with a reduced number of subcultures (2). Because no significant differences were observed between the samples maintained in slow growth conditions,these two rare plant species may be preserved by in vitro culture medium term without genetically changes at biochemical level.
Acknowledgements
This study was supported by the grant of the National Council of Scientific (CNCSIS-UEFISCSU) Romania research grant for Ph.D. student no. 227/2007.
References
- Farnsworth E.,Sahotra S. (2007) Conservation and management of rare plant species. In Encyclopedia of Earth (Eds. Cutler J. Cleveland Environmental Information Coalition,National Council for Science and the Environment),published in the Encyclopedia of Earth,Washington,D.C.
- Engelmann F. (1991) In vitro conservation of horticultural species. Acta Horticulturae 298,Postharvest Physiology of Ornamentals,327: 1-2.
- Jarret R.L.,Gawel N. (1991) Abscisic acid-induced growth inhibition of sweet potato (Ipomoea batatas L.). In vitro Plant Cell Tissue and Organ Cultures,24:13-18.
- Jonhnson N.C. (1995) Biodiversity in the balance. Approaches to setting Geographic Conservation priorities. Biodiversity Support program. Corporate Press. Landover,Maryland.
- Tutin T. G.,Heywood V. H.,Burges N. A.,et al. (eds) (1964–1980): Flora Europaea. Vol. 1–5. Cambridge Univ. Press,Cambridge
- Dihoru Ghe.,Negrean G. (2009) Cartea rosie a plantelor vasculare din Romania,Editura Academiei Romane,Bucuresti,Romania.
- Kukuła K.,Okarma Jerzy H.,Kajetan P.,Perzanowski T.,Ruzicka J.,Viera S.,Stanova L.,Vlasin T.M. (2003) In Carpathian List of Endangered Species (Witkowski Z.J.,Król W.,Solarz W.),WWF and Institute of Nature Conservation,Polish Academy of Sciences,Vienna-Krakow,67.
- Oltean M.,Negrean G.,Popescu A.,Roman N.,Dihoru Gh.,Sanda V.,Mihăilescu S. (1994) Lista roşie a plantelor superioare din România. Studii,Sinteze,Documentatii de Ecologie,Academia Romana – Institutul de Biologie,Bucureşti.
- Păunescu A.,Holobiuc I. (2003) Conservation of the endemic species Dianthus callizonus Schott et Kotsky using in vitro techniques. Rev. Roum. Biol.,sér. Biol. Végét.,48(1): 3-7.
- Blîndu R.,Holobiuc I. (2007) Contributions to ex situ conservation of rare plants from Piatra Craiului Massif using biotehnology. Conference Proceedings the 1st International Conference Environment – Natural Sciences – Food Industry in European,1: 483-788.
- Murashige T.,Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant,15(3): 473-497.
- Ng S.Y.C.,Ng N.Q. (1991) Reduced growth storage of germplasm. In In vitro methogs for conservation of plant genetic resources,JH Dodds,Chapman and Hall,London.
- Dale P.J. (1980) A method for in vitro storage of Lolium multiflorum Lam. Ann. Bot.,45: 497-502.
- Henshaw G.G.,O’Hara J.F.,Stamp J.A. (1985) Cryopreservation of plant cells and organs In KK Kartha,ed.,CRC Press,Boca Raton,FL.
- Ruredzo T.J.,Hanson J. (1991) In vitro conservation. In Crop genetic resources of Africa (F Attere,E Zedan,NQ Ng,P Perrino,(eds.),1,Trinity Press,U.K.
- Halmagyi A.,Butiuc-Keul A. (2007) Conservarea resurselor genetice vegetale,Ed. Todesco,Cluj – Napoca.
- Holobiuc I.,Blîndu R. (2006) Improvement of the micropropagation and in vitro medium-term preservation of some rare Dianthus species. Contributii Botanice,41(2): 143-151.
- Holobiuc I.,Blîndu R.,Mitoi M.,Helepciuc F.,Cristea V. (2009) The establishment of an in vitro gene bank in Dianthus spiculifolius Schur and Dianthus glacialis ssp. gelidus (Schott Nym. Et Kotschy) Tutin: I. The initiation of a tissue collection of the cultures in minimal growth conditions. Annals of Forest Research,52: 14-26.
- Holobiuc I.,Mitoi M.,Blindu R.,Helepciuc F. (2010) The establishment of an in vitro gene bank in Dianthus spiculifolius Schur. and D. glacialis ssp. gelidus (Schott Nym. et Kotschy) Tutin: II. Medium-term cultures characterization in minimal growth conditions. Romanian Biotechnological Letters,15(2): 5111-5119.
- Gaspar T.,Penel,C.,Hagege,D.,Grepin,H.,(1991) Peroxidases in plant growth,differentiation and development processes. In Biochemical Molecular and Physiological aspects of Plant peroxidases (Lobarzewski,J.,Grepin,H.,Penel,C.,Gaspar,T.),Univ. M. Currie-Sklodowska,Lublin,Poland and Univ. of Geneve,Switzerland,249-280.
- Gaspar T.,Kevers,C.,Hausman,F.,Penel,C.,Jouve,L.,Martin-Tanguy,J.,Aribaud,M.,Greppin,H. (1996) Peroxidase as an indissociable factor of auxin and polyamine metabolisms in the induction of rooting and flowering. In Plant Peroxidases: Biochemistry and Physiology (Obinger,C.,Burner,U.,Ebermann,R.,Penel,C.,Greppin,H.),University of Geneva,226-234.
- Davletova S.,Rizhsky L.,Liang H.,Shengqiang Z.,Oliver D.J.,Coutu J.,Shulaev V.,Schlauch K.,Mittler R. (2005) Cytosolic ascorbate peroxidase I is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell,17: 268-281.
- Malecka A.,Jarmuszkiewicz W.,Tomaszewska B. (2001) Antioxidative defense to lead stress in subcellular compartments of pea root cells. Acta Biochimica Polonica,48(3): 687-698.
- Jarret R.L.,Gawel N. (1991) ABA-induced quiescence of sweetpotato in vitro. Hortsci,26(6): 737.
- Rao K. (2004) Plant genetic resources: Advancing conservation and use through biotechnology. African Journal of Biotechnology,3(2): 136-145.
- Galzy R. (1969) Recherchez sur la croissance de Vitis rupestris scheele sain et court noice cultivé in vitro à differentes temperatures. Am Phytopathol,1: 149.
- Fukai S.,Goi M. Tanaka M. (1991) Cryopreservation of shoot tips of Caryophyllaceae ornamentals. Euphytica,56(2): 149-153.
- Banciu C.,Carasan M.E.,Brezeanu A. (2009) In vitro propagation of the endangered species Marsilea quadrifolia L. - morphological and biochemical analysis of the regenerates. Romanian Biotechnological Letters,14(1): 4139-4145.
- Holobiuc I.,Blîndu R.,Carasan M.,Helepciuc F.,Voichiţă C.,Negrean G. (2008) In vitro conservation strategy in Veronica multifida ssp. capsellicarpa (Dubovik) A. Jelen. Romanian Journal of Biology - Plant Biology,53(2): 71-81.
- Botau D.,Danci M.,Danci O. (2005) In vitro medium term preservation of different Romanian landraces. Acta Biologica Szegediensis,49(1-2): 41-41.
- Malaurie B.,Pungu O.,Trouslot M.F. (1995) Effect of growth regulators concentrations on morphological development of meristem - tips in Dioscorea cayenensis – D. rotundata complex and D. praehensilis. Plant Cell Tissue Organ Cult,41(3): 229-235.
- Sharma N.,Chandel K.P.S. (1992) Low temperature storage of Rauvolfia serpentina Benth. ex. Kurz: an endangered,endemic medicinal plant. Plant Cell Reports,11: 200-203.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences