Immobilization of ÃÆà ½Ãâñ-amylase from Bacillus subtilis SDP1 isolated from the Rhizosphere of Acacia cyanophlla Lindey
Eda Bal, Orkun Pinar, Dilek Kazan, Hasan Umit Öztürk, Ahmet Alp Sayar
1Marmara University, Faculty of Engineering, Department of Bioengineering, Goztepe Campus, 34722, Kadikoy, Istanbul, Turkey
2Tubitak MRC Genetic Engineering and Biotechnology Institute PK: 21, 41470 Gebze, Kocaeli, Turkey
- Corresponding Author:
- Dilek Kazan
Tel: +90(216)3480292
Fax: +90(216)3480293
E-mail: dkazan@marmara.edu.tr
Received date: February 10, 2016; Accepted date: February 17, 2016; Published date: February 23, 2016
Citation: Bal E, Pinar O, Kazan D, et al., Immobilization of α-amylase from Bacillus subtilis SDP1 isolated from the Rhizosphere of Acacia cyanophlla Lindey. Electronic J Biol, 12:2
Abstract
In the present work, Bacillus subtilis SDP1 α-amylase was immobilized in alginate beads using entrapment and physical adsorption methods. Immobilized SDP1 α-amylase in alginate beads was used to hydrolyze soluble starch and potato, wheat and maize starch. Optimum temperature and pH of immobilized enzyme were determined and compared to free enzyme. Physical adsorption technique gave the better efficiency than entrapment technique for SDP1 α-amylase. However, immobilization did not affect optimum temperature (60°C) and pH (7.0) of free enzyme, immobilization of SDP1 amylase change composition of hydrolysis products. The results showed that immobilized SDP1 α-amylase synthesized maltoriose and maltose after starch hydrolysis and maltotriose converted to maltose and glucose during the hydrolyze proceeded. Since the starch hydrolysis process was carried out at 25°C, production of maltose and glucose from starch using immobilized SDP1 amylase was successfully applied, this technique and its hydrolysis products can be used in food industry.
Keywords
Bacillus subtilis; SDP1; α-amylase; Immobilization; Calcium alginate; Starch hydrolysis
1. Introduction
Starch is one of main carbohydrates synthesized by plants in plastids as a result of photosynthesis and a major storage product of many economical important plants [1-3]. It often consists of two molecular entities, namely; amylose and amylopectin. Amylose is a linear molecule and comprise of 500-600 glucose units which is linked by 1-4 glycosidic linkages [4,5]. Glycosidic bonds between glucose units tend to be angled and that gives amylose a helix function. Two amylose molecules form a double helix structure with hydrogen bonds [6]. Amylopectin is the largest molecule in the nature and the number of glucose unit in amylopectin is from 2,000 to 2,00,000. It has a cluster like organization which consists of short 1-4 linked linear chains of 10-60 glucose units and 1-6 linked side chains with 15-45 glucose units [7,8].
Amylases are the starch degrading enzymes and they have a great significance in biotechnology with applications ranging from food, baking, brewing, fermentation, detergent applications, and textile resizing and paper industries to analysis in medicinal and clinical chemistry [9-11]. Alpha-amylases (EC 3.2.1.1) are belong to hydrolytic endo-enzymes and they hydrolyze starch molecules from 1-4-glycosidic bonds presents in inner part long chain carbohydrates randomly, ultimately yielding maltotriose (G3) and maltose (G2) from amylose, limited dextrin and glucose (G1) from amylopectin [12]. Alpha-amylases are one of the mostly used enzymes around the world for different purpose and they represent approximately 30% of the world enzyme market share [13]. They can be produced by nearly all living organisms but for industrial applications bacterial amylases, especially produced from Bacillus genus, are mostly used. After hydrolysis any molecule which may occur can be used in different industries.
In these hydrolytic products Maltooligosaccharides (MOS) are the most important ones. MOSs are the macromolecules which formed of 2-10 D-glycopranose units linked by alpha-1,4-glycosidic bonds [14,15] and there is a great interest about MOSs around the world because of their ability to use in food and pharmaceutical industries as coating agents, viscosity providers, floor carries, sweetener and crystallization inhibitors [16]. Nominally, in baking industry, crystallization of starch in dough causes retrogradation process of bread. To prevent retro gradation of bread maltotriose (G3), maltotetrose (G4) and maltopentose (G5) are commonly used as an antifoaming agent [17]. Besides of all, to prevent stalling of bread and to prolong shelf life of the products maltose (G2) use had been shown more beneficial because of its smaller size and ability to diffuse easily [15]. The discoveries of maltooligosaccharides have 30% less sweetness than sugar and compared to corn syrup, their usability in producing functional foods gains importance. Probiotic features of MOSs are frequently investigated in recent days. In literature, it was shown that MOSs increase the number of Bifidobacteria in human colon owing to absorption of MOSs in the small intestine without reaching the colon intact. Also, they decrease the level of putrefactive bacteria such as Clostridium perfringers and Enterobacteriaceace [18].
Besides MOS, maltose is usually produced during starch hydrolysis. Because of its low viscosity, colorless feature and mild sweetness, maltose is also widely used in food and pharmaceutical industry. Purified maltose from maltose syrups is also used in different industrial applications such as manufacture of antibiotics, vaccines [19]. Another important product of complete hydrolysis of starch is glucose. Since glucose is the main carbon source for sugar metabolism in living organisms and it is used as a carbon source for many fermentation processes to produce biomaterials, it is very crucial to produce glucose at the end of starch hydrolysis.
The use of enzyme in a free form is very expensive, because the enzyme can be used only one time and cannot be recovered from the reaction medium easily at the end of the reaction. A lack of availability of the enzyme and limited stability under operational conditions affects the expenditures [20,21]. Around 62.2$ million are spent in each year for the starch refining industry [22]. To overcome all that limitations and reduce production cost, enzyme immobilization is used. It can be defined as the attachment of free or soluble enzyme to different types of support or incorporated onto/into an inert, insoluble material resulting in reduction or loss of mobility of the enzyme [23-25]. Two things are very important in immobilization process; a carrier and the enzyme. Support material can be organic or inorganic but it has to be hydrophilic, inertness towards enzymes ease of derivation, biocompatible, resistance to microbial attack with physical resistance and low cost. Depending on the enzyme characteristic, immobilization method and appropriate support material can be chosen. According to UPAC, immobilization techniques fall into 4 four categories; i) covalent bonding, ii) intramolecular cross-linking, iii) adsorption, iv) entrapment [26].
Immobilization by covalent bonding technique based on the covalent bonds between available functional groups on carrier surface and amino group of the enzyme [27,28]. During immobilization by entrapment, no reaction occurs between support and enzyme, a crossed linked polymeric network is formed around the enzyme [29,30]. Both techniques have advantages and disadvantages according to enzyme characteristic and reaction conditions. The alpha-amylase enzyme that studied in this work obtained from Bacillus subtilis SDP1 which was isolated from the rhizosphere of Acacia cyanophlla Lindey from Cukurova region of Turkey. This enzyme has 61 kDa molecular weight, does not require Ca2+ for activity, works ranging from 5.0 to 9.0 pH and best at 60°C. The aim of this work was to characterize immobilized SDP1 amylase on alginate beads and determine the product profile of different starch hydrolyzed by immobilized α-amylase from B. subtilis SDP1.
2. Material and Method
2.1 Preparation of alginate beads and immobilization
Immobilization of SDP1 alpha-amylase on alginate beads was performed by using entrapment and physical adsorption techniques according to methods described by Singh et al. [31]. To perform entrapment technique, an equal volume of enzyme solution and sodium alginate solution were mixed to obtain final alginate concentration in the mixture ranged of 2% (w/v). The mixture was then extruded drop wise through a syringe (0.80 x 38.0 mm) into 2% (w/v) CaCl2 solution with a gently stirred. After beads occurred, they stored at 4°C in 6 mM CaCl2 solution. For physical adsorption, the alginate beads were prepared with the same method mentioned above. In this work, 2%, 3%, 4% and 5% (w/v) alginate solutions were prepared and compared to their ability to binding amylase enzyme. In this work alginate-enzyme ratio was used as 2:1. Enzyme solution was mixed with alginate beads with 2:1 alginate-enzyme ratio and incubated at 25°C for 30 minutes for immobilization. After incubation, beads were removed and washed several times to remove unbound enzyme.
In order to determine the immobilization efficiency, the following equation was used and the immobilization efficiency was defined here as the amount of enzyme bound to matrix.
Immobilization Efficiency (%) = (EoVo-EfVf) / (EoVo) x 100 (1)
Where Eo: The initial amylase activity (IU/ml); Ef: The amylase activity (IU/ml) after immobilization; Vo: The initial volume of amylase solution (ml) and Vf: The volume of filtrate (ml)
2.2 Enzyme assay for immobilized SDP-1 amylase
The immobilized α-amylase activity was determined by measuring the reducing sugar released from soluble starch [32,33] using the method specified by Miller [34]. To determine the immobilized enzyme activity, 1 g immobilized alginate beads were mixed with 1% (w/v) 900 μl starch solution prepared using phosphate buffer (20 mM, pH 7.0). The mixture was incubated at 50°C for 10 min, then alginate beads were removed from solution and the amount of maltose produced from starch hydrolysis was determined by the dinitrosalicylic method (DNS). In this procedure, maltose was used as a standard. One unit of enzyme activity was defined as the amount of enzyme liberating 1 μml of reducing sugar per minute from soluble starch at 50°C and pH 7.0. All activity assays were carried out in triplicate. Standard deviation did not exceed 5% of the average values. The Coomassie Blue Dye Binding method was used for the protein determination with bovine serum albumin (BSA) as standard [35].
2.3 Determination of optimum temperature and pH of immobilized SDP1 amylase
Optimum temperature was determined by assaying activity in the temperature between 25°C and 80°C. The temperature at which maximum activity was observed was taken as 100% and relative activities at different temperatures were calculated. The effect of pH on immobilized α-amylase activity was investigated by measuring the enzyme activity in reaction mixtures at different pH values range between ‘pH 3.0 to 10.0’ using 20 mM Glycine-HCl buffer for pH 3.00-4.00, 20 mM Sodium acetate buffer for pH 5.00-6.00, 20 mM Potassium phosphate buffer for pH 7.00-8.00 and 20 mM Tris-HCl buffer for 9.00- 10.00. The pH value at which maximum activity was obtained was taken as 100% and relative activities at different pH were calculated.
2.4 Determination of the hydrolytic products
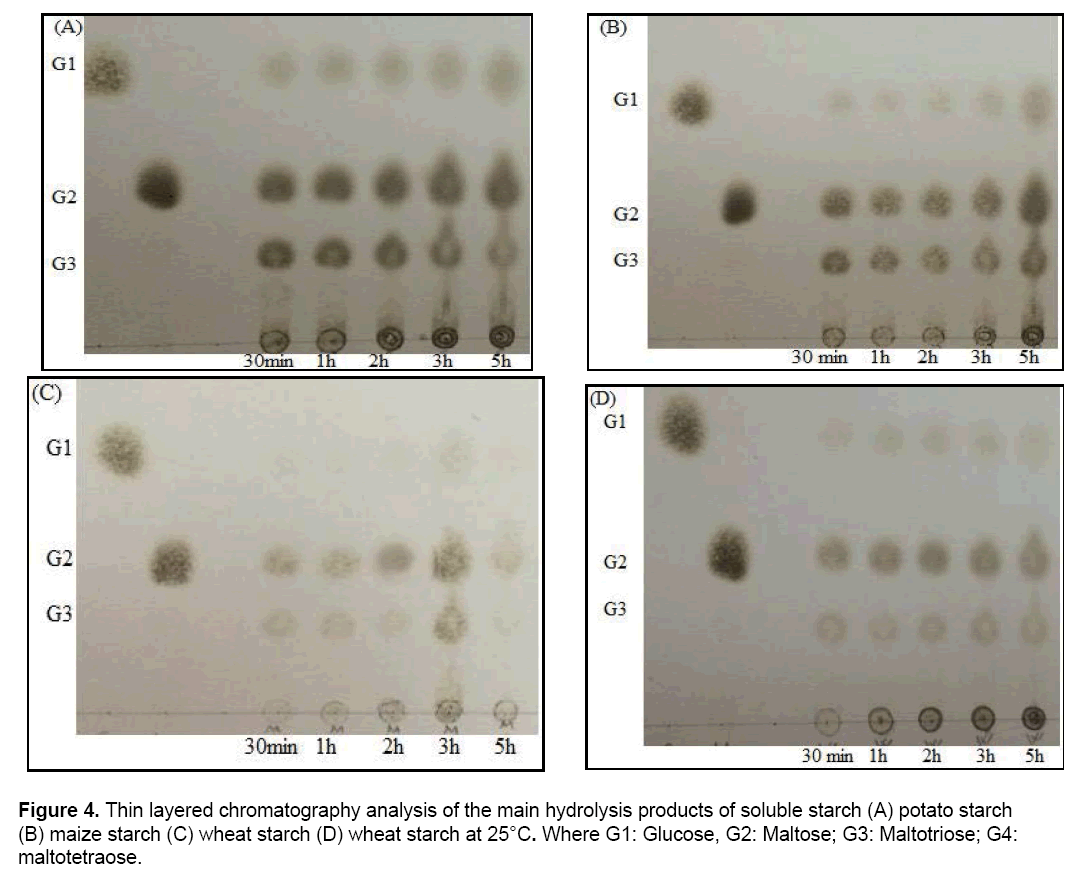
Thin Layer Chromatography (TLC) was used to determine hydrolytic end products of different starch sources as corn, potato, wheat and chemically soluble starch. Starch solutions were prepared with 1% (w/v) in 20 mM phosphate buffer, pH 7.0. Immobilized alginate beads and the substrate mixtures were incubated at 25°C at different time intervals (30 min to 5 hours). Hydrolytic products were spotted on TLC silica gel plates (60 F254, 20 x 20 cm, Merck, Darmstadt, Germany) and were run into solvent system which include chloroform/acetic acid/water within the ratio as 60:70:10. H2SO4/ethanol mixture (5:95, v/v) was sprayed the layers and incubated at 120°C for 10 minutes to make the spots visual.
3. Result and Discussion
In our previous work [36], it was reported that hydrolysis of soluble starch using native SDP1 amylase resulted in the production of maltose, maltotriose and maltotetraose. Although, similar pattern was observed with wheat starch hydrolysis, maltose was the major hydrolysis products when we used wheat starch as a raw material. Therefore, SDP1 amylase could be used to produce maltose besides maltotriose and maltotetraose. In addition to production of MOS and maltose, calcium independent nature and moderate temperature stability make Bacillus SDP1 amylase as a promising candidate for food industry.
In these industries, hydrolysis processes economically become more viable, when amylase enzyme can be reused via immobilization [37]. Different immobilization methods can be used for enzyme immobilization. One of the immobilization methods is entrapment method which prevents loss of enzyme activity after immobilization. Moreover, entrapment methods protect process from microbial contamination and increase the enzyme stability. Another immobilization technique is physical adsorption and comparing to other techniques, it is relatively easy, rapid and safe technique [38]. Therefore, we attempted to immobilize SDP1 amylase by entrapment and physical adsorption in alginate beads.
3.1 Immobilization
In order to determine the amount of enzyme bound to matrix, binding efficiency of entrapment and physical adsorption methods are calculated and compared. According to results, it was observed that immobilization of SDP1 amylase was not successfully held by entrapment technique. After several washes, almost all the entrapment beads released the enzyme in the core. In this step, lower immobilization yield in entrapment method could be explained with the problem in the size of alginate bead pores. While enzyme should retain in the gel, the substrate and end-products also should be small enough to pass through the pores of alginate gel matrix. Therefore, at this point, larger pores could have caused larger leakage of the enzyme from gel and products could not have diffuse out of the gel properly [38,39].
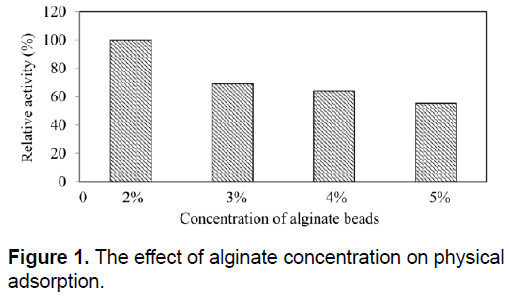
On the other hand, physical adsorption showed a much better ability to retain enzyme after washing series compared to entrapment technique. For physical adsorption, immobilization efficiency was calculated as %50. In physical adsorption, the binding forces between the enzyme and the matrix were strong comparing with entrapment techniques. To determine the best alginate concentration for SDP1 α-amylase, activity of alginate beads prepared using different alginate concentrations were determined and relative activities were calculated. As shown in Figure 1, comparing to other alginate concentrations, alginate beads having 2% alginate has highest amylase activity. Increasing alginate concentration in alginate beads caused to decrease in SDP1 amylase activity. The results indicate that increasing the concentration rate of alginate caused to reduction of surface pores and obstructed the amylases on the surface of beads. In the literature, 2% and 3% concentration rates are the mostly chosen ones [20,40]. Therefore, we selected 2% alginate concentration for further studies. Decrease in activity with increasing alginate concentration could be explained by the excessive packing of the enzyme, which renders their active sites less accessible to the substrate [31].
In order to find the best conditions for physical adsorption, SDP1 amylase was incubated with alginate beads at different time intervals (30 min, 1, 2, 4 and 8 hours). After certain incubation periods, not only the activity of immobilized enzyme was determined but also immobilization efficiencies were calculated. Incubation period did not affect the efficiency of physical adsorption. The immobilization efficiency ranged from 51% to 55%. According to these results, 30 min incubation time was chosen for further investigations.
3.2 Properties of immobilized SDP1 amylase
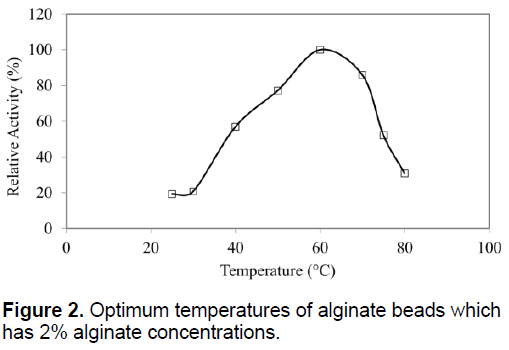
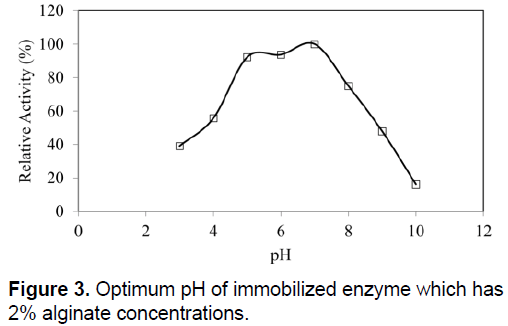
The optimum temperatures of SDP1 amylase were not affected by physical adsorption. The similar optimum temperature with native enzyme as 60°C was determined (Figure 2). Immobilized SDP1 amylase showed activity in the pH range of 3.0 to 9.0 with its optimum at pH 7.0 (Figure 3). However, the relative activity of immobilized SDP1 amylase was 92% and 94% at pH 5.0 and 6.0. This range a little bit wider than native SDP1 α-amylase and also the beads performed their higher enzymatic activities in acidic conditions as shown in the Figure 3.
3.3 Hydrolysis of different starch using immobilized SDP1 amylase
Immobilized SDP1 amylase was used in the hydrolysis of soluble starch, wheat, potato and maize starch. It can be seen from Figure 4(A), hydrolysis product from soluble starch with immobilized SDP1 amylase at 25°C were glucose, maltose and maltotriose. As seen from the Figure 4(A), immobilized SDP1 amylase initially produced maltose, maltotriose and small amount of glucose. Increasing hydrolysis period caused to increase maltose and glucose while maltotriose from soluble starch was decreased. The end product profiles of soluble starch hydrolysis were determined as maltose and glucose. The formation of maltotriose by immobilized SDP1 amylase was decreased during the prolonged incubation period. The product distribution obtained from soluble starch by immobilized enzyme was different from product obtained from soluble starch by free SDP1 enzyme [36]. Free amylase from Bacillus subtilis SDP1 did not produce glucose from soluble starch, however immobilized SDP1 amylase produced glucose from soluble starch.
In literature, the end products of starch hydrolysis via the action of amylase were illustrated as glucose and maltose by thin-layer chromatographic analysis on silica gel plates [41]. The hydrolysis products as maltose and maltotriose were obtained with the potato starch by immobilized SDP1 amylase (Figure 4B). Both maltose and maltotriose formation were increased with time and at the end of 5 h, glucose formation was also observed. The immobilized SDP1 amylase did not hydrolyze maize starch (Figure 4C) and wheat starch (Figure 4D) efficiently compared to the soluble starch and potato starch. Immobilized SDP1 amylase mostly produced maltose from maize and wheat starch. In our previous work, the hydrolysis products of the wheat starch with free SDP1 amylase was maltose [36]. Similar results were obtained with immobilized SDP1 amylase (Figure 4D). Moreover, maltose produced using immobilized SDP1 amylase appeared at the first 30 min and remained quite stable during prolonged hydrolysis (5 h). In addition to maltose, glucose was also observed in the early stage of wheat starch hydrolysis (Figure 4).
4. Conclusion
Within the scope of this work, immobilized SDP1 α-amylase was used to hydrolyze soluble starch and wheat, potato and maize starch. Physical adsorption technique gave the better efficiency than entrapment technique for SDP1 α-amylase. On the other hand, physical adsorption of SDP1 amylase on alginate beads did not change the optimum pH and temperature of SDP1 amylase. However, free and immobilized SDP1 amylase showed different products of starch hydrolysis. As stated in TLC results, immobilized SDP1 α-amylase formed maltoriose and maltose mostly after starch hydrolysis and it was seen that maltotriose converted to maltose and glucose during the hydrolyze proceeded. At this point, maltooligosaccharide composition of the immobilized SDP1 amylase was different from free enzyme. Maltose and maltotriose were produced by free enzyme while maltose and glucose on served by immobilized enzyme. Since, maltose is very important additive for foods as a sweetener and preservative, glucose was used for glucose syrup production and used as raw materials for fermentation processes, immobilized form of the enzyme can be successfully used in food industry.
References
- Annison G, Topping DL. (1994). Nutritional role of resistant starch: Chemical structure vs physiological function. Ann Rev Nutr. 14: 297-320.
- van der Maarel MJ, van der Veen B, Uitdehaag JC, Leemhuis H, Dijkhuizen L. (2002). Properties and applications of starch-converting enzymes of the a -amylase family. J Biotech. 94: 137-155.
- Perez S, Bertoft E. (2010). The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive Review. Starch J 62: 389-420.
- Buleon A, Colonna P, Planchot V, Ball S. (1998). Starch granules: Structure and biosynthesis. Int. J.Biol. Macromol. 23: 85-112.
- Myers AM, Morell MK, James MG, Ball SG. (2000). Recent progress towards understanding biosynthesis of the amylopectin crystal. Plant Physiol. 122: 989-997.
- Zobel HF. (1988). Molecules to granules: A comprehensive review. Starch/Starke. 40: 1-7.
- Hizukuri S. (1986). Polymodal Distribution of the chain lengths of amylopectins and its significance. Carbohydr Res. 147: 342-347.
- Robin JP, Mercier C, Charbonniere R, Guilbot A. (1974). Lintnerized starches. Gel filtration and enzymatic studies of insoluble residues from prolonged acid treatment of potato starch. Cereal Chem. 51: 389-406.
- Pandya PH, Jarsa RV, Newalkar BL, Bhalt PN. (2005). Studies on the activity and stability of immobilized a-amylase in ordered mesoporous silicas. Microporous and Mesoporous Mater. 77: 67-77.
- Alva S, Anupama J, Savla J, et al. (2007). Production and characterization of fungal amylase enzyme isolated from Aspergillus sp. JGI 12 in solid state culture. Afr J Biotechnol. 6: 576-581.
- Singh S. (2014). A comparative study on immobilization of alpha amylase enzyme on different matrices. IJPAES. 4: 192-198.
- Rajagopalan G, Krishnan C. (2008). Alpha-amylase production from catabolite depressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresource Technol. 99: 3044-3050.
- Choubane S, Khelil O, Cheba BA. (2014). Bacillus sp. R2 and Bacillus cereus immobilized amylase for glucose syrup production. Procedia Technology. 19: 972-979.
- Park KH. (1992). Development of new carbohydrate materials. Food Sci Ind. 25: 73-82.
- Min BC, Yoon SH, Kim JW, et al. (1998). Cloning of novel maltooligosaccharide-producing amylases as antistaling agents for bread. J Agricult and Food Chem. 46: 779-782.
- Palacios HR, Schwarz PB, D’Appolonia LD. (2004). Effect of a-amylases from different sources on the retrogradation and recyclization of concentrated wheat starch gels: Relationship to bread staling. J Agricult and Food Chem. 52: 5978-5986.
- Duran E, Leon A, Barber CB. (2001). Effect of low molecular weight dextrins on gelatinization and retrogradation of starch. Eur Food Res Technol. 212: 203-207.
- Crittenden RG, Playne MJ. (1996). Production, properties and applications of food-grade oligosaccharides. Trends Food Sci Tech. 7: 353-361.
- Lin Q, Xiao H, Liu GQ, et al. (2013). Production of maltose syrup by enzymatic conversion of rice starch. Food Bioprocess Technol. 6: 242-248
- Talekar S, Chavare S. (2012). Optimization of immobilization of a-amylase in alginate gel and its comparative biochemical studies with free a-amylase. Resent Res Sci and Technol. 4: 1-5.
- Sheldon RA, Schoevaart R, van Langen LM. (2005) Cross-linked enzyme aggregates (CLEAs): A novel and versatile method for enzyme immobilization (A review). Biocatal. Biotransform. 23: 141-147.
- Sharma M, Sharma V, Majumdar DK. (2014) Entrapment of alpha amylase in agar beads for biocatalysis of macromolecular substrate. Int. Sch. Res. 2014: 8.
- Krajewska B. (2004). Application of chitin- and chitosan-based materials for enzyme immobilizations: A review Enzyme Microb Tech. 35: 126-139.
- Khan AA, Alzohairy MA. (2010). Resent advances and applications of immobilized enzyme technologies: A review Res J Biol Sci. 5: 565-575.
- Vankelecom IFJ. (2002). Polymeric membranes in catalytic reactors. Chem Rev. 102: 3779-3810.
- Brena BM, Batista-Viera F. (2010). Immobilization of enzymes: A literature survey methods in Biotechnology: Immobilization of Enzymes and Cells. (2ndedn), Guisan JM, Humana Press Inc NJ. 15-30.
- Hanefeld U, Gardossib L, Magner E. (2009). Understanding enzyme immobilization. Chem Soc Rev. 38: 453-468.
- Cao L. (2005). Carrier-Bound Immobilized Enzymes, Wiley-VCH, Weinheim, Germany. 1-5.
- Krajewska B. (2009). Ureases II: Properties and their customizing by enzyme immobilizations: A review J Mol Catal B: Enzymatic. 59: 22-40.
- Lalonde J, Margolin A. (2002). Immobilization of Enzymes: Enzyme Catalysis in Organic Chemistry. (2ndedn), Drauz K and Waldmann H, Wiley-VCH,Weinheim, Germany. 163-184.
- Singh P, Gupta P, Singh R, Sharma R. (2012). Activity and stability of immobilized alpha-amylase produced by Bacillus acidocaldarius. Int J Pharm Life Sci. 3: 2247-2253.
- Nelson N. (1944). A photometric adaptation of the Somogyi method for the determination of glucose. J Bio Chem. 153: 375-380.
- Somogyi M. (1945). A new reagent for the determination of sugars. J. Bio. Chem. 160: 61-68.
- Miller GL. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 31: 426-428.
- Sedmak JGS. (1977). A rapid, sensitive and versatile assay for protein using Coommassie Brilliant Blue G250. Anal Biochem. 79: 544-552.
- Ozturk UH, Denizci AA, Ogan A, Kazan D. (2014). A maltooligosaccharides producing alpha-amylase from Bacillus subtilis SDP1 isolated from rhizosphere of Acacia cyanophylla Lindley. Food Biotechnol. 28: 309-332.
- Fernandes P. (2010). Enzymes in food processing: a condensed overview on strategies for better biocatalysts. Enzyme research. 2010: 862537.
- Riaz A, Qader SAU, Anvar A, Iqbal S. (2009). Immobilization of thermostable alpha-amylase on calcium alginate beads from Bacillus subtilis KIBGE-HAR. Aust J Basic Appl Sci. 3: 2883-2887.
- Dey G, Bhupinder S, Banerjee R, (2003). Immobilization of alpha-amylase produced by Bacillus circulans GRS 313. Braz Arch Biol Technol. 46: 167-176.
- Ertan F, Yagar H, Balkan B. (2007). Optimization of a-amylase immobilization on calcium alginate beads. Prep. Biochem and Biotech. 37: 195-204.
- Ray RC, Kar S, Nayak S, Swain MR. (2008). Extracellular a-amylase production by Bacillus brevis MTCC 7521. Food Biotechnology. 22: 234-246.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences