Formation of Persisting Cell Wall Deficient Forms of Mycobacterium bovis BCG during Interaction with Peritoneal Macrophages in Guinea Pigs
Nadya Markova, Lilia Michailova, Vesselin Kussovski, Mimi Jourdanova
Institute of Microbiology,Bulgarian Academy of Sciences,26.G.Bonchev st,1113 Sofia,Bulgaria
Abstract
In order to study whether and how M. bovis BCG can persist in vivo, series of events during interaction of live BCG bacilli with resting or preactivated with Lentinan peritoneal macrophages in guinea pigs were evaluated. After intraperitoneal administration of BCG, samples of peritoneal lavage fluid from guinea pigs were obtained at day 1, 14 and 45. Interactions between BCG bacilli and peritoneal macrophages were observed by transmission electron microscopy. Examination of some BCG bacilli inside macrophages revealed fate and morphological peculiarities typical of cell wall deficient bacterial L-forms as well as different modes of L-form multiplication. In correspondence to the observed L-form conversion phenomenon, BCG L-form cultures were isolated from animals on 45 day after challenge. The demonstration that BCG bacilli can convert to cell wall deficient forms(Lforms) inside resting or pre- activated macrophages in vivo suggests that this phenomenon could significantly enhance BCG survival and persistence ability.
Keywords
Mycobacterium bovis BCG; L-forms; persistence; peritoneal cells; electron microscopy; guinea pigs.
1. Introduction
The use of Mycobacterium bovis bacillus Calmette- Guerin vaccine (BCG) against tuberculosis is well established [1-6]. Although the vaccination policies differ greatly between countries,BCG vaccine is among the most widely used vaccines in the world. As far as BCG has been administered mainly to children,it is unclear whether BCG protection lasts sufficiently long after administration. Despite the massive use of BCG vaccine for many years,its efficacy has been a subject of variable scientific debates [3,4]. Since M. bovis BCG is an attenuated live strain,little is known about how long it can survive in the vaccinated persons. Reports about detection and isolation of BCG bacilli from patients with AIDS many years after their vaccination [7-9] give rise to questions about the mechanisms by which BCG bacilli persist in vivo for a long time. Many papers comment on the immune response to BCG including antigen processing and trafficking events,but only a few are concerned with the fate and mechanisms of persistence of live BCG bacilli in vivo [10-13].
In order to investigate whether and how M. bovis BCG can persist in vivo,our study was aimed at evaluating the series of events during interaction of live BCG bacilli with resting or pre-activated peritoneal macrophages in guinea pigs. We tried to emphasize on some specific points concerning loss of bacterial cell walls and morphological transformation of BCG bacilli displaying an intriguing aspect of L-form conversion phenomenon in vivo.
2. Materials and Methods
2.1 Experimental design
Male and female guinea pigs weighing 200-250g were used. The animals were obtained from National Centre for laboratory animals,Sofia and the experiments were performed with permission of Bulgarian Animal Use Committee. A total of 60 animals (5 animals per interval for each experimental group) were used. The animals were divided into four groups: ①combined treatment with Lentinan and BCG (Len+BCG); ②only with Lentinan (Lentinan); ③only with BCG (BCG); ④control with saline. The first and the second groups were inoculated intraperitoneally (i.p.) with Lentinan (Ajinomoto,Japan),3 times at 2 days intervals with a dose of 1mg/kg. The third and fourth groups (control) were i.p. given 0.2 ml of saline at the same intervals. Two days after last treatment with Lentinan or saline the first and third groups were challenged i.p. with 1x108 viable BCG bacilli (strain Sofia SL-222) in a volume of 0.5 ml. Guinea pigs were decapitated after a slight ether anaesthesia at 1,14 and 45 days after last treatment and samples of peritoneal lavage fluid were obtained for evaluation of several parameters.After centrifugation,the peritoneal cells were washed,re-suspended in saline and counted by Cobas Helios cell counting system (Hoffman La Roche). The peritoneal macrophages were obtained from lavage fluid by standard procedures and enriched by adherence to plastic. Non adherent and dead cells were removed by washing with PBS. Purity of the cells (>85%) was confirmed by α-naphthyl-acetate esterase staining. Viability of the cells (>90%) was determined by trypan blue dye exclusion.
2.2 Transmission electron microscopy (TEM)
The electron microscopic examination included cell samples from peritoneal cavity exudates,taken at the intervals indicated above. The cells were isolated in ice-cold 0.85% (w/v) NaCl,centrifuged for 20 min. at 1500 rpm,washed in 0.1 M cacodylate buffer supplemented with 0.1% (w/v) MgSO4 and 4.5% (w/v) sucrose,pH 7.2,and fixed in 2.5% (v/v) glutaraldehyde in the same buffer for 2 h at 4°C. After washing with the same buffer,the macrophages were post-fixed in 1 % (w/v) OsO4 in 0.1 M symm-kollidin buffer (Fluka),pH 7.2,for 2 h at 4°C. After dehydration in graded ethanol series and propylene oxide,the cells were embedded in Epon- Araldite (Fluka) for polymerization at 56°C; 48 h. Ultrathin sections were made on a Reichert-Jung Ultracut microtom,stained with uranylacetate and lead citrate,and observed with a Zeiss 10 C electron microscope at 60kV.
The microbial samples of the BCG control broth culture and the broth culture isolated from spleen on day 45 after BCG challenge were centrifuged for 20 min. at 4000 rpm,washed in the same cacodylate buffer,but the supplemented sucrose was 7.5 %. The concentration of glutaraldehyde was 4 %,and post-fixative 1% OsO4 was in the same cacodylate buffer. All other procedures were the same as indicated above.
2.3 H2O2 release by peritoneal macrophages
The H2O2 released by peritoneal macrophages was determined by the method of Pick and Keisari [14]. The assay is based on the horseradish peroxidase (HRPO)-mediated oxidation of phenol red by H2O2 resulting in increased absorbance at 610 nm. Macrophage monolayers were established by the addition of 1mL cell suspension at a concentration of 3.0 x 106 macrophages mL-1 in Eagle’s minimum essential medium (MEM; Difco) containing 10% fetal calf serum (Gibco) to 30 mm diameter plastic Petri dishes (tissue culture grade) and incubation for 1 h at 37°C in 90% air-10% CO2 to allow the adherence of macrophages to the dish surface.
After removing nonadherent cells,peritoneal macrophages were covered with 1mL phenol red solution,containing 140mM NaCl; 10mM potassium phosphate buffer,pH 7.0; 5.5mM dextrose; 0.28mM phenol red and 50 μgmL-1 HRPO (Sigma,Germany).
After incubation for 180 min at 37°C,the supernatants were centrifuged,alkalized with 10 μL 1N NaOH and absorbance was measured at 610 nm. The release of H2O2 was calculated using a standard curve. In each experiment,at least three dishes of adherent peritoneal macrophages were used for determining the amount of cell protein. This was done by covering the monolayers with 1mL of 1N NaOH,allowing them to stand overnight at 37°C and performing the protein assay on the digest by the classical method of Lowry et al. [15]. Results were expressed as nmoles H2O2 per mg macrophage protein per 180 min incubation.
2.4. Isolation of live BCG bacilli from spleen
Live BCG bacilli were isolated from spleens at days14 and 45 using standard technique. Spleens were aseptically removed and homogenized in distilled water. Then aliquots of spleen homogenates (0.2 ml) were simultaneously inoculated into specially supplemented with 1% yeast extract and 3% NaCl Dubos broth for isolation of L-forms and onto Löwenstein-Jensen (LJ) medium after serial 10-fold dilutions for enumeration of BCG bacilli (CFUs) with typical morphology,and incubated for 45 days at 37°C.
2.5. Statistical analysis
The results were expressed as mean values ± SEM. In order to assess the significance of differences within experiments Student’s t-test was used. Statistical significance was defined as P<0.05. Experiments were repeated three times.
3. Results
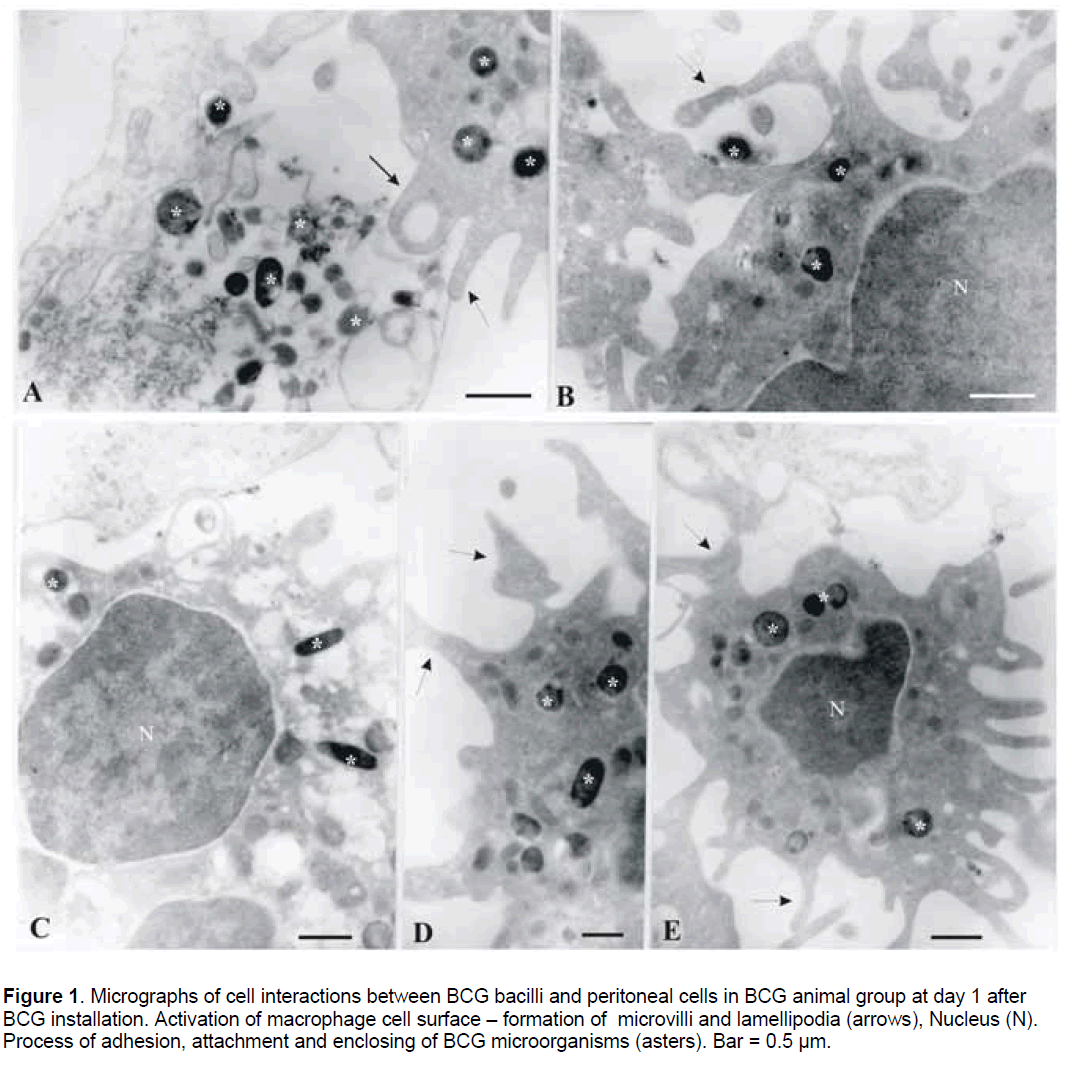
Analysis by TEM of peritoneal cell samples revealed the in vivo interactions between BCG bacilli and phagocytes on day 1,14,and 45 after challenge. At day 1 after BCG installation,the interactions of BCG bacilli with resting peritoneal cells demonstrated initial phases of phagocytosis including attraction,adhesion and attachment of bacteria to the phagocytes and processes of bacterial enclosing and engulfment (Figure 1A-1E). Groups of free bacilli were found in the extra-cellular space (Figure 1A).
Figure 1. Micrographs of cell interactions between BCG bacilli and peritoneal cells in BCG animal group at day 1 after BCG installation. Activation of macrophage cell surface – formation of microvilli and lamellipodia (arrows), Nucleus (N). Process of adhesion, attachment and enclosing of BCG microorganisms (asters). Bar = 0.5 µm
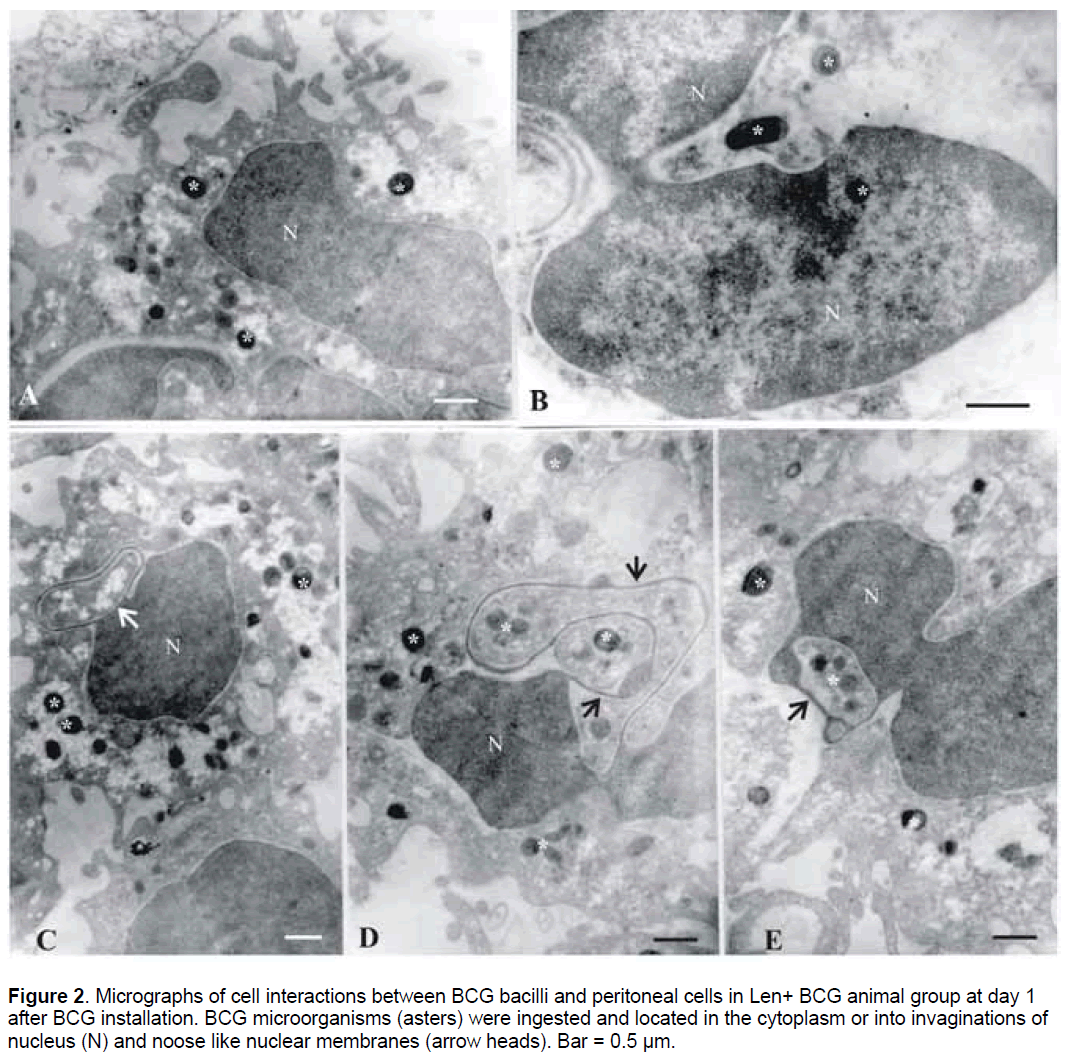
It should be noted that at the same time point,Lentinan – preactivated macrophages showed high grade of phagocyte cell surface activation and more intensive uptake of BCG bacilli (Figure 2 ). The increased number of peritoneal exudative cells corresponded to TEM observation of numerous activated phagocytes (mononuclear and polymorphonuclear phagocytes). Most of them were in state of division and with enlarged nuclei (Figure 2 B,2E). Microorganisms were ingested and found situated in electron-light parts of the phagocyte cytoplasm (Figure 2A,2C). Of special interest was the observation that in some instances,BCG bacilli were seen to be located into the invaginations of nucleus (Figure 2A,2B),inside the nucleus (Figure 2B),or within nuclear membrane noose-like formations (Figure 2C-2E). Free bacteria outside the cells were not registered in Len+BCG group.
Figure 2.Micrographs of cell interactions between BCG bacilli and peritoneal cells in Len+ BCG animal group at day 1 after BCG installation. BCG microorganisms (asters) were ingested and located in the cytoplasm or into invaginations of nucleus (N) and noose like nuclear membranes (arrow heads). Bar = 0.5 µm.
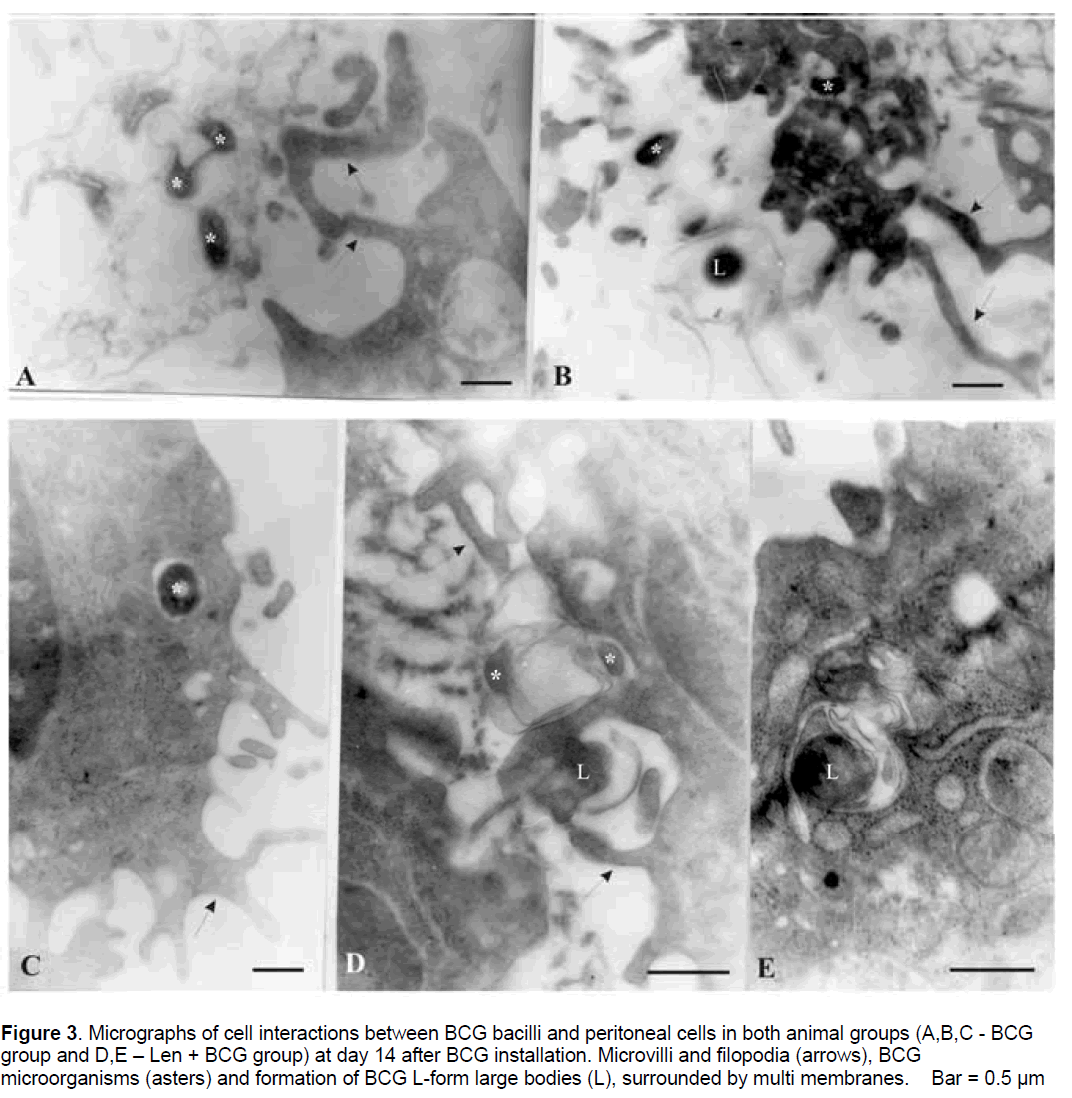
Selected moments of in vivo cell interactions at day 14 in both groups (with and without Lentinan treatment) are presented in Figure 3 .
Figure 3.Micrographs of cell interactions between BCG bacilli and peritoneal cells in both animal groups (A,B,C - BCG group and D,E – Len + BCG group) at day 14 after BCG installation. Microvilli and filopodia (arrows), BCG microorganisms (asters) and formation of BCG L-form large bodies (L), surrounded by multi membranes. Bar = 0.5 µm.
The finding of free bacterial forms in the intercellular space,situated between phagocytes and membranous remains suggested that probably viable BCG bacilli were released from destructed macrophages (Figure 3A,3B). Subsequent bacterial attachment,adhesion and enclosing by macrophage pseudopodia and ingestion were observed. Bacterial forms were seen inside the vacuoles in the cytoplasm of the macrophages (Figure 3C). It is remarkable that at this time point electron micrographs showed an interesting and surprising event in Len +BCG group,such as the formation of BCG L-form large bodies inside peritoneal macrophages. BCG L-form bodies were located inside vacuoles and even though they were pleomorphic,some of them were of relatively large size. Many of them were surrounded by multi membranes (Figure 3D,3E).
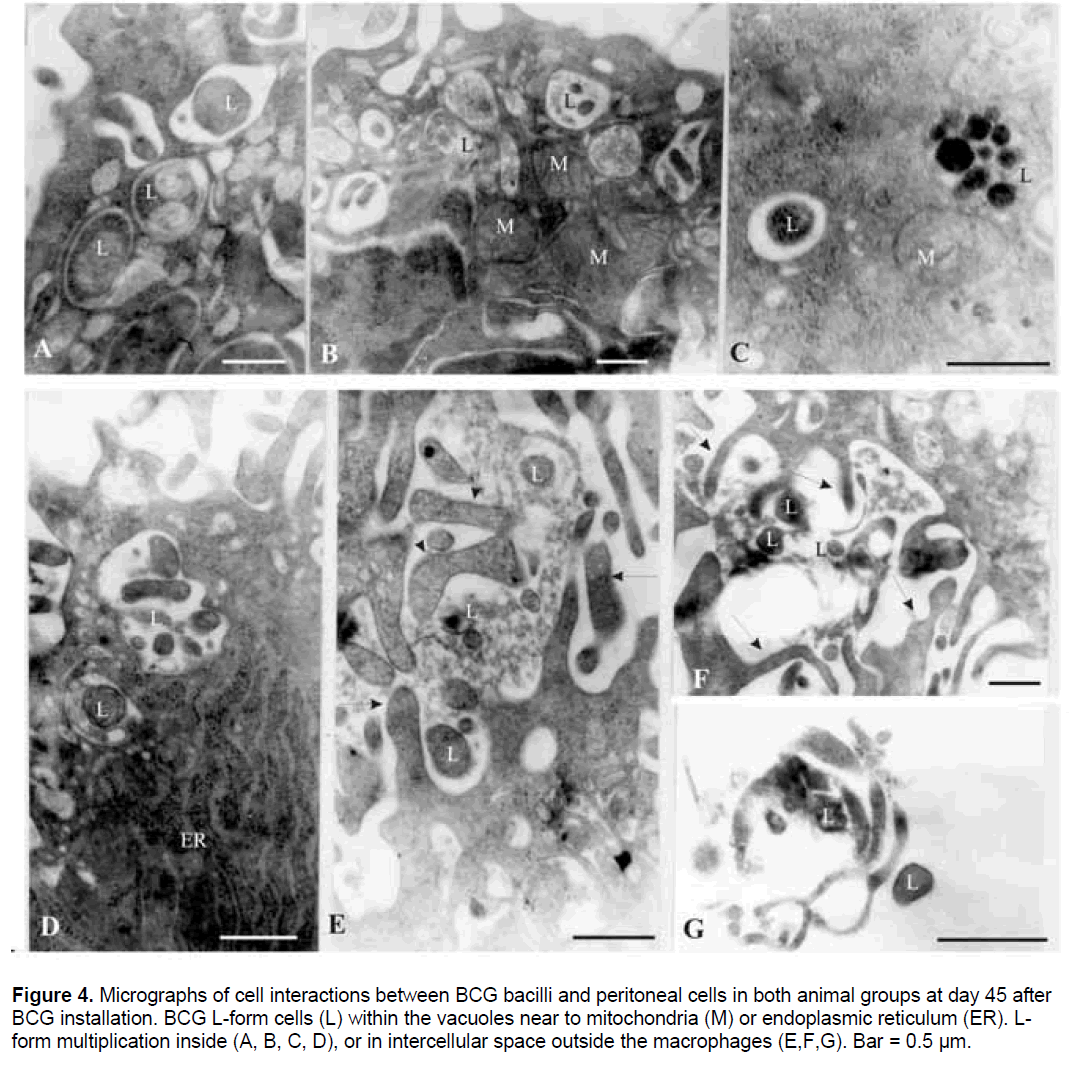
Figure 4.Micrographs of cell interactions between BCG bacilli and peritoneal cells in both animal groups at day 45 after BCG installation. BCG L-form cells (L) within the vacuoles near to mitochondria (M) or endoplasmic reticulum (ER). Lform multiplication inside (A, B, C, D), or in intercellular space outside the macrophages (E,F,G). Bar = 0.5 µm.
At day 45 after BCG installation,high activation of peritoneal phagocytes with formation of many pseudopodia and vacuoles was observed in both animal groups (Figure 4). Numerous micoorganisms of different size and shape were contained in such vacuoles (Figure 4A-4D). The size of L-form cells varied from about 0.2μm to 30μm in diameter. Examination of some BCG bacterial cells inside or outside macrophages revealed a fate and morphological peculiarities typical of cell wall deficient L- forms. While the percent of the formed L-forms at day 14 was about 15%,at day 45 we did not find any BCG bacilli with normal morphology and all observed bacteria were in L-form state. Signs of specific mode of L-form multiplication - budding,binary fission and formation of elementary bodies inside mother cells were recognized (Figure 4A-4C). Fusion of small phagosomes containing Lforms and formation of larger ones was seen as well (Figure 4A,4B,4D). Additional point of interest was the observation that many mitochondria (M) with enlarged size and endoplasmic reticulum dilation (ER) were clustered around and close to Lforms (Figure 4B,4D). The observed process of organelle translocation appeared to be related to the intracellular life of L- forms - survival and multiplication (Figure 4B,4D). Microbial digestion,respectively a process of complete phagocytosis of L-forms was not observed. The occurrence of Lforms in the intercellular space again at day 45 after challenge (Figure 4E-4G) suggested that L-forms probably exploited apoptotic-like pathways as means of returning to the extracellular environment and for subsequent rounds of new entry and uptake by macrophages.
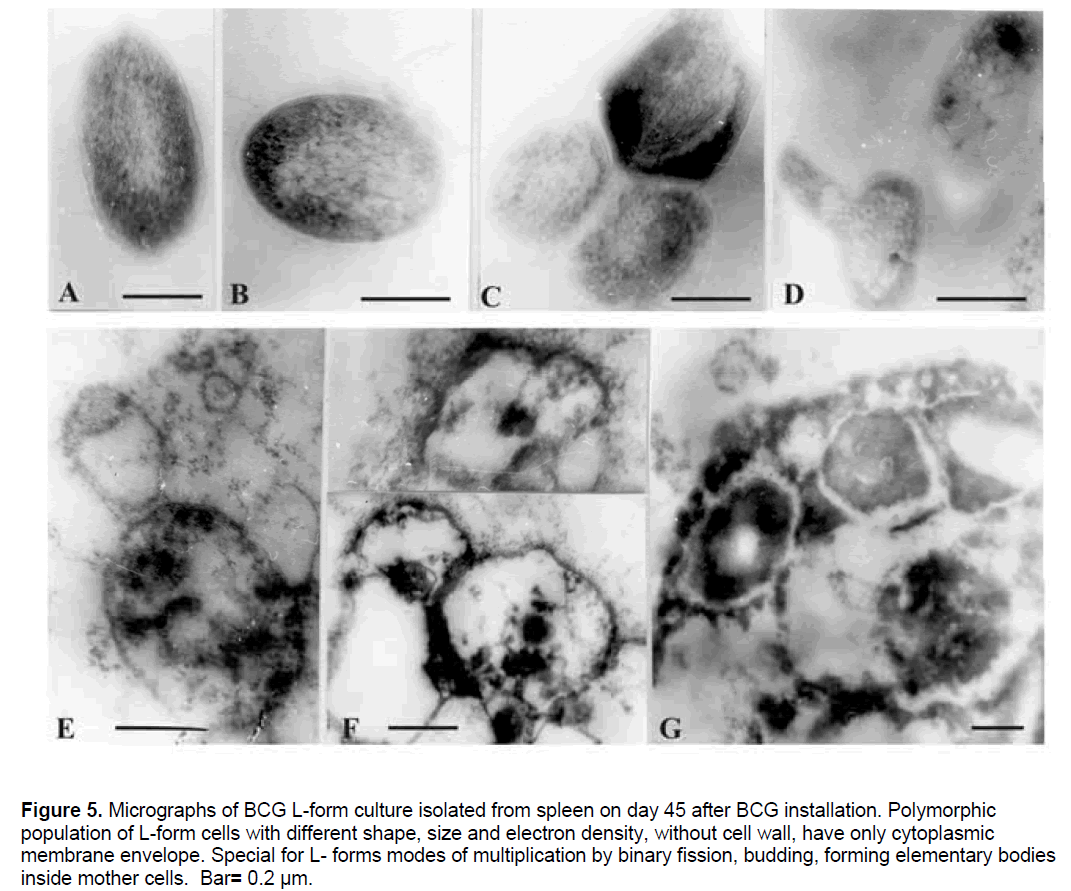
In correspondence to the observed by electron microscopy phenomenon of L-form formation (Figure 3,4),BCG L-form cultures were isolated from spleens of both animal groups (BCG and Len + BCG) on 45 day after challenge. Electron microscopic micrographs of L-form culture isolated from spleen of Len + BCG treated animal demonstrated polymorphic population of cells with different size and electron density (Figure 5). The morphology of L-form cells from this culture was similar to the observed and recognised L-forms during interaction with peritoneal phagocytes.
Figure 5.Micrographs of BCG L-form culture isolated from spleen on day 45 after BCG installation. Polymorphic population of L-form cells with different shape, size and electron density, without cell wall, have only cytoplasmic membrane envelope. Special for L- forms modes of multiplication by binary fission, budding, forming elementary bodies inside mother cells. Bar= 0.2 µm.
Numerous polymorphic ovoid cells of different size,shape and electron density,without cell wall and only with unit membrane envelope were seen. Typical modes of L-form multiplication - binary fission (Figure 5c),budding (Figure 5 D,5E),as well as forming of small electron dense elementary or large bodies outside or inside the membranous cells (Figure 5E,5F),and inside so called “mother” cells (Figure 5G) were also present.
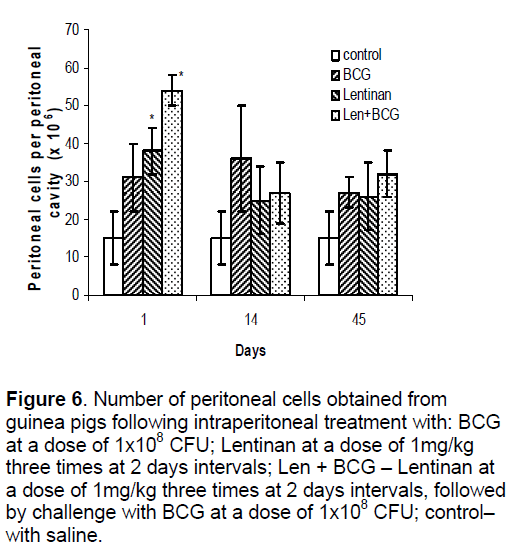
Peritoneal cell response in both models (with and without treatment with Lentinan) following BCG installation was assessed by establishing cell number and macrophage H2O2 production. As shown in Figure 6 ,statistically significant increase (approximately 3 times) in the number of peritoneal exudative cells was measured at the first interval of examination in the animals with Lentinan treatment (Len+BCG),while in BCG and Lentinan groups the number of peritoneal cells enhanced approximately 2 times compared to the control. In contrast to saline control group,peritoneal macrophages collected from animals treated with Lentinan alone or with Len+ BCG were greatly stimulated to produce H2O2 on the first day of examination. At days 14 and 45,the highest values were found in BCG and Len+BCG groups (Figure 7).
Figure 6.Number of peritoneal cells obtained from guinea pigs following intraperitoneal treatment with: BCG at a dose of 1x108 CFU; Lentinan at a dose of 1mg/kg three times at 2 days intervals; Len + BCG – Lentinan at a dose of 1mg/kg three times at 2 days intervals, followed by challenge with BCG at a dose of 1x108 CFU; control– with saline.
Figure 7.Production of H2O2 by peritoneal macrophages obtained from guinea pigs following intraperitoneal treatment with: BCG at a dose of 1x108 CFU; Lentinan at a dose of 1mg/kg three times at 2 days intervals; Len + BCG – Lentinan at a dose of 1mg/kg three times at 2 days intervals, followed by challenge with BCG at a dose of 1x108 CFU; control – with saline.
BCG colonies with typical normal morphology were isolated on Löwenstein –Jensen (LJ) medium from spleens of BCG and Len + BCG treated animals at days 14 and 45 and CFUs were enumerated (Table 1). It was found that the BCG counts were significantly reduced in Len + BCG group,compared with BCG group. The isolation of L-form cultures were isolated from both groups (BCG ; Len + BCG) on 45 day after challenge provides evidence that BCG bacilli undergo transition to cell wall defective variants in vivo and are viable. Enumeration of L-form CFU was impossible because L-form culture required for isolation initial enrichment in liquid Dubos broth.
4. Discussion
Great progress has been recently made toward the understanding of mycobacteria host cell interactions. Different aspects of mycobacterial survival inside macrophages,cell morphology and phagocytosis have been studied [13,16-19]. However,little is known about the behavior and mechanisms by which M. bovis BCG bacilli remain live for long time in vivo.
In the present study,we examined the time course of changes in morphology of BCG bacilli,loss of bacterial cell walls and conversion to L-forms during phagocytosis by peritoneal macrophages in guinea pigs. Although peritoneal macrophages are not typical niche for mycobacteria,we attempted to inhibit the classical bacterial forms and to induce occurrence of cell wall deficient forms by using an unusual unfavorable location (peritoneal cavity) of infection. In order to study the interaction of BCG bacilli with resting and activated macrophages,we set up two experimental models – with and without Lentinan pre-activation. Lentinan,a (1-3)-beta-Dglucan is a well known immunomodulator [20].
Our observations through electron microscopy showed uptake of BCG bacilli and typical signs of the initial stage phagocytosis at the first day of infection. No changes in bacterial morphology were found at this time point. It is interesting to note that the rate of BCG bacilli uptake by Lentinan activated peritoneal macrophages was apparently enhanced at this time point and that BCG bacilli were seen to be situated in close contact with or inside cell nucleus. Similar intranuclear location has been reported for R. rickettsii [21]. Although various intracellular pathogens can survive in diverse set of compartments within macrophages [22],microorganisms contained within the nucleus appear to be a relatively rare phenomenon. It is not clear how bacilli enter the nucleus. However,the observation that after treatment with Lentinan peritoneal macrophages form nuclear membrane “nooses” and dilated internal membranes indicates functional changes of phagocytes and possible increased permeability of nuclear membranes. As has been extensively studied by many authors,mycobacteria prefer intraphagosomal (early endosomes) location in macrophages [13,16-19,22].
Intriguingly,our electron microscopic observation at the late intervals of infection revealed an interesting phenomenon of L-form formation inside macrophages. As shown in figures,pleomorphic and relatively large BCG L-form bodies were found inside vacuoles. Micrographs of pairs or groups of L-form cells of varying size and electron density indicated replication of L-forms by specific modes such as budding,binary fission and formation of elementary bodies. It is also of significance to note that digestion of L-forms,respectively complete phagocytosis was not observed. Some intraphagosomally located L-forms inside macrophages were surrounded by multimembranes and so packed within membranes they were released to the extracellular space. The observed cycle of L-form attachment and engulfment by new phagocytes at the late stage of infection suggests that L-forms probably exploit apoptotic-like pathway as means of returning to the extracellular environment and for subsequent rounds of new entry and uptake by macrophages. It is generally assumed that apoptosis has developed as one of host defence mechanisms against infection,but it is not completely clear what advantages apoptosis can provide to bacteria [22]. Obviously,such apoptosis-like pathway may protect L-forms from humoral and cellular host defense factors during their trafficking from intracellular to extracellular compartment and vice versa.
Recent studies in the field of mycobacterial genetics and immunology have shown that virulent mycobacterial strains elicit much less macrophage apoptosis than avirulent and attenuated strains [23,24]. It is interesting that induced by Mycobacterium bovis BCG apoptosis has been associated with reduced viability of infecting BCG bacilli [23].
Our data demonstrate that BCG bacilli can convert to cell wall deficient form (L-form) inside both resting and Lentinan- preactivated macrophages during infection in guinea pigs. The isolation of Lform cultures from spleens at day 45 of infection provides evidence that cell wall deficient forms formed in vivo are viable. A number of authors have presented evidence that cell-wall defective variants can be formed within macrophages [25-27]. Thacore and Willett have reported about formation of spheroplasts of M. tuberculosis within tissue cultures cells [25].
As far as L-form conversion phenomenon (cell wall deficiency) is considered as a phenomenon of adaptation when bacteria are exposed to different damaging factors,particularly in face of host defense mechanisms,it is possible that guinea pig peritoneal macrophages might provide an intracellular environment in which BCG bacilli can undergo transition to cell wall defective variants. Lforms resided within phagocytic vacuoles and some of them underwent multiplication.
An interesting aspect of intracellular L-phase growth was the observed by us formation of multilayered membranes around L-form cells. Whether these membranes are of bacterial or host cell origin and what their function is remains unknown. Cole reported interesting ultrastructural observations about the ability of L-form cultures to produce membranous aggregates appearing as concentric myelinlike figures [28].
On the other hand,similar multilamella (myelinlike) structures containing bacterial and other debris have been observed in infected with BCG bacilli and induced for autophagy macrophages by Gutierrez et al. [19]. According to the authors,these structures have been typical of autophagosomes and the phenomenon of autophagy which are considered as specific innate defense mechanisms against intraphagosomal microbes.
As far as cell wall deficiency facilitates the bacterial survival under unfavorable conditions,Lforms of different bacterial species have been shown to survive and persist for a long time inside macrophages due to the ineffectual phagocytosis,digestion and clearance [29-32]. The finding that of all the bacteria,L-forms predominate and are crucial to the survival of mycobacteria in vivo [27,33] needs to be taken into account when developing and putting in use new viable mycobacterial vaccines. L-forms of M. bovis BCG bacilli have been found in the blood of persons vaccinated against TB with BCG vaccine. [34]. All this provides us with insights into the importance of L-form conversion phenomenon for the behavior,persistence and safety of live BCG vaccine.
In conclusion,the results shown here could provide the basis for better understanding the behaviour,intra- and extra-cellular trafficking of live Mycobacterium BCG bacilli in vivo and for elucidating the mechanisms by which they can remain viable for a long time. L-form conversion phenomenon and its occurrence in vivo suggest one of the possible pathways by which they can survive,replicate and persist within the body for a long period. The cell wall deficiency could be assumed as manifestation of the dormant/latent state of M. bovis BCG inside hosts. Further investigations are needed to determine the significance of this phenomenon.
5. Acknowledgements
The authors thank Ajinomoto,Tokyo,Japan,for providing Lentinan for experiments.
References
- Guerin,C.,Rosenthal,S.R. (1957) The history of BCG: early history. In: BCG Vaccination against Tuberculosis (Rosenthal,S.R. ed.) Churchill,London. pp. 48-57.
- Colditz,G.A.,Brewer,T.F.,Berkey,C.S.,et al. (1999) Efficacy of BCG vaccine in the prevention of tuberculosis. Metaanalysis of the published literature. JAMA,271: 698-702.
- Bryder,L. (1999) We shall not find salvation in inoculation: BCG vaccination in Scandinavia,Britain and the USA,1921-1960. Soc Sci Med,49: 1157-1167.
- Andersen,P. (2006) Vaccine strategies against latent tuberculosis infection. Trends in Microbiology,15:7-13.
- Rodrigues,L.C.,Diwan,V.K.,Wheeler JG. (1993) Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol,22: 1154-1158.
- Roth,A.E.,Stensballe,M.,Gary,M.,Aaby,P. (2006) Beneficial non-targeted effects of BCG-Ethical implications for the coming introduction of new TB vaccines? Tuberculosis,86: 397-403.
- Armbruster,C.,Junker,W.,Vetter,N.,et al. (1990) Disseminated bacilli Calmette-Guerin infection in an AIDS patient 30 years after BCG vaccination. J Infect Dis,162: 1216.
- Smith,E.,Thybo,S.,Bennedsen,J. (1992) Infection with Mycobacterium bovis in a patient with AIDS: a late complication of BCG vaccination. Scand J Infect Dis,24: 109-110.
- Reynes,J.,Perez,C.,Lamaury,I.,et al. (1989) Bacille Calmette-Guérin adenitis 30 years after immunization in a patient with AIDS. J Infect Dis,160: 729.
- Méderlé,I.,Bourguin,I.,Ensergueix,D.,et al. (2002) Plasmidic versus insertional cloning of heterologous genes in Mycobacterium bovis BCG: impact on in vivo antigen persistence and immune responses. Infect Immun,70: 303-314.
- Abadie,V.,Badell,E.,Douillard,P.,et al. (2005) Neutrophils Rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood,106: 1843-1850.
- Fulton,S.,Reba,S.,Martin,T.,et al. (2002) Neutrophil-mediated mycobactericidal immunity in the lung during Mycobacterium bovis BCG infection in C57/BL6 mice. Infect Immun,70: 5322-5327.
- Grode,L.,Seiler,P.,Baumann,S.,et al. (2005) Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette- Guérin mutants that secrete listeriolysin. J Clin Invest,115: 2472–2479.
- Pick,E.,Keiseri,Y. (1980) A simple colorimetric method for measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods,38: 161-170.
- Lowry,O.H.,Rosebrough,N.J.,Farr,A.L.,et al. (1951) Protein measurement with the folin phenol reagents. J Biol Chem,193: 265-275.
- Baumann,S. A.,Nasser,E.,Kaufmann,S.H.E. (2006) Progress in tuberculosis vaccine development. Curr Opin Immunol 18: 438-448.
- Warner,D.F.,Mizrahi,V. (2007) The survival kit of M.tuberculosis. Nature Medicine,13: 282-284.
- Lamichhane,G.,Bishai,W. (2007) Defining the ‘survivasome’ of M.tuberculosis. Nature Medicine,13: 280-282.
- Gutierrez,M.G.,Master,S.S.,Singh,S.B.,et al. (2004) Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119: 753-766.
- Maeda,Y.,Chihara,G. (1999) Lentinan and other antitumor polysaccharides. In: Immunomodulatory agents from plants (Wagner,H.H. ed.). Basel,Birkhauser,Verlag,pp. 203-221.
- Wisseman,C.L. Jr,Edlinger,E.A.,Waddel,A.D.,et al. (1976) Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect Immun.14: 1052-1064.
- Rosenberger,C.M.,Finlay,B.B. (2003) Phagocyte sabotage: Disruption of macrophage signalling by bacterial pathogens. Nature Reviews | Mol Cell Biol 4: 385-396.
- Riendeau,C.J.,Kornfeld,H. (2003) THP-1 Cell Apoptosis in Response to Mycobacterial Infection. Infect Immun,71: 254-259.
- Keane,J.,Remold,H.G.,Kornfeld,H. (2000) Virulent Mycobacterium tuberculosis Strains Evade Apoptosis of Infected Alveolar Macrophages. J Immun,164: 2016-2020.
- Thacore,H.,Willett,H.P. (1966) The formation of spheroplasts of Mycobacterium tuberculosis in tissue cultures cells. Am Rev Resp Dis,93: 786-796.
- Michailova,L.,Stoitsova,S.,Markova,N.,et al. (2000) Interaction of alveolar macrophages with Staphylococcus aureus and induction of microbial Lforms during infection in rats. Int J Med Microbiol,290: 259-267.
- Mattman,L.H. (2001) Mycobacterium tuberculosis and atypicals. In: Cell wall deficient forms: Stealth Pathogens. 3rd eds (Mattman,L.H. ed.). Boca Raton Fla,CRC Press,pp.189-197.
- Cole,R.M. (1971) Some implications of the comparative ultrastructure of bacterial L-forms. In: Mycoplasmas and the L-forms of bacteria (Madoff,S. ed.) Gordon and Breach Scientific Publishers,New York,pp 49-83.
- Mihailova,L.,Markova,N.,Radoucheva,T.,et al. (1993) Cell interaction of Listeria monocytogenes Lforms and peritoneal exudative cells in rats. Can J Microbial,39:1014-1021.
- Michailova,L.,Markova,N.,Radoucheva,T.,et al. (2000) Atypical behaviour and survival of Steptococcus pyogenes L-forms during intraperitoneal infection in rats. FEMS Immun Med Microbiol ,28: 55-65.
- Markova,N.,Mihailova,L.,Vesselinova,A,et al. (1997) Cell wall-deficient forms (L-Forms) of Listeria monocytogenes in experimentally infected rats. Zbl Bakt 286: 46-55.
- Michailova,L.,Kussovski,V.,Radoucheva,T.,et al. (2007) Persistence of Staphylococcus aureus L-forms during experimental lung infection in rats. FEMS Microbiol Lett ,268:88-97.
- Michailova,L.,Kussovski,V.,Radoucheva,T.,et al. (2005) Morphological variability and cell-wall deficiency in ‘heteroresistant’ strains. Int J Tuberc Lung Dis,9: 907-914.
- Xalabarder,C. (1958) Electron microscopy of tubercle bacilli. Excerpta Med. Sect XV Chest Dis,11: 467-473.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences