Fatigue in Long Coronavirus Disease 2019 A Review of its Mechanism and Management
Junji Moriya1 , Takeshi Nakahashi1, Takuya Sakamoto2, Tsugiyasu Kanda3,4*, Yuji Kasamaki3,4
1Department of General Medicine, Kanazawa Medical University, Ishikawa, Japan 2Medical Research Institute, Kanazawa Medical University, Ishikawa, Japan 3Department of Community Medicine, Kanazawa Medical University, Ishikawa, Japan 4Department of General Medicine, Kanazawa Medical University Himi Municipal Hospital, Him, Japan
Published Date: 2024-03-19DOI10.36648/1860-3122.20.1.106
Junji Moriya1, Takeshi Nakahashi1, Takuya Sakamoto2, Tsugiyasu Kanda3,4*, Yuji Kasamaki3,4
1Department of General Medicine, Kanazawa Medical University, Ishikawa, Japan
2Medical Research Institute, Kanazawa Medical University, Ishikawa, Japan
3Department of Community Medicine, Kanazawa Medical University, Ishikawa, Japan
4Department of General Medicine, Kanazawa Medical University Himi Municipal Hospital, Him, Japan
- *Corresponding Author:
- Tsugiyasu Kanda
Department of Community Medicine, Kanazawa Medical University, Ishikawa,
Japan
Tel: +81-076-286-2211;
E-mail: kandat@kanazawa-med.ac.jp
Received date: February 17, 2024, Manuscript No. IPEJBIO-24-18666; Editor assigned date: February 20, 2024, PreQC No. IPEJBIO-24-18666 (PQ); Reviewed date: March 05, 2024, QC No. IPEJBIO-24-18666; Revised date: March 12, 2024, Manuscript No. IPEJBIO-24-18666 (R); Published date: March 19, 2024, DOI: 10.36648/1860-3122.20.1.106
Citation: Moriya J, Nakahashi T, Sakamoto T, et al. (2024) Fatigue in Long Coronavirus Disease 2019: A Review of its Mechanism and Management. Electronic J Biol 20(1): 1-7
Abstract
Fatigue emerges as a prevalent symptom in individuals affected by coronavirus disease 2019 (COVID-19). Chronic unexplained symptoms become apparent in the post-acute phase of the illness, collectively known as long COVID-19. A sustained fatigue symptom prevalence rate of 21.6% was reported in 120 studies. Notably, emerging data on new omicron variants indicate a 50% reduction in the risk of developing long COVID. The potential risk factors associated with fatigue were type 2 diabetes, Epstein-Barr virus viremia and lower cortisol levels. Female sex, older age, post-traumatic stress disorder and intensive care unit admission were associated with severity. Although some treatments have demonstrated efficacy in specific populations, their universal applicability remains uncertain. The role of physical exercise in managing fatigue is a subject of controversy. The most extensive cohort study to date reported a reduction from 68% at 6 months to 55% at 2 years in individuals experiencing fatigue or muscle weakness. The prevalence of long COVID continues to escalate, underscoring the imperative for enhanced diagnosis and management strategies. Robust clinical trials are essential to elucidate the underlying biological mechanisms.
Keywords
Fatigue; Long COVID; Pathogenesis; Risk; Management
Introduction
Fatigue emerges as the most frequent symptom in patients infected by coronavirus disease 2019 (COVID-19). The ongoing pandemic has shed light on the persistent burden of symptoms even after the acute stage. Systematic reviews indicate a prevalence of fatigue in long COVID ranging from 2.5% to 74%, with a mean of 21.6% [1] where fatigue of long COVID is identified as a major symptom [2].
Post-recovery, more than 60% of COVID-19 survivors experience difficulties in performing daily activities, self-care and social interactions. This lingering impact complicates the return to work, even in individuals without apparent symptoms [3]. This sequela is particularly significant, underscoring the insufficient understanding of symptoms such as fatigue. A cohort study in Chinese hospital involving 1,733 COVID-19 patients revealed that approximately 63% experienced fatigue, persisting for up to 6 months [4]. This review aimed to explore the proposed mechanisms, risk factors, biomarkers and management strategies related to fatigue related with long COVID, under a specific focus on insights from Japan.
Review of Literature
History
Post-viral fatigue was initially identified during the Spanish flu pandemic affected by the influenza A virus in 1918, which resulted in over 20 million deaths [5]. Persistent and unsolved symptoms was observed in the post-COVID-19 phase, collectively known as post-acute COVID-19 syndrome, post-COVID-19 syndrome, or long COVID-19. In this review, the term “long COVID” was used.
Fatigue, a prevalent symptom, is commonly experienced during short-term illness, typically dissipating as the illness concludes. However, in certain cases, fatigue persists, unaffected by rest and its underlying cause remains unclear. This enduring fatigue reduces energy levels, hampers the ability to perform tasks and impairs focus, thereby impacting the quality of life and mental well-being.
Fatigue can encompass both a particular feeling of malaise and loathing to activity or objectively reduced performance [5]. It manifests in both mental and physical features. Importantly, the fatigue is not a universally definite sensation. Therefore, a thorough assessment is necessary to essential to explain the complaints of “fatigue,” “tiredness,” or “exhaustion” and to differentiate the deficiency of energy from damage of sleepiness or motivation, which might serve as indicators pointing toward specific diagnoses [6].
Clinical features
The World Health Organization accounted that long COVID-19 arises in individuals with an antiquity of severe acute coronavirus 2 infection, typically occurring 3 months from the symptom by COVID-19, ongoing for 2 months and defying explanation through an alternative diagnosis [7].
One hundred twenty studies were thoroughly reviewed, with the duration of follow-up ranging from 12 weeks to 12 months. Among more than 20 symptoms, fatigue emerged as the most prevalent, persisting at a rate of 21.6% (range: 2.5%-74.7%) [8].
Post-acute infectious symptoms align with the diagnosis of Myogenic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). ME/CFS is characterized by general exertion prejudice, primarily presenting as immunological and neurological symptoms attended by chronic fatigue unrelieved by sleep or relaxation. Patients with this illness involvement a deteriorating of symptoms following emotional, cognitive or physical energy beyond their endured limitation. These symptoms exacerbation, termed “post-exertional malaise” or “post-exertional symptom exacerbation,” may continue for several days, weeks or months. These indications can be associated with permanent decline. The fatigue symptom in this context may serve as a protective mechanism for a compromised neurological system, such as cognition or mentality, while awaiting recovery [9].
Virus variant, vaccination and anti-viral drugs
The emergence of new omicron variants is reportedly linked to a 50% reduction in the risk of progressing long COVID [10]. COVID-19 vaccination has demonstrated a decrease in long COVID incidence, particularly notable after the second dose (odds ratio: 0.64). Despite vaccination efforts, ongoing long COVID cases, including those investigated in Japan, have not shown a significant reduction [11,12]. Long COVID was more prevalent and associated with more severe symptoms among individuals infected before the pre-Omicron era [13]. Recent research suggested that anti-viral agents, such as nirmatrelvir/ ritonavir, may reduce the risk to long COVID [14].
Pathogenesis
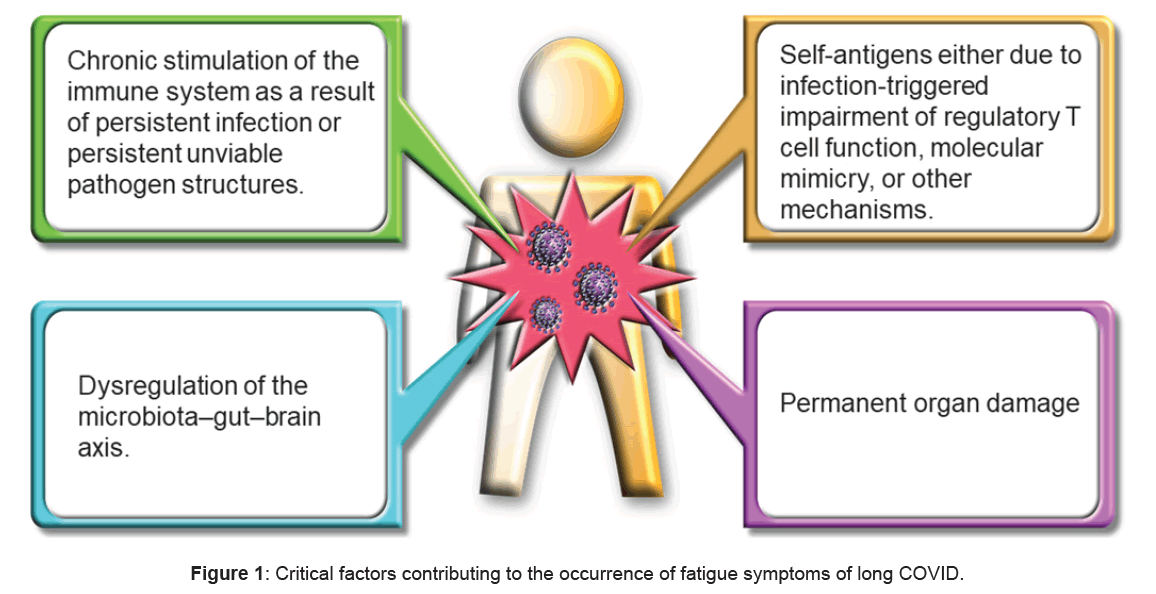
In terms of pathogenesis, several potential mechanisms have been proposed. First, persistent infection or the presence of unviable pathogen structures may lead to chronic immune system stimulation. Second, the alternative modes of immune induction may include targeting self-antigens, due to infection-initiated damage of regulatory T-cell role, molecular mimicry, or other mechanisms [3]. Third, the microbiota-gut-brain axis may have been imbalanced. Fourth, permanent organ damage may have occurred (Figure 1). These progressions are not equally exclusive and may coexist in mixture or be utterd with mixed intensity in dissimilar PAIs subsets. The illustration was generated using BioRender.com. Gottschalk et al. have proposed that the mitochondrial impairment of metabolic pathways triggers the fatigue symptom through the apoptosis of infected muscle cells [15,16].
A combined analysis of magnetic resonance imaging and positron emission tomography revealed an association between more pronounced fatigue and a reduced volume of the frontal area of the brain, with no observed changes in brain metabolism [17]. Moreover, the impairment of intracoronary GABAergic circuits has been linked to persistent fatigue and cognitive dysfunction. Neuroinflammation may induce GABAergic and fatigue [18].
Risk factors
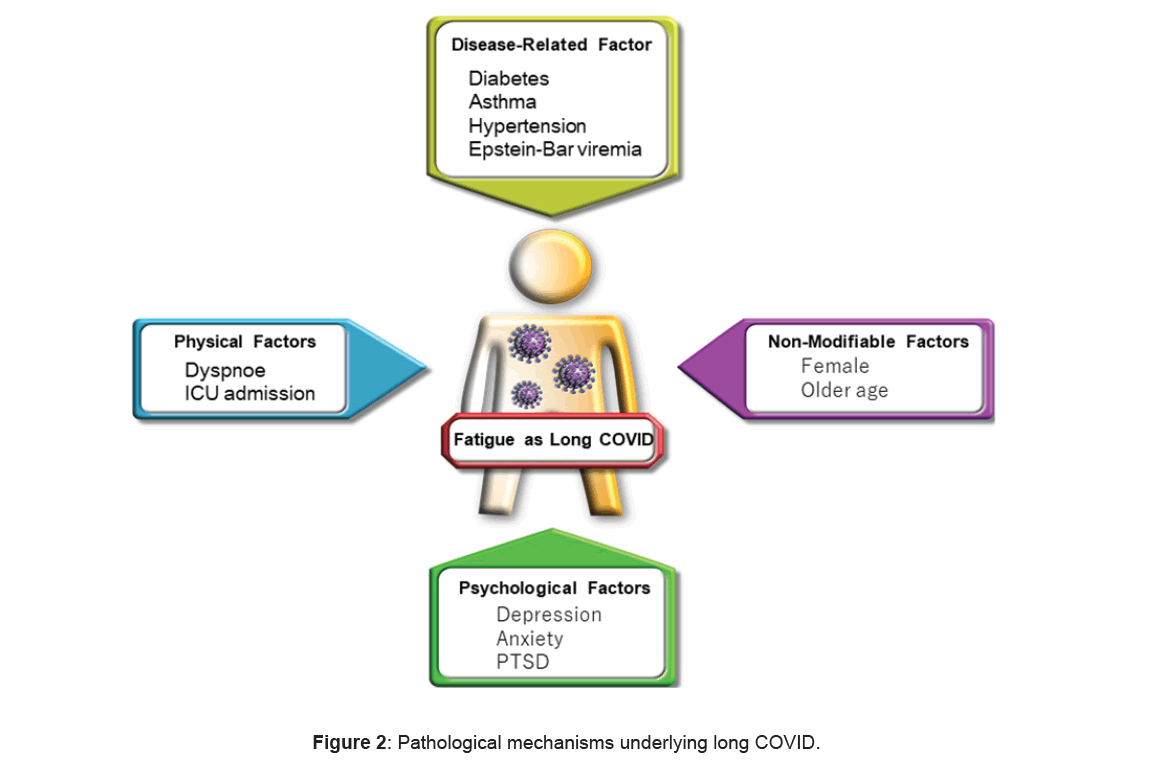
Persistent dyspnea was associated with fatigue. The potential risk factors for fatigue included type 2 diabetes and Epstein-Barr (EB) virus viremia [19]. Klein J et al. reported that participants with long COVID showed high titers of antibodies against the EB virus and lower levels of cortisol [20].
Female sex emerged as a risk for fatigue [1,9,21]. The reasons for this association remain unclear, but it is hypothesized that women may experience higher levels of psychological stress, increased depression and anxiety, poorer sleep worth and an inflammatory adaptation. Although some studies found an association between older age and fatigue severity, others did not [21]. Additionally, baseline depressive or anxiety conditions were linked to higher levels of fatigue. Moreover, posttraumatic stress disorder was associated with the severity of fatigue [22].
Hospitalization did not lead to an increased frequency of fatigue symptoms compared with non-hospitalization. However, admission to the Intensive Care Unit (ICU) was related with an elevated risk of fatigue (Figure 2) [23].
In Japan, the risk factors for long COVID included female sex, ages of middle (41-64 years), oxygen obligation and critical state in hospitalization [2].
Marker
A biomarker evaluation in patients with ME/CFS may have relevance to long COVID, counting saliva tests, electric impedance blood tests, erythrocyte distortion, sex-specific plasma lipid outlines and flexibles related to isocapnic buffering. The use fullness of developed and validated biomarkers that may be utilized for the diagnosis of long COVID is not adequately underlined. These markers will not only aid in establishing the diagnosis but will also be valuable for accurately assessing treatment replys [24].
The positivity of anti-nuclear antibodies was associated with fatigue and the circulating Tumor Necrosis Factor alpha (TNF-α) level was used as a predictor of fatigue at 12 months [25]. Circulating anti-nuclear autoantibodies in COVID-19 survivors were predictive of long COVID [26]. The D-dimer and C-reactive protein levels were correlated with cognitive deficits and professional effect. This relation was refereed by fatigue and breathless, with neither profile significantly facilitated by depression or anxiety. Individuals with higher levels of D-dimer shows the risk of venous thromboembolism [24].
Discussion
Treatment
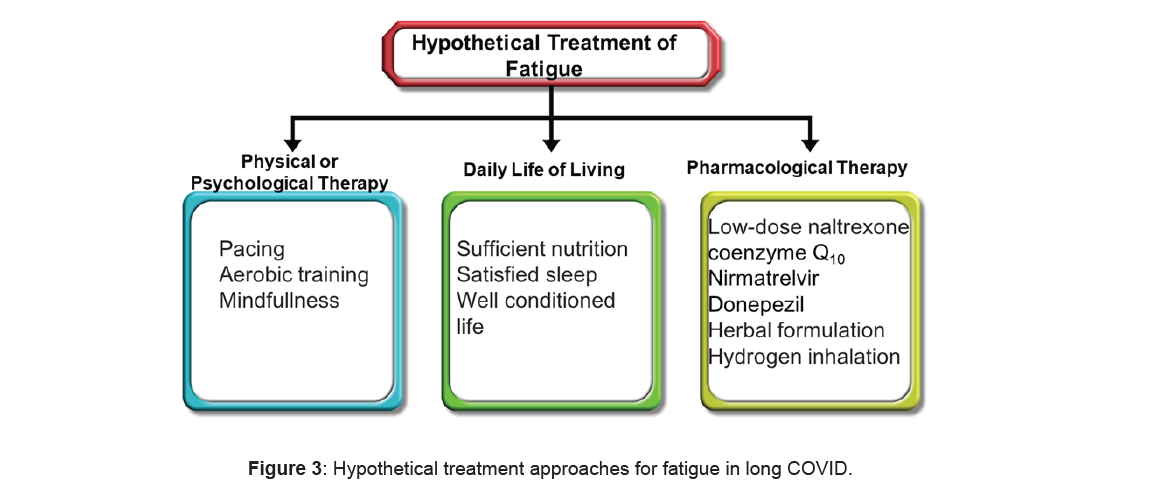
Interventions targeting positive factors have been proven effective for specific subgroups of patients in long COVID (Figure 3) [27]. Numerous approaches for ME/ CFS have demonstrated success for individual patients with long COVID. These interventions included stepping and symptom-matched pharmacological choices. Examples include β-blockers for Postural Orthostatic Tachycardia Syndrome (POTS), low-dose naltrexone for brain inflammation, injective immunoglobulin and nonpharmacological selections including increasing salt intake for POTS, elimination diets for gastrointestinal symptoms and cognitive stepping for cognitive impairment [28]. Low-dose naltrexone is administered for ME/ CFS [29] and has also demonstrated efficacy for long COVID. Similarly, low-dose aripiprazole was effective for fatigue, un-refreshing sleep and brain fog through dopamine modulation [30]. The administration of H1 and H2 antihistamines, frequently using protocols for mast cell activation syndrome and incorporating famotidine, are used to improve a broad spectrum of symptoms [31].
Kampo medicine is a potential pharmacological treatment for long COVID, particularly addressing general fatigue. Hochuekkito emerged as the most frequently prescribed Kampo medicine [32].
Apheresis is also improving long COVID symptoms, [33] since it is hypothesized to eradicate microclots and reduces autoantibodies [34]. Various supplements, including coenzyme Q10 and D-ribose, are shown potential in caring for long COVID and ME/CFS. Further study may be warranted to explore their full benefits [35].
Noninvasive Brain Stimulation (NIBS) methods present promising therapeutic strategies to alleviate individual pathology of long COVID-related fatigue. Preliminary evidence suggests the efficiency of NIBS in long COVIDrelated fatigue [36].
The administration of donepezil to mice improved fatigue by increasing the production of acetylcholine. This improvement is significant as a reduction in the acetylcholine level was associated with neuroinflammation and fatigue [37]. The reduced levels of donepezil in mice recovered neuroinflammation, malaise and depressive clinical signs [38].
Mindfulness meditation has demonstrated efficacy in reducing fatigue and deficient sleep. In individuals after viral infections, it not only reduces fatigue but also enhances immunity and mitigates inflammatory-driven pathogenesis [39].
Progressive epigenetics has implications for long COVID and its treatment. DNA methylation, a molecular epigenetic change, resembles developing structural conditions and changed gene alternations. Epigenetic features such as mindful movements play a crucial role in environmental enrichment. Contemplated interventions are vital for moderating immune function, specifically for long COVID.
The role of physical exercise in managing fatigue remains controversial. Although some studies indicated that submaximal exercise can reduce fatigue, others suggested that physical activity may exacerbate symptoms, especially fatigue [40,41].
Some nutrients are crucial for reducing long COVID fatigue syndrome, such as essential fatty acids, vitamin B group and vitamin C, zinc, magnesium, folic acid, L-tryptophan, L-carnitine and coenzyme Q10. Antioxidants may also offer support, drawing from research on chronic fatigue syndrome [42,43]. The daily consumption of arginine and carnitine, magnesium, zinc may advance physical symptoms in long COVID. Additionally, a supplement comprising cysteine and essential amino acids; vitamins B6 and B1 and malic, succinic and citric acids was beneficial on nutritional status, better recovery and capable life in long COVID-19 with persistent fatigue [44,45]. Recent studies showed that plant-based food may alleviate fatigue symptoms in long COVID (Table 1) [46].
| Symptoms and/or biological mechanism | Treatments | Supporting evidence | Comments |
|---|---|---|---|
| Fatigue and neurocognitive impairment | Nirmatrelvir | - | Reduced risk of long COVID with unvaccinated, vaccinated, and boosted [14]. |
| Pain, fatigue, neurological symptoms | Low-dose naltrexone | ME/CFS and other literature | Substantial anecdotal reports of success within the patient community [30]. |
| Fatigue, unrefreshing sleep, brain fog | Low-dose aripiprazole | ME/CFS literature | Dopamine-modulating drugs like aripiprazole may improve fatigue and cognitive symptoms in ME/CFS [31]. |
| Fatigue, brain fog, abdominal disorders, and increased heart rate | Histamine receptors blockade | - | Mast cell activation may play a role in the pathophysiology of long-COVID [32]. |
| Fatigue | Donepezil | - | Increased production of acetylcholine [39]. |
| Fatigue in CFS | Kampo, hochuekkito | - | Treatment for mild to moderate COVID-19 [33]. |
| Fatigue | Coenzyme Q10, D-ribose | ME/CFS literature | - |
| Fatigue | Coenzyme Q10 and alpha lipoic acid | Chronic Covid syndrome | Antioxidant effects for CFS [43,44]. |
| Fatigue | Non-invasive brain stimulation | - | The methods are safe and well-tolerated and allow for large-scale use in clinical practice [37]. |
| Debilitating symptoms of patients with CFS | Apheresis | CFS | Remove microclots and autoantibodies [34]. |
| Postexertional malaise | Pacing | ME/CFS literature | Exercise, cognitive behavioural therapy and graded exercise therapy are contraindicated. |
Table 1: Summary of candidate treatments.
Patient care provided in clinics
Several tools are available to assist individuals in coping with long COVID and accessing social and health care. Numerous support groups provide a platform for individuals experiencing long COVID symptoms to connect. The National Institute of Mental Health offers valuable tips for managing stress during pandemics [47]. Additionally, the UCLA Health homepage showed the details of counseling and support, medications and physical therapy. Stanford University Hospital established the Post-Acute COVID-19 Syndrome Clinic, while Brigham and Women’s Hospital launched a new, multidisciplinary COVID Recovery Center. These facilities are dedicated to assessing post- COVID symptoms and providing comprehensive care to help patients achieve the best possible recovery [48-50].
Prognosis
In the longest cohort study conducted, a 68% decrease in fatigue or muscle weakness was observed at 6 months, decreasing to 55% at 2 years. Notably, the percentage of depression or anxiety dropped from 23% at 6 months to 12% at 2 years in China. Return to work was achieved by 88% of the patients within 12 months. Similar movements were detected in hospitalized patients in Italy, where 40.5% of patients reported symptoms at 12 months, with fatigue being the most common [51,52]. After 2 years, long COVID was observed in 36.1% of 230 Italian patients [8,53].
Conclusion
Education
To effectively equip the future generation of healthcare professionals and researchers, medical universities need to enhance the education on viruses and infectioninitiated illnesses, extended pandemics, including long COVID and ME/CFS. Capability evaluations must encompass these illnesses. As of 2013, only 6% of medical schools comprehensively addressed ME/CFS in treatment conferences, research and curricula, which has resulted in challenges related to care, correct diagnosis, investigation and particular treatment.
Within >2 years of research, the prevalence of long COVID continues to rise, highlighting the pressing need for further investigation. Current diagnoses and management approaches have proven insufficient. Confidence in clinical trials is essential to elucidate the biological mechanisms underlying long COVID and inform more effective treatments.
Conflict of Interests
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author contributions
JM and TN were responsible for collecting the references and organizing the manuscript. TS managed and prepared the figures. TK critically reviewed the manuscript. YK managed and contributed various ideas to enhance the content of this paper.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing
References
- Woodrow M, Carey C , Ziauddeen N, et al. (2023) Systematic review of the prevalence of long COVID. Open Forum Infect Dis 10: ofad233.
[Crossref], [GoogleScholar], [Indexed]
- Terai H, Ishii M, Takemura R, et al. (2023) Comprehensive analysis of long COVID in a Japanese nationwide prospective cohort study. Respir Investig 61: 802-814.
[Crossref], [GoogleScholar], [Indexed]
- Ceban F, Ling S, Lui LMW, et al. (2022) Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun 101: 93-135.
[Crossref], [GoogleScholar], [Indexed]
- Huang L, Yao Q, Gu X, et al. (2021) 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 398: 747-758.
[Crossref], [GoogleScholar], [Indexed]
- Trilla A, Trilla G, Daer C (2008) The 1918 "Spanish flu" in Spain. Clin Infect Dis 47: 668-673.
[Crossref], [GoogleScholar], [Indexed]
- Sharpe M, Wilks D (2002) Fatigue. BMJ 325: 480-483.
- Soriano JB, Murthy S, Marshall JC, et al. (2022) A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 22: e102-e107.
[Crossref], [GoogleScholar], [Indexed]
- Choutka J, Jansari V, Hornig M, et al. (2022) Unexplained post acute infection syndromes. Nat Med 28: 911-923.
[Crossref], [GoogleScholar], [Indexed]
- Antonelli M, Pujol JC, Spector TD, et al. (2022) Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 399: 2263-2264.
[Crossref], [GoogleScholar], [Indexed]
- Wada A, Higashiyama M, Kurihara C, et al. (2022) Protective effect of luminal uric acid against indomethacin-induced enteropathy: Role of antioxidant effect and gut microbiota. Dig Dis Sci 67: 121-133.
[Crossref], [GoogleScholar], [Indexed]
- Watanabe A, Iwagami M, Yasuhara J, et al. (2023) Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 41: 1783-1790.
[Crossref], [GoogleScholar], [Indexed]
- Thaweethai T, Jolley SE, Karlson EW, et al. (2023) Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA 329: 1934-1946.
[Crossref], [GoogleScholar], [Indexed]
- Xie Y, Choi T, Al-Aly Z (2023) Association of treatment with nirmatrelvir and the risk of Post-COVID-19 condition. JAMA Intern Med 183: 554-564.
[Crossref], [GoogleScholar], [Indexed]
- Choutka J, Jansari V, Hornig M, et al. (2022) Unexplained post-acute infection syndromes. Nat Med 28: 911-923.
[Crossref], [GoogleScholar], [Indexed]
- Gottschalk CG, Peterson D, Armstrong J, et al. (2023) Potential molecular mechanisms of chronic fatigue in long haul COVID and other viral diseases. Infect Agent Cancer 18: 7.
[Crossref], [GoogleScholar], [Indexed]
- Deters JR, Fietsam AC, Gander PE, et al. (2023) Effect of post-COVID-19 on brain volume and glucose metabolism: Influence of time since Infection and fatigue status. Brain Sci 13: 675.
[Crossref], [GoogleScholar], [Indexed]
- Campos MC, Nery T, Starke AC, et al. (2022) Post-viral fatigue in COVID-19: A review of symptom assessment methods, mental, cognitive, and physical impairment. Neurosci Biobehav Rev 142: 104902.
[Crossref], [Indexed], [GoogleScholar]
- Su Y, Yuan D, Chen DG, et al. (2022) Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185: 881-895.
[Crossref], [Indexed], [GoogleScholar]
- Klein J, Wood J, Jaycox J, et al. Distinguishing features of Long COVID identified through immune profiling. Nature 623: 139-148.
[Crossref], [Indexed], [GoogleScholar]
- Rudroff T, Workman CD, Bryant AD (2022) Potential factors that contribute to post-COVID-19 fatigue in women. Brain Sci 12: 556-559.
[Crossref], [Indexed], [GoogleScholar]
- Poole-Wright K, Guennouni I, Sterry O, et al. (2023) Fatigue outcomes following COVID-19: A systematic review and meta-analysis. BMJ Open 13: e063969.
[Crossref], [Indexed], [GoogleScholar]
- Unoki T, Sakuramoto H, Uemura S, et al. (2021) Prevalence of and risk factors for post-intensive care syndrome: Multicenter study of patients living at home after treatment in 12 Japanese intensive care units, SMAP-HoPe study. PLoS One 16: e0252167.
[Crossref], [Indexed], [GoogleScholar]
- Davis HE, McCorkell L, Vogel JM, et al. (2023) Long COVID: Major findings, mechanisms and recommendations. Nat Rev Microbiol 21: 133-146.
[Crossref], [Indexed], [GoogleScholar]
- Son K, Jamil R, Chowdhury A, et al. (2023) Up regulated interleukin-6, C-reactive protein,TNF-α may be potential biomarker of Long COVID. Eur Respir J 61: 2200970.
- Lai YJ, Liu SH, Manachevakul S, et al. (2023) Biomarkers in long COVID-19: A systematic review. Front Med (Lausanne) 10: 1085988.
[Crossref], [Indexed], [GoogleScholar]
- Taquet M, Skorniewska Z, Hampshire A, et al. (2023) Acute blood biomarker profiles predict cognitive deficits 6 and 12 months after COVID-19 hospitalization. Nat Med 10: 2498-2508.
[Crossref], [Indexed], [GoogleScholar]
- Bateman, L, Bested AC, Bonilla HF, et al. (2021) Myalgic encephalomyelitis/chronic fatigue syndrome: Essentials of diagnosis and management. Mayo Clin Proc 96: 2861-2878.
[Crossref], [Indexed], [GoogleScholar]
- Pitt B, Tate AM, Gluck D, et al. (2022) Repurposing Low-Dose Naltrexone (LDN) for the prevention and treatment of immunothrombosis in COVID-19. Eur Heart J Cardiovasc Pharmacother 8: 402-405.
[Crossref], [Indexed], [GoogleScholar]
- Crosby LD, Kalanidhi S, Bonilla A, et al. (2021) Off label use of Aripiprazole shows promise as a treatment for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A retrospective study of 101 patients treated with a low dose of Aripiprazole. J Transl Med 19: 50.
[Crossref], [Indexed], [GoogleScholar]
- Salvucci F, Codella R, Coppola A, et al. (2023) Antihistamines improve cardiovascular manifestations and other symptoms of long-COVID attributed to mast cell activation. Front Cardiovasc Med 10: 1202696.
[Crossref], [Indexed], [GoogleScholar]
- Takayama S, Yoshino T, Koizumi S, et al. (2023) Conventional and Kampo Medicine Treatment for Mild-to-moderate COVID-19: A multicenter, retrospective, observational study by the Integrative Management in Japan for Epidemic Disease (IMJEDI Study-observation). Intern Med 62: 187-199.
[Crossref], [Indexed], [GoogleScholar]
- Hohberger B, Harrer T, Mardin C, et al. (2021) Case report: Neutralization of autoantibodies targeting G-protein-coupled receptors improves capillary impairment and fatigue symptoms after COVID-19 infection. Front Med (Lausanne) 8: 754667.
[Crossref], [Indexed], [GoogleScholar]
- Tselmin S, Julius U, Jarzebska N, et al. (2022) COVID-19 and therapeutic apheresis. Horm Metab Res 54: 571-577.
[Crossref], [Indexed], [GoogleScholar]
- Barletta MA, Marino G, Spagnolo B, et al. (2023) Coenzyme Q10 + alpha lipoic acid for chronic COVID syndrome. Clin Exp Med 23: 667-678.
[Crossref], [Indexed], [GoogleScholar]
- Linnhoff S, Koehler L, Haghikia A, et al. (2023) The therapeutic potential of non-invasive brain stimulation for the treatment of Long-COVID-related cognitive fatigue. Front Immunol 13: 935614.
[Crossref], [Indexed], [GoogleScholar]
- Leng A, Shah M, Ahmad SA, et al. (2023) Pathogenesis underlying neurological manifestations of long COVID syndrome and potential therapeutics. Cells 12: 816.
[Crossref], [Indexed], [GoogleScholar]
- Oka N, Shimada K, Ishii A, et al. (2023) SARS-CoV-2 S1 protein causes brain inflammation by reducing intracerebral acetylcholine production. iScience 26: 106954.
[Crossref], [Indexed], [GoogleScholar]
- Porter N, Jason LA (2022) Mindfulness meditation interventions for Long COVID: Biobehavioral gene expression and neuroimmune functioning. Neuropsychiatr Dis Treat 18: 2599-2626.
[Crossref], [Indexed], [GoogleScholar]
- Araújo BTS, Barros AEVR, Nunes DTX, et al. (2023) Effects of continuous aerobic training associated with resistance training on maximal and submaximal exercise tolerance, fatigue and quality of life of patients post-COVID-19. Physiother Res Int 28: e1972.
[Crossref], [Indexed], [GoogleScholar]
- Arienti C, Cordani C, Lazzarini SG, et al. (2022) Fatigue, post-exertional malaise and orthostatic intolerance: A map of Cochrane evidence relevant to rehabilitation for people with post COVID-19 condition. Eur J Phys Rehabil Med 58: 857-863.
[Crossref], [Indexed], [GoogleScholar]
- Barrea L, Grant WB, Frias-Toral E, et al. (2022) Dietary recommendations for Post-COVID-19 syndrome. Nutrients 14: 1305.
[Crossref], [Indexed], [GoogleScholar]
- Ghram A, Ayadi H, Knechtle B, et al. (2022) What should a family physician know about nutrition and physical exercise rehabilitation' advices to communicate to 'long-term COVID-19' patients? Postgrad Med 134: 143-147.
[Crossref], [Indexed], [GoogleScholar]
- Galluzzo V, Zazzara MB, Ciciarello F, et al. (2022) Fatigue in Covid-19 survivors: The potential impact of a nutritional supplement on muscle strength and function. Clin Nutr ESPEN 51: 215-221.
[Crossref], [Indexed], [GoogleScholar]
- Landi F, Martone AM, Ciciarello F, et al. (2022) Effects of a new multicomponent nutritional supplement on muscle mass and physical performance in adult and old patients recovered from COVID-19: A pilot observational case-control study. Nutrients 14: 2316.
[Crossref], [Indexed], [GoogleScholar]
- Picone P, Sanfilippo T, Guggino R, et al. (2023) Neurological consequences, mental health, physical care and appropriate nutrition in Long-COVID-19. Cell Mol Neurobiol 43: 1685-1695
[Crossref], [Indexed], [GoogleScholar]
- U.S. Department of Health and Human Services (2023) Long COVID.
- (2024) Long COVID Treatment. UCLA Health.
- (2024) Post-Acute COVID-19 Syndrome (PACS) Clinic. Stanford Medicine.
- (2024) COVID Recovery Center. Brigham and Women's Hospital.
- Huang L, Li X, Gu X, et al. (2022) Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir Med 10: 863-876.
[Crossref], [Indexed], [GoogleScholar]
- Fumagalli C, Zocchi C, Tassetti L, et al. (2022) Factors associated with persistence of symptoms 1 year after COVID-19: A longitudinal, prospective phone-based interview follow-up cohort study. Eur J Intern Med 97: 36-41.
[Crossref], [Indexed], [GoogleScholar]
- Peghin M, de Martino M, Palese A, et al. (2023) Post-COVID-19 syndrome 2 years after the first wave: The role of humoral response, vaccination and reinfection. Open Forum Infect Dis 10: ofad364
[Crossref], [Indexed], [GoogleScholar]
- Peterson TM, Peterson TW, Emerson S, et al. (2013) Coverage of CFS within U.S. medical schools. Univers J Public Health 1: 177-179.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences