Effect of Foliar Application of Jasmonic Acid on Hypericin Content of St. JohnÃÆâÃâââ¬Ãâââ¢s Wort ( Hypericum perforatum L.)
B. Hamedi, A. Ghasemi Pirbalouti, P. Moradi
1Research Center for Medicinal Plants & Ethno-veterinary,Department of Medicinal Plants,Islamic Azad University,Shahrekord Branch,Iran
2Medicinal Plants Program,Natural Science Collage,University of Massachusetts,MA,USA
3Department of Horticulture,Saveh Branch,Islamic Azad University,Saveh,Iran.
- Corresponding Author:
- A. Ghasemi Pirbalouti
Tel: +98-381-3361093
Fax: +98-381-3361060
E-mail: ghasemi955@yahoo.com
Abstract
St. John’s wort (Hypericum perforatum L.) belongs the family Hypericaceae, is one of important medicinal plants in the world. This herb has used in traditional folk medicine for the treatment of various ailments. Jasmonic acid (JA) and its methyl ester, methyl jasmonate (MJ), is regarded as endogenous regulator that plays important roles in regulating stress responses, plant growth and development. A pot experiment was conducted to assess the effect of foliar application of JA on herbage yield, and hypercin content in the tetra-hydrofuran extract from the aerial parts of H. perforatum. Experimental treatments included (I) water foliar application (control), (II) water + acetone foliar application (as a solvent), (III-VI) 50, 100, 200, and 400 μL/L JA. Statistical analysis indicated that there were no significant differences among treatments for total dry matter. The results indicted the different levels of the foliar application of JA do have highly significant impacts on hypericin content. The highest amount of hypericin obtained from foliar application 200 μL/L JA with 0.8% (w/w) hypericin/extract in both harvesting times (before and after the flowering stages). Finally, foliar application JA could be used as a source for rapid and increased production of hypericin, as an important secondary metabolite in H. perforatum with a wide range of biologically active.
Keywords
Methyl jasmonate,tetra-hydrofuran extract,secondary metabolite,harvesting time.
1. Introduction
St. John’s wort (Hypericum perforatum L.) belongs to the family Hypericaeae,is a medicinal herb that has for centuries been utilized in folk medicine for a range of purposes [1]. This herb is under intensive investigation for its wound-healing,antidepressant,diuretic,sedative,anti-inflammatory,antiviral,antibacterial,and antitumor effects and it has been used to treat neurological disorders and traumas [2-4]. H. perforatum contains a wide range of biologically active secondary metabolites that belong to different chemical groups. Naphthodianthrones (hypericins),phloroglucinols (hyperforins),xanthones,essential oil,phenolic acid,and flavonoids (flavonols,biflavones and procyanidins) have been identified in extracts of the plant [5,6]. Several pharmacological studies have confirmed that these compounds are the main contributors to the antidepressant activity of the species through various mechanisms of action [7]. The presence of red-pigmented naphthodianthrone derivatives is characteristic to several species of the genus Hypericum [8,9]. The naphthodianthrones detected in H. perforatum,include hypericin and its 2-methoxy derivative pseudohypericin as well as their precursor’s protohypericin and protopseudohypericin. Hypericins are most abundantly present in petals,stamens of flowers,and leaves of H. perforatum [10]. Hypericins accumulate in the dark glands of H. perforatum [11]. The levels of hypericin and pseudohypericin in the Hypericum species can vary under different environmental parameters,but the precise environmental factors influencing the biosynthesis of naphtodianthrones and the partitioning with other secondary metabolisms are not well understood. It has been reported that naphtodianthrone production can be influenced by plant and environmental factors,including light intensity,nitrogen availability,temperature,seasons,and growing region. For example,an increased light intensity and decreased nitrogen supply resulted in a parallel increase of the number of leaf dark glands and hypericin content [12].
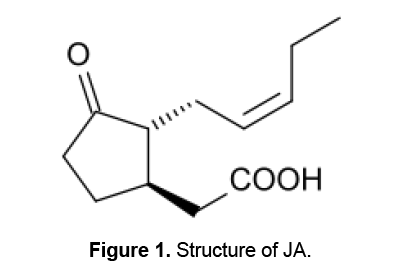
(-)-Jasmonic acid [(3 R,7 R,9Z)-jasmonic acid; (-)-(JA)] (Figure 1) and its methyl ester were first identified in the essential oils of jasmine (Jasminum grandiflorum L.),and rosemary (Rosmarinus officinalis L.) and their chemical structures were subsequently determined [13,14]. JA and its volatile methyl ester,MJ,collectively termed Jasmonates,are regarded as endogenous regulators that play important roles in regulating stress responses,plant growth and development [15]. Jasmonates are involved in many critical functions,including defense against insects and pathogens by inducing phytoalexin production,protection from abiotic stresses,immunity,and plant growth and development,suggesting that they have critical roles in plant physiology [16].
Few studies have been carried out to investigate the effect of foliar application of JA and/or MJ on the accumulation of secondary metabolites in medical plants,especially the Hypericum species. Therefore,the current study was done to find out the convenient rate of JA foliar application for optimum quality of H. perforatum..
2. Materials and Methods
2.1 Chemicals
Jasmonic acid (JA) was purchased from Sigma– Aldrich Co. (Steineheim,Germany). Acetone,ethanol,and tetrahydrofuran were bought from Merck Co. (Darmstadt,Germany).
2.2 Plant material and field site description
H. perforatum seeds collected from the Bakhtiari Zagros Mountains (latitude. 31° 20ʹ to 31° 50ʹ; longitude. 50° 50ʹ to 51° 05ʹ; 2700 to 3000 m above sea level),Chaharmahal va Bakhtiari province,Iran. Plant identity was confirmed by H.A. Shirmardi,and voucher specimen has been placed in the Herbarium of Research Center of Agriculture and Natural Resources,Chaharmahal va Bakhtiari,Iran (No. 1335). In spring 2011 in plastic greenhouse conditions,25 seeds (0.001 g) of H. perforatum were sown in each plastic pot and after six weeks were thinned to four healthy seedlings per pot. The pots transferred to the filed in Shahrekord (latitude 32° 20´ N,longitude 50° 51´ E,altitude 2061 m above sea level),southwestern Iran on May 2011. Plastic white pots with a top diameter of 20 cm and a depth of 25 cm were filled with clay loam soil. Type of study area climate by Emberger’s climatology method is cold and semiarid (annual precipitation = 340 mm and average annual temperature = 12.2 °C). In this study,no inorganic fertilizer and systemic pesticide was used during the entire experiment,and weed control was done manually. For the first month (established phase),a watering level equivalent to 60-75% of soil field capacity was applied once a day. For the subsequent months,the plots were irrigated when 55-60% of soil available water was depleted.
2.3 Treatments
Experimental treatments were arranged in a completely randomized design (CRD) with six replications. Experimental treatments,included (I) water foliar application (control),(II) water + acetone (10:1) foliar application (as a solvent),(III-IV) 50 (JA 1 ),100 (JA 2 ),200 (JA 3 ),and 400 (JA 4 ) μL/L JA. Treatments were foliar-sprayed on four the plants per pot (200 ml of the solution per pot) in twice on July 17th at the vegetative stage of growth (93 days after sowing),and August 6th at the before blooming stage of growth (113 days after sowing). The aerial parts (leaves,and stem) of three pots in each treatment harvested before at the flowering or the early blooming stage of growth (127 days after sowing). In addition,the aerial parts (leaves,stem,and flower) of three pots in each treatment harvested at 10-50% flowering stages of growth (141 days after sowing).
2.4 Extract preparation
Air-dried and powdered the aerial parts of H. perforatum were macerated at room temperature with 500 ml of ethanol: water (80:20) for 48 h. The extractions continued two times and then were concentrated in a rotary evaporator under reduced pressure (Model Zirbus 302®,Italy). The extract samples were stored in universal bottles and refrigerated at 4 °C prior to use.
2.5 Extraction and quantification of hypericin
Extraction and hypericin determination were performed as described by British Pharmacopeia [17]. Test solution in a 100 ml flask; introduce 0.8 g of the powdered plant,60 ml of a mixture of 20 volumes of water R and 80 volumes of tetrahydrofuran R and a magnetic stirrer. Boiled the mixture in a water-bath at 70 °C under a reflux condenser for 30 min. Centrifuged (2 min at 700 g) and decanted the supernatant into a 250 ml flask. The residue with 60 ml of a mixture of 20 volumes of water R and 80 volumes of tetrahydrofuran R. Heat again under a reflux consider for 30 min. Centrifuge (2 min at 700 g) and decanted the supernatant. Combined the extracts and evaporate to dryness. The residue with 15 ml of methanol R with the help of ultrasound and dilute to 25 ml with the same solvent. Centrifuge again,filter 10 ml through a syringe filter (0.2 μm). Discard the first 2 ml of the filtrate. Introduce 5.0 ml of the filtrate into a measuring flask and dilute to 25 ml with methanol R. Measure the absorbance at 590 nm of the test solution,by comparison with comparison liquid. Calculate the percentage content to total hypercinis,expressed as hypericin,using the flowing expression:
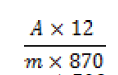
where,A= absorbance at 590 nm; m= mass of the drug to be examined in plant extract,in grams.
2.6 Analysis of data
The data was statistically analyzed using one–way ANOVA by the stat SPSS (17.0). Means of hypericin content compared by Duncan’s multiple range test at p ≤ 0.05 level.
3. Results and Discussion
3.1 Effect of Light Variation on Leaf Growth of Lettuce
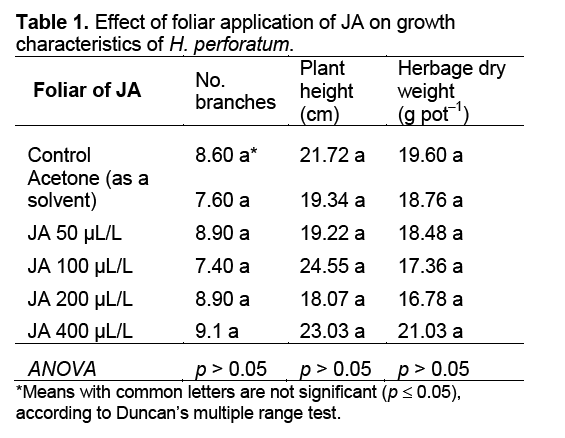
Statistical analysis indicated that there were no significant differences among treatments for dry matter,number of branches,and plant height of H. perforatum (Table 1). Generally,results of this study indicated that the foliar application of JA no significant influenced on growth and development of H. perforatum. Similarly,other researcher [18,19] also reported the foliar application of JA had no significant effect on herbage dry weight of Thymus daenensis Celak,and Salvia officinalis L. under greenhouse conditions.
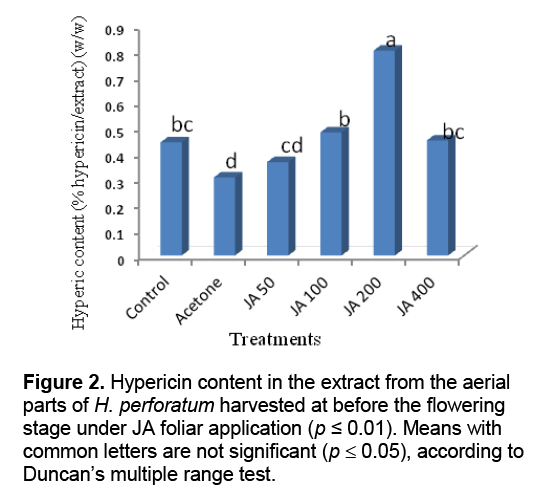
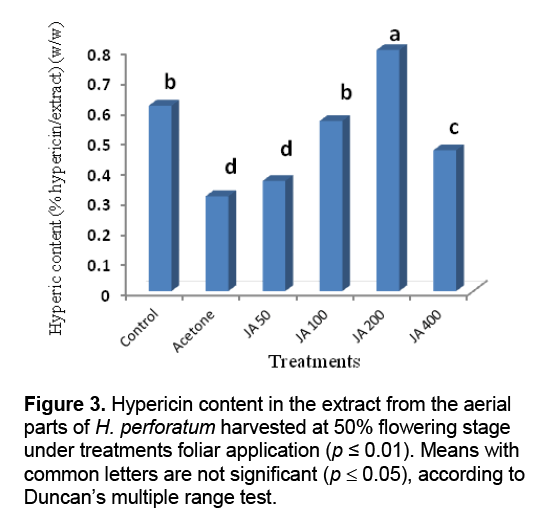
Result of statistical analysis indicated that different levels of the foliar application of JA had significant impacts on hypericin content in the extract of H. perforatum harvested before and 50% flowering stages (Figure 2 and Figure 3 ). The highest hypericin contents in the tetrahydrofuran extracts of the aerial parts of H. perforatum was obtained from the foliar application of 200 μL/L JA with 0.8% (w/w) hypericin/extract in both harvesting times (before and 50% flowering stages) (Figure 2 and Figure 3 ). Previous studies indicated that the foliar application of JA was increased some monoterpenes compounds (carvacrol,thymol,and α-pinene) in the essential oils of Salvia officinalis [19],and T. daenensis [18]. The JA signaling pathway is generally regarded as an integral signal for biosynthesis of many plant secondary products [20]. Increasing evidence indicates that JA-induced changes in secondary metabolism constitute a ubiquitous plant defense response [20,21]. Cell cultures of H. perforatum treated with JA and incubated in the dark showed increased growth and hypericin production as compared with the cultures grown under light conditions [22]. JA and its derivatives are known to stimulate production of some secondary metabolites in medicinal and aromatic plants [23]. It is interesting to note the production of hypericin,a light-mediated insecticide [24],is stimulated upon JA treatment,since JA is known to be a key signaling compound activated upon insect feeding [25]. JA has also been implicated as the signal molecule responsible for increased synthesis of nicotine,an insecticidal compound produced by Nicotiana sylvestris upon leaf wounding [26].
The lowest hypericin contents in the extracts obtained from the foliar application water + acetone foliar application (as a solvent) with 0.3% (w/w) hypericin/extract for both harvesting times. Similarly,Ghasemi Pirbalouti et al. [27] reported the foliar application of water + acetone (as a solvent) decreased contents of polyphone,flavenoid,and carotenoid in the extract of Calendula officinalis L. cultivated in same conditions with the current study. The negative effect of foliar application of acetone might be attributed to toxic effect of acetone on metabolism and cell structure of the plant
4. Conclusion
Plant secondary metabolites are unique sources for pharmaceuticals,food additives,flavors and other industrial materials. Hypercin is important secondary metabolites with a wide range of biologically active which extract from the Hypericum species. Results of current study indicated that JA is an effective elicitor for the production of hypericin in aerial parts of H. perforatum. JA could induce secondary metabolite production leading to naphtodianthrone biosynthesis. Increased hypericin content in the plants grown under the foliar application of JA (200 μL) may be attributed to stressed,which would activate the synthesis of secondary metabolites. The current study shows that the signaling compound JA,a new plant growth regulator,induces the production of hypericin. Finally,JA could be used as foliar application of hypericin production.
Acknowledgements
The authors gratefully acknowledge Shahrekord Branch Branch,I.A.U.,Iran for financial support of this research.
References
- Nathan PJ. (2001). Hypericum perforatum (St John’s Wort): a non-selective reuptake inhibitor? A review of the recent advances in its pharmacology. J. Psychopharmacol. 15: 47−54.
- Di Carlo G,Borrelli F,Ernst E,Izzo AA. (2001). St John’s wort: Prozac from the plant kingdom. Trends Pharmacol Sci. 22: 292−297.
- Bilia AR,Gallori S,Vincieri FF. (2002). St. John’s wort and depression. Efficacy,safety and tolerability - an update. Life Sci.70:3077−3096.
- Butterweck V. (2003). Mechanism of action of St John´s wort in depression: what is known? CNS Drugs 17: 539−562.
- Patočka J. (2003). The chemistry,pharmacology,and toxicology of the biologically active constituents of the herb Hypericum perforatum L. J. App Biomed. 1: 61−70.
- Tatsis EC,Boeren S,Exarchou V,Troganis AN,Vervoort J,Gerothanassis IP. (2007). Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry. 68:383−393.
- Medina MA,Martínez-Poveda B,Amores-Sánchez MI,Quesada AR. (2006). Hyperforin: More than an antidepressant bioactive compound? Life Sci. 79:105−111.
- Kitanov GM. (2001). Hypericin and pseudohypericin in some Hypericum species. Biochem Syst. Ecol. 29:171−178.
- Crockett SL,Schaneberg B,Khan IA. (2005). Phytochemical profiling of new and old world Hypericum (St. John’s Wort) species. Phytochem Analysis.16: 479−485.
- Zobayed SMA,Afreen F,Goto E,Kozai T. (2006). Plant–environment interactions: accumulation of hypericin in dark glands of Hypericum perforatum. Ann Bot. 98:793−804.
- Hölscher D,Shroff R,Knop K,Gottschaldt M,Crecelius A,Schneider B,Heckel DG,Schubert US,Svatoš A. (2009). Matrix-free UV-laser desorption/ionization (LDI) mass spectrometric imaging at the single-cell level: distribution of secondary metabolites in Arabidopsis thaliana and Hypericum species. Plant J. 60:907−918.
- Briskin DP,Gawienowski MC. (2001). Differential effects of light and nitrogen on production of hypericins and leaf glands in Hypericum perforatum. Plant Physiol. Biochem. 39:1075−1081.
- Demole E,Lederer E,Mercier D. (1962). Isolement et détermination de la structure du jasmonate de méthyle,constituant odorant caractéristique de l'essence de jasmin. Helv. Chim. Acta. 45(2): 675-685.
- Crabalona L. (1967). Presence of levorotatory methyl jasmonate,methyl cis-2-(2-penten-l-yl)-3-oxocyclopentenyl acetate,in the essential oil of Tunesian rosemary. CR Acad. Sci. (Paris) Ser. C. 264: 2074-2076.
- Ghasemi Pirbalouti A,Sajjadi SE,Parang K. (2014). A review (research and patent) on jasmonic acid and its derivatives. Archiv der Pharmazie.347: 229-239.
- Avanci NC,Luche DD,Goldman GH,Goldman MHS. (2010). Jasmonates are phytohormones with multiple functions,including plant defense and reproduction. Genet Mol Biol 9:484–505.
- British Pharmacopeia. (2009). British Pharmacopeia Volume III,Herbal Drugs and Herbal Drug Preparations St John’s Wort.
- Ashrafi M,Ghasemi Pirbalouti A,Rahimmalek M,Hamedi B. (2012). Effects of foliar application of jasmonic acid (JA) on the essential oil chemical composition of Thymus daenesis Celak. J. Herbal Drugs 3:75-90.
- Rahimmalek M,Azad S,Yadegari M,Ghasemi Pirbalouti A. (2012). Effect of jasmonic acid and salicylic acid on phytochemical properties of Salvia officinalis L. J. Herbal Drugs. 2: 89-94.
- Zhao J,Davis LC,Verpoorte R. (2005). Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 23: 283-333.
- Goossens A,Häkkinen ST,Laakso I,Seppanen-Laakso T,Biondi S,Sutter VD,Lammertyn F,Nuutila AM,Söderlund H,Zabeau M,Inzé D,OksmanCaldentey KM. (2003). A functional genomics approach toward the understanding of secondary metabolism in plant cells. Proc. Natl. Acad. Sci. U.S.A. 100: 8595–8600.
- Walker TS,Bais HP,Vivanco JM. (2002). Jasmonic acid-induced hypericin production in cell suspension cultures of Hypericum perforatum L (St. John’s wort). Phytochemistry. 60:289−293.
- Kurin Sanz M,Hernandez XE,Tonn CE,Guerreio E. (2000). Enhancement of tessaric acid production in Tessaria absinthioides cell suspension cultures. Plant Cell Rep. 19: 821–824.
- Guillet G,Podeszfinski C,Regnault-Roger C,Arnason JT,Philogene BJR. (2000). Behavioral and biochemical adaptations of generalist and specialist herbivorous insects feeding on Hypericum perforatum (Guttiferae). Environ Entomol. 29:135–139.
- Walling LL. (2000). The myriad plant responses to herbivores. J Plant Growth Regul. 19:195–216.
- Zheng ZP,Krumm T,Baldwin IT. (1997). Structural requirements of jasmonates and mimics for nicotine induction in Nicotiana sylvestris. J. Chemical Ecol. 23: 2777–2789.
- Ghasemi Pirbalouti A,Mosavi Haris SA,Tirgir F,Hamedi B. (2012). Effect of jasmonic acid and salicylic acid on polyphenol and flavenoids in extract of Calendula officinalis L. flower. J. Herbal Drugs. 3:175-180..

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences