Effect of Climate Change on Banj Oak (Quercus leucotrichophora A. Camus) Dominated Forest in Kumaun, Central Himalaya
Sanjay Kumar, Neha Chopra
Department of Botany, D S B Campus, Kumaun University, Nainital, India.
Received date: April 10, 2017; Accepted date: October 04, 2017; Published date: October 11, 2017
Citation: Kumar S, Chopra N. Effect of Climate Change on Banj Oak (Quercus leucotrichophora A. Camus) Dominated Forest in Kumaun, Central Himalaya. Electronic J Biol, 13:4
Abstract
Background: Phenology is the study of growth of buds; leaf flushing, anthesis, fruiting and leaf fall in relation to seasons or years with climatic factors. Phenological events of three trees and shrubs species were made during 2008-2009 in a banj oak (Quercus leucotrichophora A. Camus) dominated forest located between 1700-2200 m elevation in Kumaun Himalaya. After winter dormancy the growth initiation occurred when temperature began to rise continuously and resulted in bud break. Materials and methods: Monthly counts of leaves, flowers, fruits and shoot measurement were made on 150 tagged twigs on ten individuals of each species for initiation, completion and duration of different phenological events. Results: In most species, leaves emerged during spring time (February-March). Across all species, peak number of leaves per shoot (0.34-1.03), peak leaf area per shoot (9.56-52.27 cm2), shoot extension growth (3.43-25.46 cm) and shoot extension period (16-28 weeks) varied considerably. Conclusion: Comparisons with earlier studies indicated that certain phenological events have started to occur early. However, some phenophases did not show any shifts.
Keywords
Phenology; Climate change; Litter fall; Kumaun Himalaya
1. Introduction
The third assessment report from the IPCC projected that the earth’s average surface temperature will increase by 1.4 to 5.8°C between 1990 and 2100, if no major efforts are undertaken to reduce the emissions of greenhouse gases. This is significantly higher than what the panel predicted in 1995 (1.8-3.5°C). The predicted impact of warming in the Himalayan will be much higher [1]. The general phenological aspects of leafing, flowering and fruiting in tropical tree species are fairly known [2-8]. However, few reports are available on phenological studies in forest ecosystems of Central Himalaya and north eastern India [9-13].
Global climate change may force variation in timing, duration and synchronization of phenological events in tropical forests [14]. Tropical trees are expected to respond variously to changes in rainfall and temperature because they differ widely in respect to adaptations to seasonal drought and cues for bud break of vegetative and flower buds [15]. Several studies have shown significant variation (advanced or delayed) in onset dates of flowering and fruiting responses in tree species as a result of climatic change [16,17]. Probably the climate change impact can be better assessed at the level of functional types based on the duration of deciduousness and timing of onset of the reproductive phase (first visible flower). The need for functional types has been emphasized to evaluate and predict the nature of vegetation responses to future global change [18].
If the phenological changes in a species are not at the same pace as the climatic shifts it will lead to a mistiming of several seasonal activities [19]. In the present study we have tried to compare the shifts in timing of major phenological events in three tree and three shrub species of Quercus leucotrichophora A. Camus dominant forest. Comparisons are based on earlier studies on phenology on the some species at the same site [20].
2. Materials and Methods
2.1 Study area
The study was conducted in Nainital forest division of Kumaun Himalaya. The study site was located between 1700-1840 m. the studied tree species were banj oak (Quercus leucotrichophora) with Myrica esculenta Ham. ex D. Don and Rhododendron arboreum Smith occurring in the under canopy. The phenophase were also recorded for the following three shrub species D. cannabina Wall., D. salicifolia (D.Don) Rendle and R. ellipticus Smith.
2.2 Soils
The soils are residual, originating from slates, phyllites, sandstone, and limestone of the Krol series. Sand predominates in the soil (60-80%), while the silt and clay contents are 10-20% and 5-10%, respectively. Organic matter ranges between 10.0 % and 18.5% and available C between 2.6% and 3.8%, the soil pH ranges between 5 and 6 [21].
2.3 Climate
The climate is characterized by a summer monsoon and the year has four distinct seasons viz., monsoon (July to September), post-monsoon (October to November), winter (December to January) and summer (April to mid-June). Climatic data for 2008- 2009 were obtained from the State Observatory at Nainital. The average annual rainfall was 159.98 mm of, 60% of which was occur in the rainy season and the mean daily temperature ranged from -2.0°C to 30.5°C (Source: ARIES, Nainital).
2.4 Site selection
Site for the studied species were similar as reported by Negi [20]. In the forest site 1ha permanent plot was established. Within 1ha permanent plot ten individuals of each of the canopy, subcanopy and shrub species were randomly selected for collecting seasonal data on shoot elongation and diameter changes.
2.5 Phenological recording
Phenological records on leaf emergence and leaf drop were made from December, 2007 to December, 2009 in banj oak dominated forest where trees and shrub of a given species were extensively distributed (Table 1). The site were visited at a weekly interval during the phenophase was observed in 5-10% individuals of a species, it was considered to have initiated and the species was considered to be in the phenophase as long as that phenophase was represented by at least 5-10% individuals [20].
| Species | % Leaf Population Produced in 1st Month of Bud-Break | Leaf Recruitment Rate (per Shoot Day-1) in 1st Month of Leafing | Peak Leaf Pool Size (Number per Shoot) | Leaf Number per cm Shoot at Peak Leaf Pool Size |
|---|---|---|---|---|
| Trees | ||||
| M. esculenta | 92 | 0.41 | 14.3 ÃÆââ¬Å¡Ãâñ 0.72 | 1.03 |
| Q. leucotrichophora | 83 | 0.23 | 8.16 ÃÆââ¬Å¡Ãâñ 0.34 | 0.62 |
| R. arboreum | 81 | 0.20 | 7.88 ÃÆââ¬Å¡Ãâñ 0.54 | 0.51 |
| Shrubs | ||||
| D. cannabina | 78 | 0.26 | 8.73 ÃÆââ¬Å¡Ãâñ 0.72 | 0.81 |
| D. salicifolia | 89 | 0.22 | 7.21 ÃÆââ¬Å¡Ãâñ 0.22 | 0.97 |
| R. ellipticus | 76 | 0.24 | 8.4 ÃÆââ¬Å¡Ãâñ 0.86 | 0.34 |
Table 1. Periodicity of leaf recruitment and their number per shoot in studied species.
2.6 Measurement of shoot length
One healthy lateral branch from each stratum viz., upper, middle and lower was marked. To assess the shoot elongation, the distance from the mark to the end of the shoot was measured to the nearest millimeter approximately fortnightly starting from late winter of 2007 for a period of two years [20,22].
2.7 Measurement of shoot diameter
The diameter changes for marked branches were measured by vernier calliper in two directions at right angle to one another to compensate, to extent, for any eccentricity in the shoots. The observations for diameter changes were made each month at approximately the same time of the day on each reading date to reduce effect of thermal expansion and hydration for period of eighteen months from March, 2008 to August, 2009 [20,22].
2.8 Leaf area
On each individual two representative canopy branches, one largely with sun leaves in an upper crown position and other largely with shade leaves in a lower crown position, were labled with permanent metal tag. Thus in total ten branches each for lower and upper crown position were marked. Leaves for each of the species were collected randomly on each of the sampling date (at weekly intervals from budbreak to full leaf expansion and at monthly intervals during the leaf senescence) from the marked twigs. All collected leaves of individual species were sketched on graph paper to measure the leaf area [20,22].
2.9 Flowering and fruiting
The flowering period of a species was the duration (days) from the initiation of flowering to the completion of flowering amongst its individual. Fruiting period of species was also the duration (days) from the first fruit formation to the last amongst its individual.
2.10 Litter fall
For studying litter production in banj oak forest three plots of 31.5 × 31.5 m2 were established. The litter was measured by placing five litter traps (1 × 1 m2) on the forest floor. Each trap was 2 mm mesh nylon, supported by wooden sides with 25 cm height. Litter from these traps was collected separately in paper bags and brought in to laboratory where the sample was sorted out in to three main categories viz. (i) leaf litter (ii) wooden litter (<2 cm Diameter) and (iii) miscellaneous litter and dried in shade. Litter sampling study was done during May, 2008 to April, 2010 [23,24].
3. Results
3.1 Changes in leaf appearance
The months of leafing in 2009 were warmer than in 2008, i.e., the mean maximum temperature in February-March were 11.8 and 14.5°C, respectively in 2009 compared to 9.0 and 13.0°C in 2008. In 2009 the greater proportion of species showed leafing earlier due to the early rise in temperatures of February and March. Also, wet conditions during February-March of 2009 (45.1 and 14.8 mm rainfall in 2009 compared to 38.7 and 7.3 mm in 2008) triggered earlier leaf initiation in that year. 3 species (viz. Quercus leucotrichophora, Rhododendron arboreum and Rubus ellipticus) showed leafing 1-5 weeks earlier due to the early rise in temperature in February and March, 2009.
3.2 Leaf demography
In the first month following the bud-break, the tree species had produced 81-92% of total number of leaves. The leaf recruitment rate (number of leaves appearing in a shoot unit per day) in the first month of leafing ranged from a minimum of 0.20 in R. arboreum to maximum 0.41 in M. esculenta. The peak leaf pool size per shoot ranged between 7.88 (R. arboreum) to 14.3 (M. esculenta). Leaf density (number of leaves per cm shoot length) at the peak leaf pool size ranged from 0.51 (R. arboreum) to 1.03 (M. esculenta).
In comparison to trees, shrub species produced 76-89% of total number of leaves in the first month following the bud break. The leaf recruitment rate of shrub species was found lower compared to tree species. It ranged from 0.22 (D. salicifolia) to 0.26 (D. cannabina). Peak leaf pool size per shoot ranged between 7.21 in D. salicifolia to 8.73 in D. cannabina. Leaf density (number of leaves per cm shoot length) at the peak leaf pool size ranged from 0.34 (R. ellipticus) to 0.97 (D. salicifolia) (Table 1). The initiation of leaf expansion in tree species commenced from second week of February (R. arboreum) and continued till second week of March (Q. leucotrichophora and R. arboreum). The rate of leaf expansion was found maximum for R. arboreum (1.24 cm2 day-1) followed by M. esculenta (0.81 cm2 day-1) and Q. leucotrichophora (0.65 cm2 day-1). In shrub species, leaf expansion commenced from first week of February (D. salicifolia) to second week of April (D. cannabina). Leaf expansion rate was found maximum for D. cannabina (0.54 cm2 day-1) compared to D. salicifolia (0.34 cm2 day-1) and R. ellipticus (0.27 cm2 day-1).
3.3 Flowering and fruiting
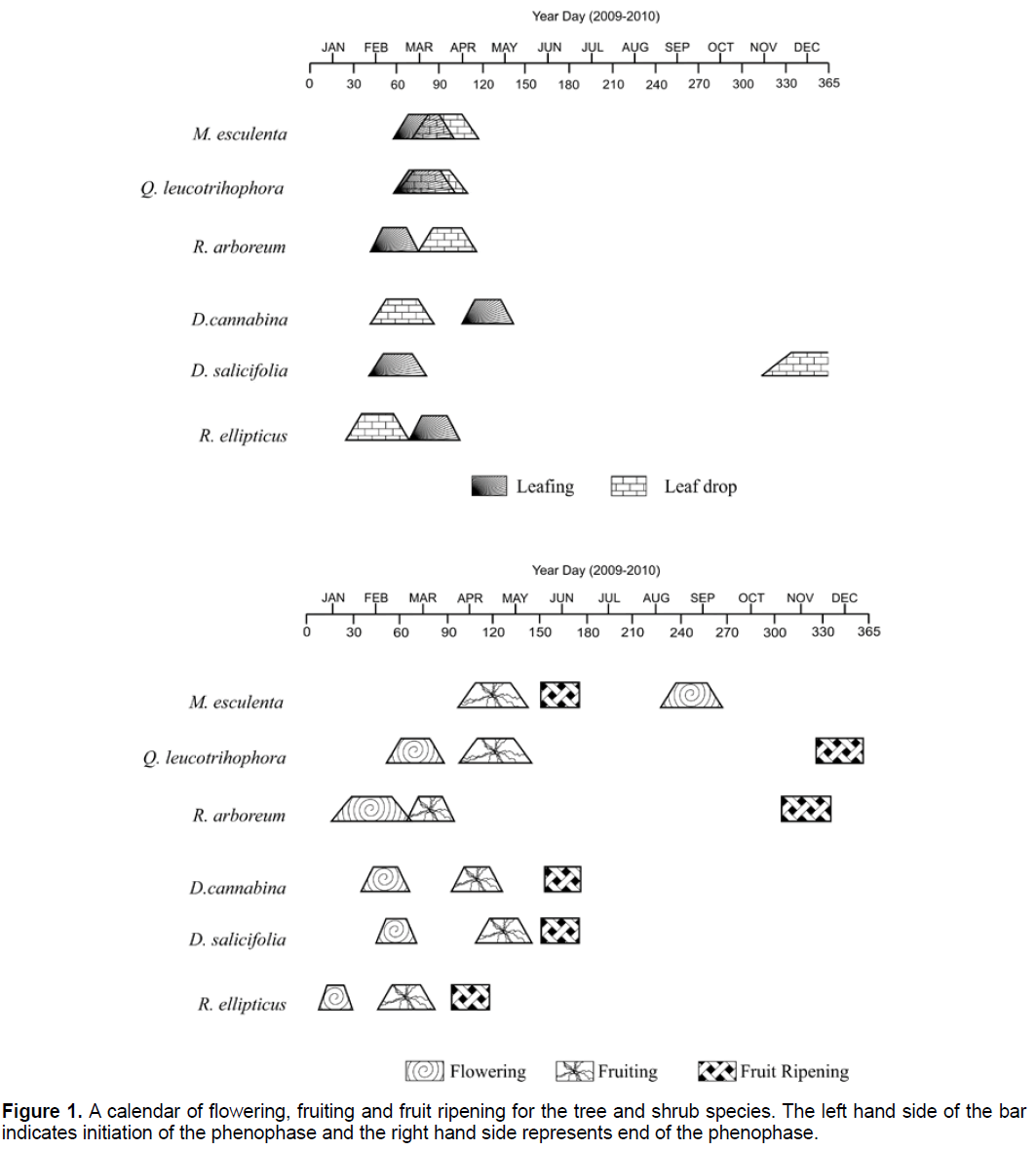
Q. leucotrichophora and R. arboreum flowered during late winter to early spring seasons (January and February) and M. esculenta flowered during the rainy season (August). The duration of flowering was observed to a maximum of seven weeks in R.arboreum. In the remaining species the flowering period ranged between five to six weeks. When comparison was made in 2009 it occurred earlier by four weeks in Q. leucotrichophora and R. arboreum and delayed by one week in M. esculenta. In R. arboreum visible fruits occurred just after the competition of flowering. In the remaining species fruits were visible after six months period in M. esculenta and after one year in Q. leucotrichophora (Figure 1).
In shrubs R. ellipiticus flowered during late winter (January) and D. cannabina and D. salicifolia flowered during the early spring (February). The flowering period ranged abound four weeks in all shrub species. Differences were also recorded in regard to the time of flower initiation between the two years of study. In 2009 it occurred earlier by one week in D. salicifolia and delayed by same period in D. cannabina. However, R. ellipticus was unaffected by this year to year variation in flowering. In D. cannabina and D. salicifolia fruiting was took place just after flowering, i.e., April. R. ellipticus differed from other species in which fruiting occurred in February (Figure 1).
3.4 Shoot demography
In Q. leucotrichophora and R. arboreum, 90% shoot elongation was completed during rainy season (July and August) and in M. esculenta it was completed by the end of rainy season (i.e., September and October). The amount of shoot elongation ranged between 8.45 cm in M. esculenta to 25.46 cm in R. ellipticus. The average percentage of the shoot elongation realized in the first month of bud break was higher for Q. leucotrichophora (72.89%).
In D. cannabina, 90% shoot elongation was completed by June, prior to the commencement of rainy season, in D. salicifolia it was completed during rainy season (July and August) and in R. ellipticus it was completed by the end of rainy. The amount of shoot elongation ranged between 20.18 cm in D. salicifolia to 25.46 cm in R. ellipticus. The average percentage of the shoot elongation realized in the first month of bud break was higher for D. salicifolia (53.05%) (Table 2).
| Rate (cm-2 dayl) | |||||
|---|---|---|---|---|---|
| Species | Date of Bud Break | Full Length of Shoot (cm) | Net Gain in Diameter (mm) | Shoot Elongation | Shoot Diameter |
| Trees | |||||
| M. esculenta | 12-April | 8.45 ÃÆââ¬Å¡Ãâñ 0.62 | 4.97 ÃÆââ¬Å¡Ãâñ 0.09 | 0.043 ÃÆââ¬Å¡Ãâñ 0.01 | 0.026 ÃÆââ¬Å¡Ãâñ 0.001 |
| Q. leucotrichophora | 11-March | 20.43 ÃÆââ¬Å¡Ãâñ 1.09 | 4.89 ÃÆââ¬Å¡Ãâñ0 .21 | 0.119 ÃÆââ¬Å¡Ãâñ 0.002 | 0.021 ÃÆââ¬Å¡Ãâñ 0.002 |
| R. arboreum | 8-May | 10.44 ÃÆââ¬Å¡Ãâñ 0.47 | 3.67 ÃÆââ¬Å¡Ãâñ 0.12 | 0.098 ÃÆââ¬Å¡Ãâñ 0.02 | 0.033 ÃÆââ¬Å¡Ãâñ 0.001 |
| Shrubs | |||||
| D. cannabina | 3-March | 20.22 ÃÆââ¬Å¡Ãâñ 2.25 | 4.71 ÃÆââ¬Å¡Ãâñ 0.26 | 0.187 ÃÆââ¬Å¡Ãâñ 0.03 | 0.044 ÃÆââ¬Å¡Ãâñ 0.01 |
| D. salicifolia | 13-March | 20.18 ÃÆââ¬Å¡Ãâñ 1.78 | 6.66 ÃÆââ¬Å¡Ãâñ 0.41 | 0.12 ÃÆââ¬Å¡Ãâñ 0.02 | 0.04 ÃÆââ¬Å¡Ãâñ 0.002 |
| R. ellipticus | 4-February | 25.46 ÃÆââ¬Å¡Ãâñ 2.53 | 4.38 ÃÆââ¬Å¡Ãâñ 0.36 | 0.089 ÃÆââ¬Å¡Ãâñ 0.001 | 0.019 ÃÆââ¬Å¡Ãâñ 0.02 |
Table 2. Different parameters of shoot length and diameter of tree and shrub species.
Of the total diameter increment, the percentage increment in one month from bud-break ranged between 65.32% in Q. leucotrichophora to 80.89% in R. arboreum. Of the total diameter increment, the percentage realized in one month from bud-break found maximum for R. arboreum (80.98%) and minimum for Q. leucotrichophora (65.32%).
Compare to trees, in shrub species the percentage increment in one month from bud-break ranged between 44.52% in R. ellipticus to 71.55% in D. cannabina. Of the total diameter increment, the percentage realized in one month from bud-break found maximum for D. cannabina (71.55) and minimum R. ellipticus (44.52%) (Table 2).
3.5 Litterfall
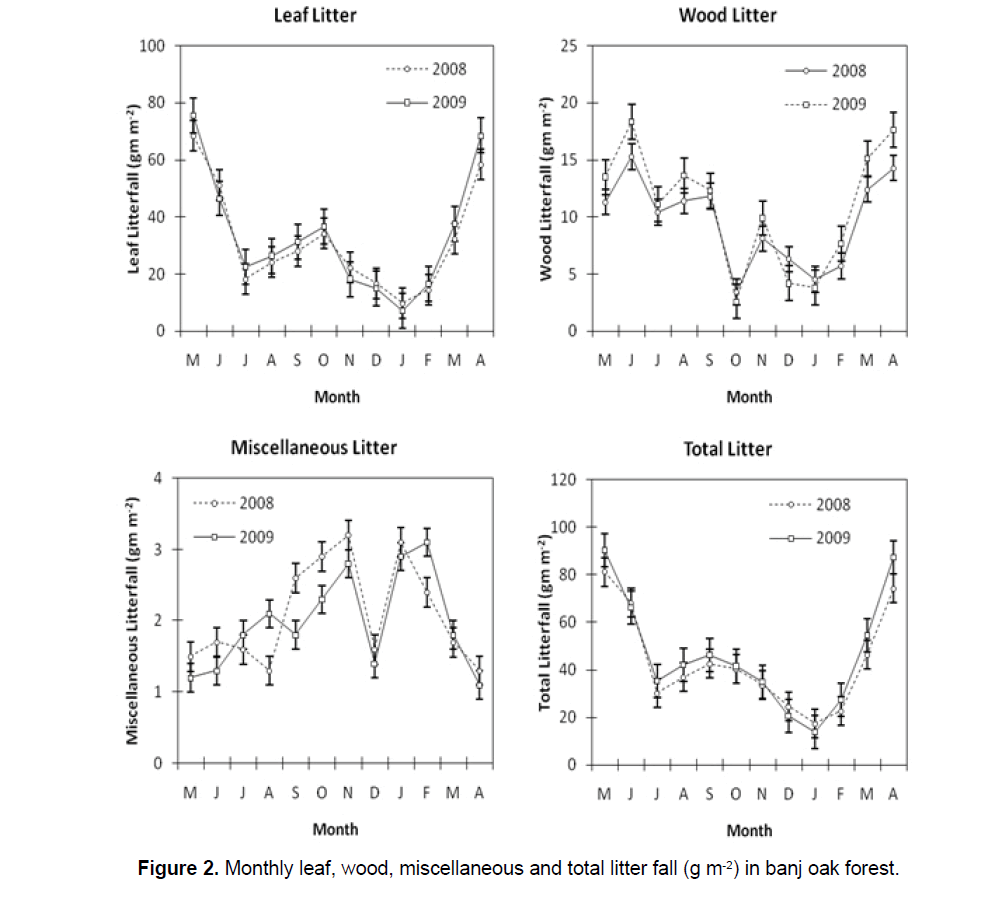
The leaf fall was greatest in the spring (February- May) season (47.29%), followed by the monsoon (19.33%), winter (19.13%) and post monsoon seasons (14.26%). The contribution of leaf litter to total annual litter production was highest during summer months; the leaf litter accounted for 18.47% (May) to 12.53% (June) of the respective total monthly fall (Figure 2).
The contribution of wood litter to total annual litter production was highest during summer seasons; the wood litter accounted for 13.74% (June) to 10.14% (May) of the respective total monthly fall. The wood fall was greatest in the summer season (36.92%), followed by the monsoon (28.86%), winter (24.4%) and post monsoon seasons (9.81%).
The contribution of miscellaneous litter to total annual litter production was highest during winter seasons; the miscellaneous litter accounted for 12.3% (January) to 6.15% (December) of the respective total monthly fall. The miscellaneous fall was greatest in the winter season (36.69%), followed by the post monsoon (23.57%), monsoon (22.96%) and summer season (16.6%). The mean monthly temperature (°C) was significantly correlated with leaf litter fall (r=0.873), wood litter fall (r=0.737) and miscellaneous litter fall (r=0.803) per month. The mean monthly rainfall (mm) also showed significant correlated with leaf litter fall (r=-0.628), wood litter fall (r=-0.858) and miscellaneous litter fall (r=0.554) per month.
The contribution of leaf fall to total annual litter in respective month was highest during summer months; the leaf litter accounted for 78.7% (April) to 84.0% (May). The wood fall contribution was highest during monsoon months; the wood litter accounted for 31.7% (August) to 27.1% (September) and highest during winter by Miscellaneous litter. The miscellaneous litter accounted for 6.7% (December) to 19.2% (January).
4. Discussion
In the studied species the growth initiation occurred in month of February and March when temperature had started to increase. R. arboreum and D. salicifolia were among the first to show growth initiation, in the second week of February and D. cannabina was the last to exhibit its growth initiation (in mid April). Studies by Njoku [25], Lawton and Akpan [26] have also implicated day length and air temperature increase as the inducer of leaf flushing, which holds true for the present study area where peak activity of bud break and leafing took place during February-March when photoperiod and temperatures were increasing [4,27]. Further, the role of isolated rain showers to initiate leafing by replenishing water content has also been frequently emphasized [28]. In the study site, though the soil moisture continues to be low from October to mid-June, the long dry spells were frequently broken by isolated rain showers (average monthly rainfall is 41.9 and 11.2 mm in February and March), possibly facilitating leaf emergence during March-April.
The numbers of flowering species increased from January and by mid February majority of the species were flowering. In M. esculenta it was delayed until first week of August. In Q. leucotrichophora and two shrub species (D. cannabina and D. salicifolia) fruiting began in the first to third week of April. However, R. ellipticus was first to initiate fruiting in February. From the standpoint of time interval between fruitition to fruit ripening, in most of the species fruit ripened within two or three months of fruitiiton except R. arboreum in which fruit ripened occurred within 6 months and Q. leucotrichophora within 15 months. In majority of species shoot elongation began with the increase of temperature in mid-March to mid- April except R. ellipticus which had first emergence of shoot in February. In five species over 90% shoot elongation was completed during rainy season (July and August) except D. cannabina prior to the commencement of rainy season.
The available data indicates that the Himalayas seem to be warming several times more than the global average rate [29,30]. The temperature rise was more during the winter and autumn than during the summer; and that increases was larger at higher altitudes [30]. A study based on the analysis of data of 43 years (period 1965-2007) indicated that the maximum temperatures have increased by 1.1°C and minimum by 1.2°C temperature [31]. This study supported the result of Liu and Chen [30].
Climate change is clearly affecting plant phenology world wide [32-35]. Our results further confirm this observation. In present study leaf initiation of M. esculenta and Q. leucotrichophora had shifted by approximately 3-4 weeks in comparison to earlier studies [20]. R. arboreum showed approximately 7-8 weeks shifts in leafing. Flowering in R. arboreum was earlier by 5 weeks and fruiting had also shifted about 2 weeks earlier when compared with earlier studies conducted by Negi [20]. This shift in flowering can largely be explained by a 0.81°C increase in the mean January and February maximum temperature over the past 10 years. Only M. esculenta was unaffected by this phenological change. However, no previous studies were available to compare phenological activities of studied shrub species (Table 3).
| Species | Leafing | Flowering | Fruiting | Fruit Ripening | ||||
|---|---|---|---|---|---|---|---|---|
| Present study | Negi [20] | Present study | Negi [20] | Present study | Negi [20] | Present study | Negi [20] | |
| M. esculenta | 4th week (February) |
1st week (April) |
2nd week (August) |
1st week (August) |
1st week (April) |
1st week (March) |
1st week (June) |
1st week (May) |
| Q. leucotrichophora | 4th week (February) |
4th week (March) |
4th week (February) |
2nd week (March) |
2nd week (April) |
3rd week (April) |
4th week (November) |
1st week (December) |
| R. arboreum | 2nd week (February) |
3rd week (April) |
2nd week (January) |
4th week (February) |
2nd week (March) |
1st week (April) |
1st week (October) |
1st week (June) |
Table 3. A summary of comparative values of various phenologic events for studied species. Each value is represented as average week of initiation of a phenophase
5. Conclusion
To conclude, it is evident that the phenophases of species are responding to climate change, i.e., increasing temperature changing rainfall pattern. The changes in early-flowering species respond to enhanced February and March temperatures to a greater degree. Such changes in flowering times in response to future climate change might significantly affect hybridization, pollination biology, and population structures and regeneration on the studied species.
References
- Ravindranath NH, Sathaye J, Shukla PR. (2006). Climate change, sustainable development and India: Global and national concerns. Curr Sci.90: 314-325.
- Borchert R. (1983). Phenology and control of flowering in tropical trees. Biotropica. 15: 81-89.
- Daubenmire R. (1972). Phenology and other characteristics of tropical semideciduous forests in north western Costa Rica. J Ecol. 60: 147-170.
- Frankie GW, Baker HG, Opler PA. (1974). Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. J Ecol. 62: 881-919.
- Opler PA, Frankie GW, Baker HG. (1980). Comparative phenological studies of shrubs and treelets in wet and dry forests in the lowlands of Costa Rica. J Ecol. 68: 167-186.
- Putz FE. (1979). A seasonality in Malaysian tree phenology. Malaysian Forester. 42: 1-24.
- Singh JS, Singh VK. (1992). Phenology of seasonally dry tropical forest. Curr Sci. 63: 684-688.
- Sun C, Kaplin BA, Kristensen KA, et al. (1996). Tree phenology in a tropical montane forest in Rwanda. Biotropica. 28: 668-681.
- Ralhan PK, Khanna RK, Singh SP, et al. (1985). Phenological characteristics of the tree layer of Kumaun Himalayan forests. Vegetation. 60: 91-101.
- Ralhan PK, Khanna RK, Singh SP, et al. (1985). Certain phenological characters of the shrub layer of Kumaun Himalayan forests Vegetation. 63: 113-120.
- Sundriyal RC. (1990). Phenology of some temperate woody species of the Garhwal Himalaya. Int J Ecol Environ Sci. 6: 107-117.
- Boojh R, Ramakrishnan PS. (1981). Phenology of trees in a sub-tropical evergreen mountain forest in north-east India. Geo-Eco Trop. 5: 189-209.
- Shukla RP, Ramakrishnan PS. (1982). Phenology of trees in a subtropical humid forest in north eastern India. Vegetation. 49: 103-109.
- Reich PB. (1995). Phenology of tropical forests: patterns, causes and consequences. Can J Bot. 73: 164-174.
- Singh KP, Kushwaha CP. (2005). Emerging paradigms of tree phenology in dry tropics. Curr Sci. 89: 964-975.
- Fitter AH, Fitter RSR. (2002). Rapid change in flowering time in British plants. Science. 296: 1689-1692.
- Chapman CA, Chapman LJ, Struhsaker TT, et al. (2005). A long-term evaluation of fruiting phenology: Importance of climate change. J Trop Ecol. 21: 31-45.
- Box EO. (1996). Plant functional types and climate at global scale. J Veg Sci. 7: 309-320.
- Visser ME, Both C, Lambrechts MM. (2004). Global climate change leads to mistimed avian reproduction. Adv Ecol Res. 35: 89-110.
- Negi GCS. (1989). Phenology & Nutrient dynamics of tree leaves in Kumaun Himalaya, Ph.D Thesis, Kumaun University. Nainital.
- Kumar S, Pande R, Deepshikha A. (2016). Shoot demography of some evergreen and deciduous tree species of Kumaun Himalaya, India, along an altitudinal gradient. Scientific Research and Essays. 11: 1-10.
- Ralhan PK. (1985). Phenology of plants in forest ecosystem of Kumaun Himalaya, Ph.D. Thesis, Kumaun University, Nainital.
- Majila BS. (1992). Phytosociology, biomass structure and primary productivity of Oak-Pine forest of Kumaun Himalaya, Ph.D. Thesis, Kumaun University, Nanital.
- Mehra MS. (1984). Litter fall and nutrient return in certain forest ecosystems of Kumaun Himalaya. Ph.D. Thesis, Kumaun University, Nainital.
- Njoku E. (1963). Seasonal periodicity in the growth and development of some forest trees in Nigeria. I. Observations on mature trees. J Ecol. 51: 617-624.
- Lawton JRS, Akpan EEJ. (1968). Periodicity in plumeria. Nature. 218: 384-386.
- Hanninen H. (1995). Effects of climatic change on trees from cool and temperate regions: An ecophysiological approach to modeling of bud burst phenology. Can J Bot. 73: 183-199.
- Borchert R, Rivera G, Hagnauer W. (2002). Modification of vegetative phenology in a tropical semi-deciduous forest by abnormal drought and rain. Biotropica. 34: 27-39.
- Shrestha AB, Wake CP, Mayewski PA, et al. (1999). Maximum temperature trends in the Himalaya and its vicinity: An analysis based on temperature records from Nepal from period 1971-1994. Journal of Climate. 12: 2775-2787.
- Liu X, Chen B. (2000). Climate warming in the Tibetan Plateau during recent decades. Int J Climatol. 20: 1729-1742.
- Rani M. (2010). Micro climatology of Almora and its environs. Kumaun Himalaya, Ph.D. Thesis, Kumaun University, Nainital.
- Myneni RB, Keeling CD, Tucker CJ, et al. (1997). Increased plant growth in the northern high altitudes from 1981 to 1991. Nature. 386: 689-702.
- Parmesan C, Yohe G. (2003). A globally coherent fingerprint of climate change impacts across natural system. Nature. 421: 37-42.
- Root TL, Price JT, Hall KR, et al. (2003). Fingerprints of global warming on wild animals and plants. Nature. 421: 57-60.
- Menzel A, Sparks TH, Estrella N. (2006). European phenological response to climate change matches the warming pattern. Glob Chang Biol. 12: 1969-1976.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences