Construction and Bioassay of Recombinant AcNPV Containing SpltNPV gp37 Fusion gene
Chongbi Li, Zhaofei Li, Guanghong Li, Yi Pang
1Biopharmaceutical Engineering Center of Zhaoqing University,Zhaoqing city,Guangdong Province,526061,China
2State Key Laboratory for Biocontrol and Institute of Entomology,Zhongshan University,Guangzhou,510275,China
Abstract
The Spodoptera litura nucleopolyhedrovirus (SpltNPV) gp37 fusion gene was inserted into the transfer vector (pBacPAK9), named pBacPAKSL37fus. The vector was cotransfected BT1-Tn5B1-4 (Hi5) cells with liner Autographa californica nucleopolyhedrovirus(AcNPV) DNA (BacPAK6).The purified, non-occluded body recombinant baculovirus (Ac-PAKSL37fus) was selected by successive 3 cycles of plaque purification. The inserted gp37 fusion gene under the control of polyhedrin gene could high-express gp37fus protein. At 24 hours post infection (hr.p.i), sf-21 cells infected with recombinant virus had a distinctive cytopathic effect compared to cells with wild type (w.t) AcNPV. Through successive 5 generation infections, cytotoxicity of the recombinant virus did not weaken. The result from infected beet armyworm larvae showed that mortality of test group infected with recombinant virus was 26 percent higher than the control group with parent virus at 60 hr p.i. The recombinant baculoviruses were co-embedded in insect with wt parent virus via subcutaneous injection. If again, beet armyworm larvae infected with the NPV isolated from dead insects, mortality of insects was 17% higher than that of the control.
Keywords
SpltNPV gp37 fusion gene,recombinant baculovirus,bioassay.
1. Introduction
Baculoviruses are double stranded DNA virus which are virtually ubiquitous in the environment and inhabit mainly infect arthropods [1]. But they do not infect human,mammal animals,plants and other vertebrate. Being referred to safety,baculoviruses are important factor of controlling insect species growth. It has a potential to pesticide alternative agricultural chemical reagents. Therefore,people are attaching importance to baculoviruses' usage. However,because narrow-range baculovirus insecticide is of little use,a critical factor for the performance of any baculovirus insecticide is to enlarge the host range of the baculovirus as pesticide. Now researchers are being focus on how to modify baculovirus to become an effective insecticide. This paper showed the construction of recombinant baculovirus through a co-transfection of SpltNPV gp37 fusion gene and AcNPV DNA and its' bioassay.
2. Materials and Methods
2.1 Construction of the plasmid
T vector,pBacPAK9 and AcNPV liner DNA (BacPAK6) (Clontech),Spodoptera litura and Spodoptera exigua larvae (2.State Key Lab),BT1-Tn-5B1-4 cells (Hi-5) was donated by Granados [2]. Strain DH5a (2.State Key Lab),Plasmid Extraction Kit (New England). SpltNPV gp37 fusion gene containing ubiquitin and gp37 genes isolated by PCR within an open reading frame (ORF) was cloned into T vector named pTSL37fus. pTSL37fus and pBacPAK9 were digested with PstI-EcoRI,respectively [3]. And then SpltNPV gp37 fusion gene was cloned into pBacPAK9 named transfer vector pBacPAK37fus.
2.2 Cotransfection
All cells were propagated at 27 in TC℃-100 medium supplemented with 10% fetal bovineserum(Gibco). Virus titre was determined by plaque assay as previously described [4]. T1-Tn-5B1-4 (Hi-5) cells were coinfected with pBacPAK37fus and liner AcNPV BacPAK6 (Clontech) using a procedure based on the manual of Clontech laboratory. As a control,Hi-5 cells were infected with wild type (w.t) AcNPV. The recombinant virus was isolated by plaque assay after cotransfection [5].
2.3 EcoRI digestion and protein gel
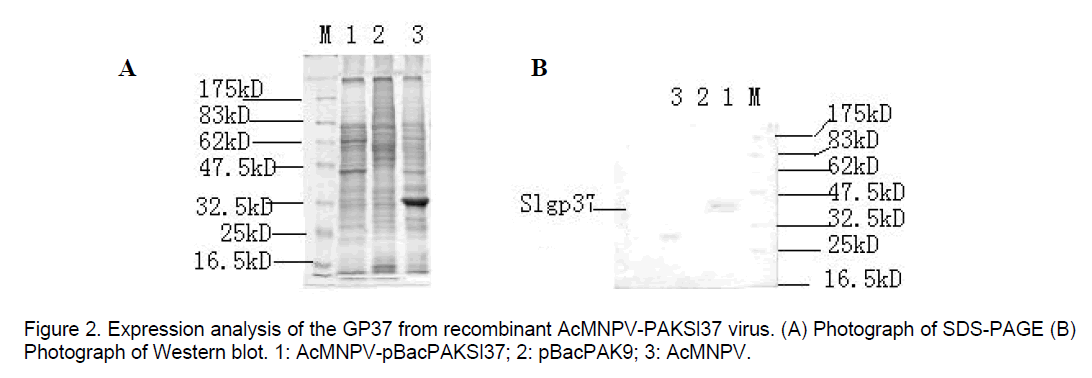
DNA was extracted from the purified recombinant viruses and parent AcNPV,respectively and were digested with EcoRI. The results of electrophosis were compared with each other. In addition,Spodoptera frugiperda cells infected were harvested 48 hours post infection (hr p.i) for analysis. Cells pellets were resuspended in electrophoresis sample buffer,and expression of SpltNPV gp37 fusion gene was detected by SDS-PAGE and Western-blot (TrpE-gp37 antiserum donated by Dr.G.F. Rohrmannin).
2.4 Infections and Spodoptera litura and Spodoptera exguia larvae
Quantities of the viruses in infecting were determined by the method of Volkmann [6]; 1ug protein corresponded to 1.8x1010 virus particles. In this study,each larva was challenged with 1x109 virus particles by subcutaneous injection. Toxicity of the viruses was assayed by mortality of the larvae p.i compared to the control infected with wt AcNPV. Co-embedding of recombinant viruses and w.t AcNPV. The recombinant viruses were unfavourable to practical usage owing to not producing polyheda. Therefore,it was conceived that the recombinant viruses would be coembedded in polyhedra from w.t AcNPV. Briefly,recombinant viruses and w.t AcNPV particles were mixed in vitro as a ratio of 4:1 and 9:1. Beet armyworm larvae at 3 days of age were infected with the mixture of two virus particles by way of subcutaneous injection. Nucleopolyhedrovirus (NPV) would be isolated from dead insects p.i. Then the beet armyworm larvae at the same age were infected through feeding the forage smeared with the virus particles,30 larvae each group. First group was infected with w.t AcNPV,second one infected with mixture of 4:1,third one with the mixture of 9:1. Infecting quantities of the viruses were 5.6x106 virus particles each larvae in 3 groups. In the event,infective rates were compared between groups according to mortality of the larvae p.i.
3. Results
3.1 Construction of recombinant virus having SpltNPV gp37 fusion gene
Recombinant plasmids were prepared with an insertion of the SpltNPV gp37 fusion gene in the pBacPAK9. The selected plasmids were cotransfected with w.t AcNPV DNA (BacPAK6) into Hi-5 cells. Because BacPAK6 was a convenient virus for practice and could express β-galactosidase and produce plaques that could be stained blue with x-gal,recombinant virus was easily selected from infectious supernatants by plaque assay in the presence of x-gal. Restriction analysis of DNA extracted from recombinant virus and w.t AcNPV indicated that relevant fragment of polyhedrin gene was lost in AcNPV-PAKSL37fus DNA because of insertion of SpltNPV gp37 fusion gene into AcNPV genome. This relevant fragment containing polyhedrin gene with EcoRI sites was 6.5kb long in w.t AcNPV genome (Figure 1).
Futhermore,SDS-PAGE analysis confirmed that recombinant virus produced gp37 fusion protein detectable amounts of the Mr 37kD. On SDS-PAGE,the cells infected with w.t AcNPV show a striking polyhedrin protein band (Figure 2A). But expression of SpltNPV gp37 fusion gene in sf-21 cells infected with recombinant viruses could be verified by Western-blot,and GP37 protein could not be detected in w.t AcNPV-infected cells only 29kD polyhedrin protein detectable (Figure 2B).
3.2 Morphology and cytopathology of cells infected with AcNPV-PAKSL37fus
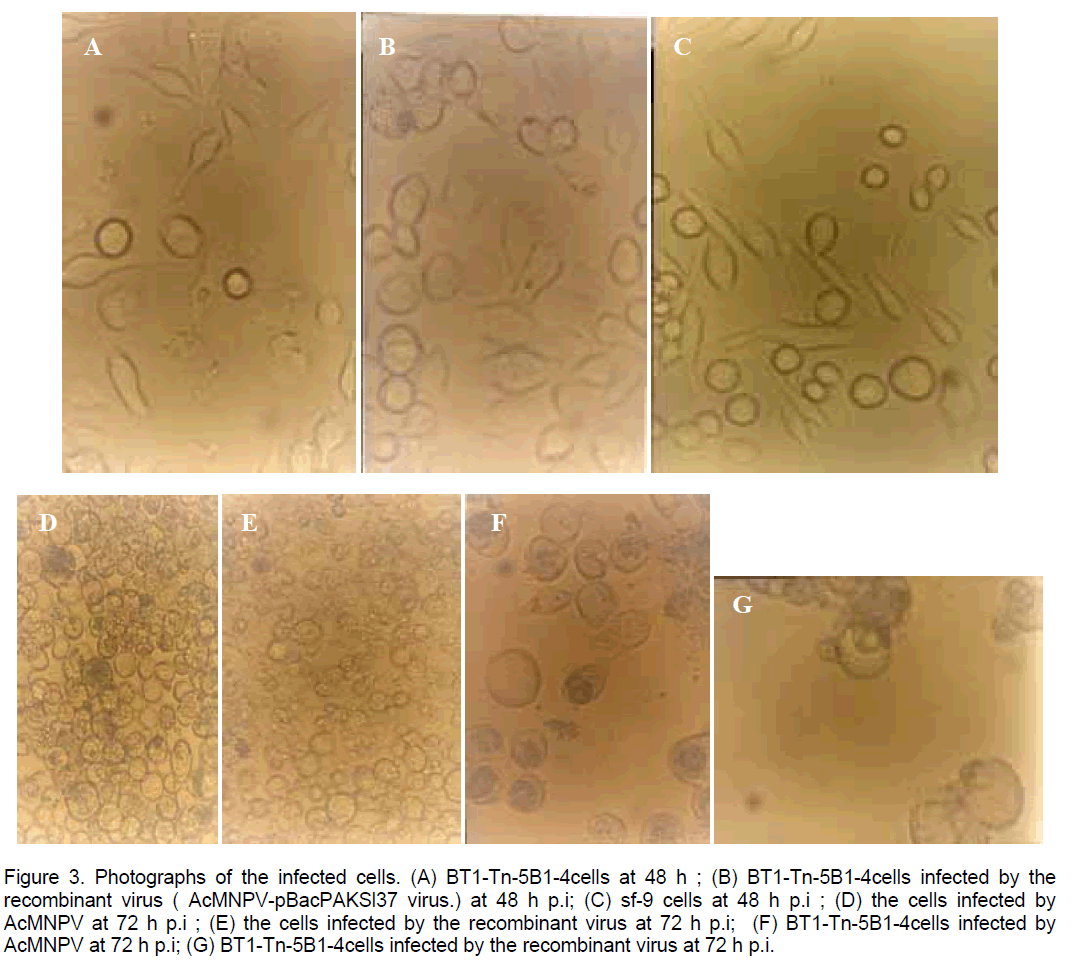
Phase microscopic examination of cells showed that the AcNPV-PAKSL37fus had distinctive cytopathologic effects compared to cells infected with w.t AcNPV (Figure 3 ). At 48 hr p.i,cells infected with AcNPV-PAKSL37fus had large swollen nuclei (Figure 3 B). A striking cytological observation was that sf cells infected with the virus having gp37 fusion gene began to lyse by 72hr p.i,whereas the cells infected with w.t AcNPV did not progress to cell lysis (Figure 3 D,E). And the cytopathological effects were more strikingly at 72 hr p.i (Figure 3 D-G). Both of recombinant virus and w.t AcNPV could infect sf-21 or Hi-5 cells (Figure 3A-G). But they could not infect SL-ZSU cells.
Figure 3. Photographs of the infected cells. (A) BT1-Tn-5B1-4cells at 48 h ; (B) BT1-Tn-5B1-4cells infected by the recombinant virus ( AcMNPV-pBacPAKSl37 virus.) at 48 h p.i; (C) sf-9 cells at 48 h p.i ; (D) the cells infected by AcMNPV at 72 h p.i ; (E) the cells infected by the recombinant virus at 72 h p.i; (F) BT1-Tn-5B1-4cells infected by AcMNPV at 72 h p.i; (G) BT1-Tn-5B1-4cells infected by the recombinant virus at 72 h p.i.
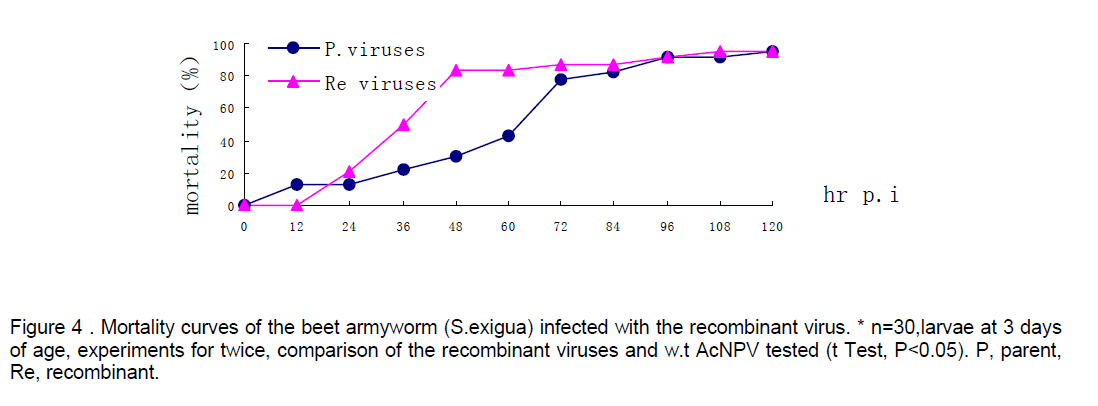
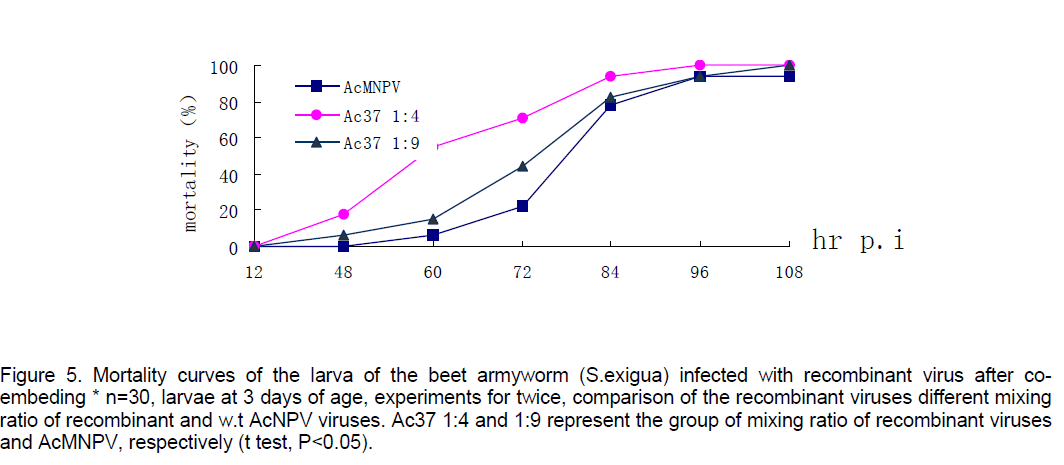
Mortality of beet armyworm larvae infected with AcNPV-PAKSL37fus. At 60 hr p.i,bioassay showed that recombinant virus could increase mortality of the larvae infected 26% compared to parent viruses-AcNPV. Mortality of the larvae infected indicated that the larvae infected with recombinant viruses began to die earlier and more severely than the control group (n=30,t test p<0.05,Figure 4). However,both of baculoviruses could not effectively infect Spodoptera litua larvae. After the recombinant viruses and parent viruses being coembedded in beet armyworm larvae at 3 days of age,the recombinant viruses with polyhedra isolated from dying insects infected with mixed viruses also increased mortality of larvae infected 17% compared to w.t AcNPV. Differences between test group and control (t test,p<0.05). And the recombinant viruses embedded with ratio of 1:4 had a more effective infection than that with 1:9 (Figure 5 ).
Figure 5. Mortality curves of the larva of the beet armyworm (S.exigua) infected with recombinant virus after co-embeding * n=30, larvae at 3 days of age, experiments for twice, comparison of the recombinant viruses different mixing ratio of recombinant and w.t AcNPV viruses. Ac37 1:4 and 1:9 represent the group of mixing ratio of recombinant viruses and AcMNPV, respectively (t test, P<0.05).
4. Discussion
Our approach to exploit new insecticides for biocontrol is the employment of recombinant baculovirus. Though the recombinant baculovirus was not used in practice,the experiments indicated that the infectivity of recombinant baculoviruses for the beet armyworms (S.exigua) was superior to wt AcNPV. The reason is that new polyhedron formed after the recombinant baculovirus being coembed with wt AcNPV particles in beet armyworm larvae. We know that the NPV polyhedron is primarily composed of a single protein,termed polyhedrin. This protein is expressed under an exceptionally strong promoter at a late stage of infection. Since the polyhedrin promoter is so active,the polyhedrin protein accounts for more than 50% of the cell mass at a late stage of infection by observing the polyhedra under light microscopy. In our experiment,the infected larvae showed the typical signs and symptoms of NPV disease and protein extracted from OBs harvested from dead insects showed the polyhedrin and GP37. Thus,it is the reason of the insertion of SpltNPV gp37 fusion gene into AcNPV genome; the recombinant virus could contain two gp37 genes. One is from SpltNNPV and the other is from AcNPV. And foreign gp37 fusion gene could express GP37 fusion protein in high level because of the control of polyhedrin promoter compared to gp37 gene of w.t AcNPV without the control of polyhedrin promoter. Since the transfer plasmid which we used was designed to produce baculovirus expression vector (BEV) in which the polyhedrin gene had been replaced by a new,chimeric gene consisting of the very late polyhedrin promoter and the sequence encoding the protein of interest positioned downstream,as described previously [7],this plasmid could most effectively and widely be used to express foreign protein. The research indicated that gp37 fusion gene in recombinant viruses could produce high level GP37 fusion protein. But gp37 gene of AcNPV itself in recombinant viruses could not produce distinctive GP37 protein. It verified that polyhedrin promoter is a strong late promoter of baculoviruses and could induce the gene to high express foreign protein. At 24 hr p.i,it is a late phase that baculovirus genes encoding occlusion-related polypeptides such as polyhedrin begin to transcribe primarily.
Additionally,this transcription requires the sequence TAAG as an essential promoter element,whereas the TAAG sequence plays a critical role in very late promoter activity. The gp37 and ubiquitin fusion genes do share a common sequence ATAAG. Since the upstream of gp37 fusion gene contains the sequence TAAG,and this gene also is positioned downstream of polyhedrin promoter,the fusion protein is produced in large quantities. We speculated that large accumulations of the fusion product would speed up the lysis resulted in severe cytopathology. Maybe GP37 fusion protein has some function of virus resolvase. Results from the cytopathology and dead insects’ p.i indicated that GP37 fusion protein played a role at the early phase p.i and verified Dr. Lius' viewpoint [8]. It also showed that GP37 fusion protein is not relevant to the baculoviruses host range because the recombinant viruses did not infected SL-ZSU cells and Spodoptera litura larvae. In order to use the recombinant viruses as an insecticide,we manage to pack the recombinant viruses in polyhedra in vivo. It is an imagination that both the recombinant viruses without polyhedra and w.t baculoviruses with polyhedra would be co-embedded in vivo in polyhedrin from dissolved w.t AcNPV. In the event that the viruses isolated from dead insects are highly efficient at infecting insects compared to w.t AcNPV,we will speculate that the recombinant viruses become enveloped within insect cell nuclei and occluded in polyhedrin. The experiment supported this anticipation.
Although the co-embedded recombinant viruses have a striking infection for insects compared to w.t AcNPV,to date,no other report is found. Therefore,we can't get a definitive conclusion,but the basis for this experiment is just a beginning to perform further studies on recombinant baculoviruses.
Acknowledgments
We thank Dr. Grannados for T1-Tn-5B1-4 (Hi-5) cells,Dr.G.F. Rohrmannin for TrpE-gp37 antiserum,Dr. Guren Zhang for statistics of the data,Miss Miner He for preparations of the cells. This project was supported by National Natural Science Foundation of China (No. 39730030 and 39800092),Zhaoqing city scientific creative project (No. 2003301) and Open Project of the state key lab for biocontrol in Zhongshan University (2004).
References
- Pang,Y. (1994) Viral Disease of Insect,Insect Pathology (Ed,Pu Zhelong),Guangdong Science & Technology Press.
- Granados,R.R.,Li,G.X.,Derksen,A.C.G.,et al. (1994) A new insect cell line from Trichoplusia ni.( BT1-Tn-5B1-4) susceptible to Trichoplusia ni single enveloped nuclear polyhedrosis virus. J. Invert. Pathol.,64: 260-266.
- Sambrook,J.,Fritsch E.F.,Maniatis,T. (1989) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory,Cold Spring Harbor,NY.
- O’ Reilly,D.R.,Brown,M.R.,Miller,L.K. (1992) Alteration of ecdysteroid metabolism due to baculovirus infection of the fall armyworm Spodoptera frugiperda: Host ecdysteroids are conjugated with galactose,Insect Biochem. Mol. Biol. 22: 313-319.
- Horton,H.M.,Burand,J.P. (1993) Saturable attachment sites for polyhedron-derived baculovirus on insect cells and evidence for entry via direct membrane fusion. J. Virol.,67: 1860-1868.
- Volkmann,L.E.,Summer,M.D.,Hsieh,C.H. (1976) Occluded and nonoccluded nuclear polyhedrosis virus grown in Trichoplusion ni:comprartive neurolization,comparative infectivity,and in vitro growth studies. J. Virol.,119: 820-832.
- Long,Q.X.,Wang,X.Z.,Xian,W.D.,et al. (1991) Expression of surface antigen gene of Hepatitis B virus in insects,Biotechnology Sinica,7: 37-46.
- Liu,J.J.,Carstens,E.B. (1996) Identification,molecular cloning,and transcription analysis of the Choristoneura fumiferana nuclear polyhedrosis virus spindale-like protein gene. Virology. 223: 396-400.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences