Calcium-Activated Chloride Channel in Rat Pulmonary Artery Smooth Muscle Cells
Shangbang Gao, Changming Wang, Weiwei Yu, Biwen Mo, Chenhong Li
1Institute of Biophysics and Biochemistry, School of Life Science and Technology, Wuhan 430074, China
2Department of Respiratory, the Affiliated Hospital of Guilin Medical College, Guilin 541001, China
3Department of Respiratory, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430074, China
Abstract
Calcium-activated chloride channels (CaCC) are crucial regulators of vascular tone by promoting a depolarizing influence on the resting membrane potential of vascular smooth muscle cells. The chloride equilibrium potential (ECl) of pulmonary artery smooth muscle cells (PASMC) is significantly more positive than the resting potential. The role of CaCC is probably to produce membrane depolarization and consequent opening of voltage-dependent calcium channels (VDCCs) to evoke contraction by influx of extracellular Ca2+. Using the whole cell patch-clamp technique, sustained Ca2+-activated Cl- currents (ICl(Ca)) evoked by K+ free pipette solutions containing 500nM Ca2+ were recorded in single rat pulmonary artery smooth muscle cell. The electrophysiological characters of CaCC and its possible contributions to the rat pulmonary artery tone were studied. In conclusion, ICl(Ca) exhibited the typical outwardly rectifying. The steady states currents and activation/inactivation time constant of ICl(Ca) have obvious voltage dependence when evoked by fixed 500nM [Ca2+]i. However, increasing the duration of a +70 mV test pulse from 200 to 1400 ms didn’t significantly augment the amplitude of the outward current relaxation and tail current. The possible reasons are discussed.
Keywords
Calcium-activated chloride channel, Pulmonary artery, Smooth muscle cells, Patch clamp, Whole cell, Electrophysiological characters.
1. Introduction
In vascular smooth muscle cells, a variety of cationic channels (Ca2+, K+, Na+) in the plasma membrane have been characterized, and their functional roles in controlling vascular tone have been extensively studied. For example, K+ channel dysfunction plays an important role in the development of pulmonary hypertension [1]. Activity of K+ channels regulates the membrane potential (Em) of PASMC and in turn elevates [Ca2+]i by opening VDCCs which is implicated in stimulating vascular SMC proliferation and inducing vasomotor tone [2]. Analogous to K+ as the predominant intracellular cation, Cl- is the most abundant intracellular and extracellular anion under physiological conditions. The contribution of Cl- channels to the regulation of membrane potential (Em), cytoplasmic free Ca2+ concentration, and vasomotor tone in PASMC is still not completely explored. Membrane depolarization is an important contributor to initiation and maintenance of arterial contraction. The mechanisms responsible for membrane depolarization induced by intracellular Ca2+ release and initial Ca2+ influx through receptor-operated Ca2+ channels are not completely known. However, activation of CaCC and inhibition of delayed rectifier K+ channels may play a pivotal role in depolarizing the cells when [Ca2+]i is increased by agonists. Furthermore, CaCC was found playing an important role in regulating the vascular tones. ICl(Ca) have been recorded from many types of smooth muscle. The majority of experiments studying the properties of ICl(Ca) in vascular smooth muscle cells have utilized the whole-cell recording technique where the resting intracellular Ca2+ concentration and the chloride equilibrium potential (ECl) is significantly more positive than the resting potential. The role of ICl(Ca) is probably to produce membrane depolarization and consequent opening of VDCCs to evoke contraction. ICl(Ca) is activated by free Ca2+ acting on the inner surface of the smooth muscle cell membrane. The Ca2+ that stimulate ICl(Ca) release from intracellular Ca2+ store or enter through VDCCs from extracellular solution. ICl(Ca) is manifest not only during the depolarizing steps, but also as an inward “tail” current (Itail) on stepping back to the holding potential. Itail may generate the depolarizing after-potential that is observed following the action potentials occurring in some smooth muscle [3]. Physiologically these after-potentials are likely to have an important influence on membrane excitability, as they will lead to further opening of VDCCs. The duration of Itail is usually greater than 100 ms, but an interesting observation is that the decay to Itail appears to be vary greatly depending on the tissue used. For example, in rat portal vein and rabbit coronary artery Itail decayed exponentially at negative potentials, and it was postulated that the dECline of Itail might represent channel kinetics [3].
ICl(Ca) has recently been described in smooth muscle cells from coronary artery, portal vein [5] and pulmonary artery [4,5].The present study investigated the electrophysiological properties of CaCC in single rat pulmonary artery’s smooth muscle cell such as the activation/inactivation kinetics, voltage-/time-dependence of ICl(Ca) evoked by fixed 500nM [Ca2+]i.
2. Materials and methods
2.1 Preparation of PASMC
Cells were prepared from the rat main pulmonary artery isolated. After dissection and removal of connective tissue the artery was rubbed with incurvate scissors softly to remove endothelial cells in D-Hanks’ balanced salt solution containing (in mg/mL) 0.4 KCl, 0.06 KH2PO4, 8.0 NaCl, 0.06 Na2HPO4, 0.35 NaHCO3, pH7.2. The tissue was then cut into small strips and incubated in 2mL D-Hanks’ balanced salt solution containing 2mg/mL collagenaseⅠfor 1hour and then added 1mL 0.15% trypsinase for 5min at 37℃ to create a single cell suspension. The digestion was stopped by 2mL DMEM supplemented with 10% fetal bovine serum and the cells were released by gentle agitation with a wide bore Pasteur pipette. Single pulmonary artery smooth muscle cells were resuspended and plated onto a glass coverslip and incubated in a humidified atmosphere of 5% CO2-95% air at 37℃ in 20% fetal bovine serum culture medium for 2-3 days before use.
2.2 Electrophysiology
Conventional whole cell patch-clamp measurements were performed using an EPC-9 patch-clamp amplifier and PULSE software (HEKA, Lambrecht, Germany). In experiments, ICl(Ca) were evoked by pipette solutions containing 500nM Ca2+ as this concentration of Ca2+ generated large and robust Cl- current in pulmonary artery smooth muscle cells [4, 5]. The pipette solution containing (mM): TEA-Cl 20; CsCl 106; HEPES 5; BAPTA 10; MgATP 3; GTPNa2 0.2; MgCl2 0.42 and pH was set to 7.2 by adding CsOH. Free [Ca2+] was set at 500nM by the addition of 7.8mM CaCl2 determined by the EQCAL buffer program. The external solution contained (mM): NaCl 126; HEPES 10; pH 7.4, glucose 11; CaCl2 1.8; MgCl2 1.2; TEA-Cl 10 and 4-aminopyridine 5. All reagents were purchased from Sigma unless other wise stated. Experiments were performed at room temperature (22-25°C).
2.2 Statistical analysis
Data analysis was carried out using IGOR PRO software (Wavemetrics, LakeOswego, OR). Averaged results were expressed as mean ± SEM. The data fitted by mono-exponential started after 5-10 ms of the pulse to avoid the capacitive current. Comparisons between means were performed using Student’s t test. Difference between groups were considered significant when P<0.05.
3. Results
Rat pulmonary artery smooth muscle cells were dialysed with a K+ free pipette solution containing 500nM Ca2+ (see Methods) in order to activate ICl(Ca). A stable inward current was evoked at the holding potential of –50 mV (Ihold) which had mean steady amplitude of 97±13 pA (n=16, Figure 1A). This current has been shown to be a Cl- current that is activated at physiological membrane potentials by intracellular calcium concentrations greater than 100nM [4,5]. ICl(Ca) elicited in the present study exhibited time-/voltage-dependent properties that were revealed by depolarization from –50 mV to +70 mV. Stepping to +70 mV produced an instantaneous current that was followed by the development of an outward current during the voltage step (Irelax+70mV). The activation time constant (τact) of ICl(Ca) was 156±18 ms (n=16) by mono-exponential fitting.Upon repolarization to –80 mV, a tail current (Itail) was recorded that dEClined over the course of the step. The time constant of deactivation (τina) was 69±8 ms (n=16). After an initial period of stabilization of the amplitude of the current at –50 mV and the kinetics of the voltage-dependent relaxations were reproducible for the duration of the experiment under control conditions.
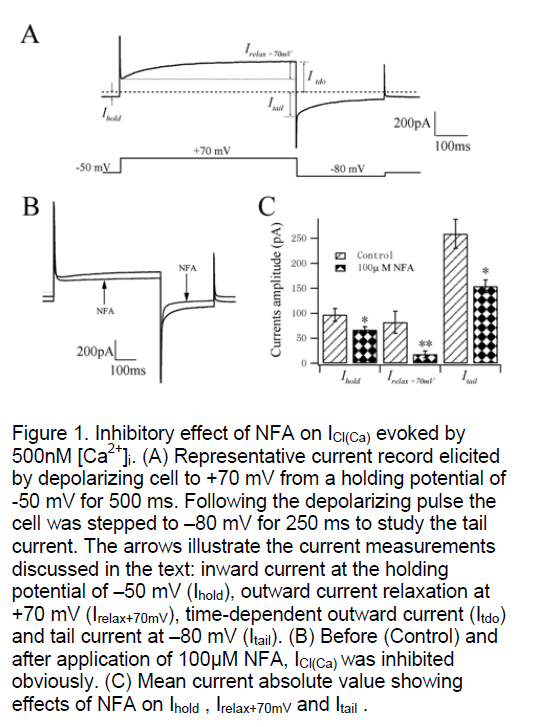
The pioneers have demonstrated that niflumic acid (NFA) inhibits ICl(Ca) in micromolar concentrations with an IC50 (concentration to reduce the amplitude by 50%) of around 2~5μM for inhibition of spontaneous transient inward currents (STICs). Using the present method of activation of ICl(Ca) with 500nM Ca2+ in the pipette solution, Ihold at –50 mV was reduced 30.9% from 97±13 pA to 67±6 pA (n=4, P<0.05, Figure 1B,C) after application of 100μM NFA. Fig. 1B shows the great inhibition of 100μM NFA on the current developed at +70 mV with the mean outward relaxation (Irelax+70mV) decreasing 78.1% from 82±22 pA to 18±7 pA (n=4, P<0.01, Figure 1C). The mean amplitude of the inward tail current recorded upon repolarization to –80 mV (Itail) was reduced 40.6% from 259±29 pA to 154±13 pA (n=4, P<0.05, Fig. 1C). These data suggest that the current we recorded using this method is sensitive to NFA which is known as a special blocker of CaCC.
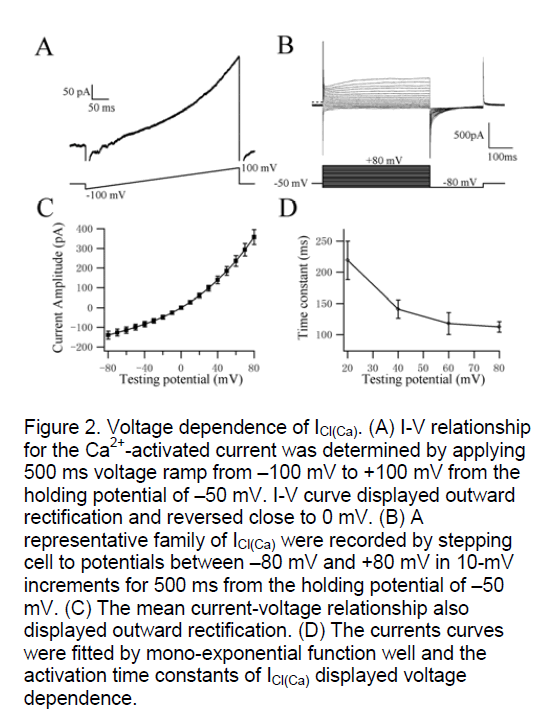
In order to investigate the voltage-dependence of activation kinetics of ICl(Ca) at different testing potentials, we used two classical protocols (ramp and steps) to record the whole cell currents. Figure 2A shows the I-V relationship for the Ca2+-activated current was determined by applying 500 ms voltage ramp from –100 mV to +100 mV from the holding potential of –50 mV. I-V curve revealed outward rectification and reversed close to 0 mV (mean reversal potential (Erev) was 3±2 mV, n=5). The theoretical chloride equilibrium potential (ECl) in these experiments was calculated to be 0.5 mV, suggesting that the Ca2+-activated currents recorded in rat pulmonary artery smooth muscle was ICl(Ca). The currents amplitude and activation time constants of Itdo by depolarizing voltage steps from –80 mV to +80 mV in 10-mV increments from a holding potential of –50 mV were recorded in freshly isolated cells (Figure 2B). Figure 2C shows the relationship between the potentials and the currents by stepping to different testing potentials, which also exhibited typical outwardly rectifying steady states (n=11). The Irelax+70mV could be fitted well by mono-exponential function. The activation time constant is dependent on voltage (n=11, Figure 2D). The testing potentials are higher the activation of CaCC is more quickly. These data indicate that CaCC have distinct voltage dependence in rat pulmonary artery smooth muscle cells when evoked by 500nM [Ca2+]i.
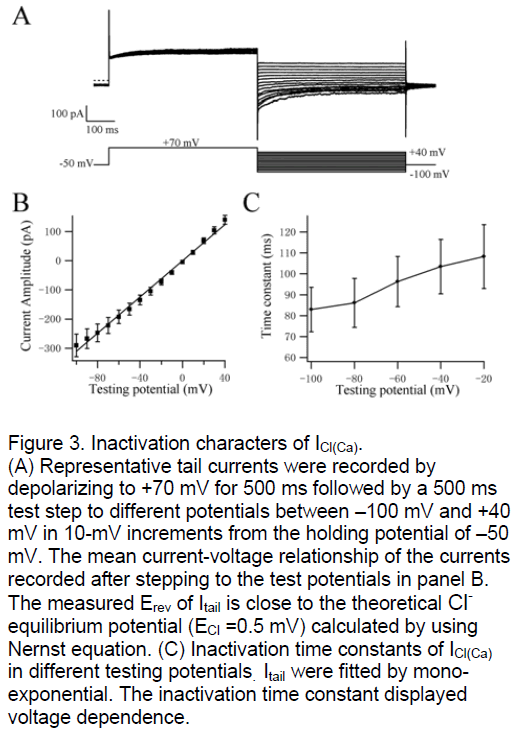
ICl(Ca) was widely discussed as a tail current in many tissues. We study the tail currents to investigate the inactivation kinetics of CaCC. The PASMC were depolarized initially from –50 mV to +70 mV for 500 ms and then stepped to test potentials from +40 mV to –100 mV in 10-mV increments for 500 ms (Figure 3A). The curve of mean current-voltage relationship was shown in Figure 3B. The measured Erev of Itail was 3.7±3 mV (n=17, Figure 3A,B) that is also close to the theoretical Cl- equilibrium potential (ECl =0.5 mV) calculated by using Nernst equation. These data confirmed that Itail carried by Cl- in deed again. The tail currents were fitted by mono-exponential function and the inactivation time constants were shown in Figure 3C. The inactivation kinetics of ICl(Ca) is voltage-dependent similar with its activation kinetics. The testing potentials are lower the inactivation of CaCC is more quickly.
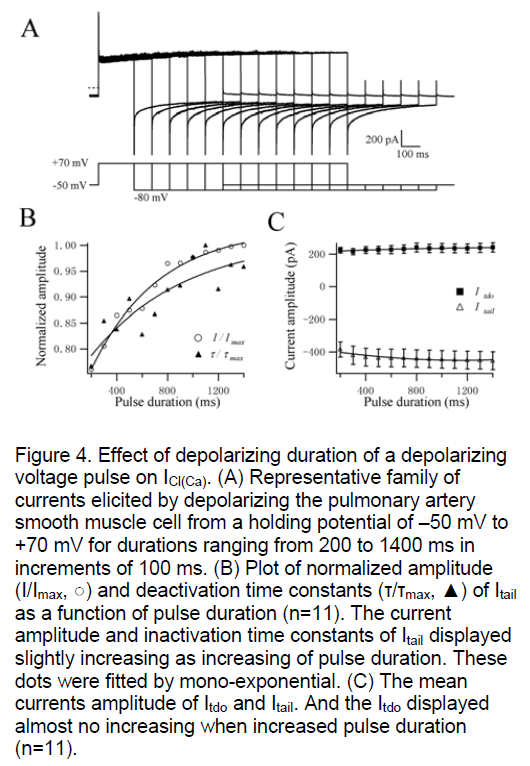
The time-dependence of CaCC was studied in single rat PASMC. Increasing the duration of +70 mV test pulse from 200 to 1400 ms (in increments of 100 ms) only slightly augmented the amplitude of Itdo and Itail (n=11, Figure 4B,C). With longer periods of prior activation, the time constants for Itail inactivation were also increased a little (n=11, Figure 4B). And, increasing the step duration had no effect on the activation time constant of Itdo at +70 mV (Figure 4A). The activation kinetics followed the same mono-exponential time course as shown in Figure 4A is similar to the results of Figure 1A. The current amplitude of Itail just augmented slightly but not significantly with a settled 500nM [Ca2+]i which activated the CaCC throughly and stably [4,5]. These results indicate that the activation and inactivation characters of CaCC evoked by a fixed 500nM [Ca2+]i didn’t have obvious time dependence which is reported in Yuan’s study. Their results were different that increasing the duration of a +10 mV test pulse significantly augmented the amplitude and inactivation time constants of Itail [12]. The possible reason was that the currents were evoked via a gradual accumulation of Ca2+ through Ca2+ influx through membrane Ca2+ channels and/ or Ca2+-induced Ca2+ release from intracellular Ca2+ stores when the cells was polarized to +10 mV. However, the concentration of [Ca2+]i in our experiments was an invariable 500nM. So it didn’t need time to accumulate of Ca2+ to elevate the [Ca2+]i concentration to evoke ICl(Ca) progressively.
3. Discussion
3.1 Electrophysiological properties of CaCC
The electrophysiological properties of CaCC are the essential study contents in present experiments. We used three classical voltage protocols to study the activation kinetics, inactivation kinetics of Itail, time dependence of ICl(Ca) evoked by a fixed 500nM [Ca2+]i in rat PASMC. In our experiments, we found that the activation kinetics of CaCC in rat PASMC have similar voltage dependent properties as some other cells. And the activation time constants fitted by mono-exponential function decreased following the increasing of voltage. The time constants of Itail were between 70~120 ms at different testing potentials, and inactivation kinetics also had voltage dependent properties. The main finding of the present work is that in rat pulmonary artery smooth muscle cells the ICl(Ca) when activated by 500nM [Ca2+]i imposed by dialysis from the pipette solution didn’t have obvious time dependent properties. This is a surprising result because in all previous reports it has been shown that CaCC behave an apparent time dependent activation. The most likely explanation for the discrepancy in the results with [Ca2+]i is a difference in the experimental conditions used. In previous studies, the CaCC were tonically activated by constantly elevated [Ca2+]i by activating the VDCCs in single cells. The cytoplasmic calcium concentration increased via a gradual accumulation of influx of extracellular Ca2+ by depolarizing cell to +10 mV. In the present work ICl(Ca) was activated by a relatively high 500nM [Ca2+]i imposed by the patch pipette solution. And the depolarizing potential we used was +70 mV that can’t open the VDCCs.
3.2 Activity of CaCC regulates PA vasomotor tone by controlling Em.
CaCC appear to exist in cells isolated from a variety of types of vessels smooth muscle cells [3, 4, 5, 10]. In PASMC the resting Em, ranging from –35 mV to –55 mV [5, 6], is more positive than the K+ equilibrium potential (Ek) but more negative than ECl- Accordingly, under these resting conditions, alteration of sarcolemmal Cl- channel activity would substantially contribute to the regulation of Em, which dominates the activity of voltage-gated Ca2+ channels. Activation of CaCC, by facilitating Cl- efflux, would thus result in membrane depolarization and activation of voltage-gated Ca2+ channels, increasing [Ca2+]i and causing pulmonary vasoconstriction. The Ca2+ concentration threshold for activation of ICl(Ca) in portal vein smooth muscle cells is 180nM, with full activation at 600nM. In rat PASMC, the resting [Ca2+]i is 50-100nM, whereas agonist-induced increases in [Ca2+]i usually range from 200 to 1000nM. Many vasoactive agents mediate vascular contraction in association with an initial transient increase in [Ca2+]i followed by a sustained [Ca2+]i plateau. The Ca2+ transient is often due to Ca2+ release from intracellular stores and serves to trigger contraction [11]. Increase in [Ca2+]i activates CaCC and elicits inward ICl(Ca). The resulting Cl- currents would cause membrane depolarization, thereby open voltage-gated Ca2+ channels and leading to additional increase in [Ca2+]i. Although the precise mechanism is not completely known, Ca2+-induced activation of CaCC like 5-TH and PE, in addition to increasing [Ca2+]i, also causes membrane depolarization and sustained vasoconstriction.
Ca2+-activated chloride channels were less studied than other chloride channels, such as volume-regulated chloride channel, or cystic fibrosis trans-membrane conductance regulator (CFTR) chloride channels. Nevertheless, CaCC, in diverse cell types, is clearly an important channel type involved in various physiological functions (cell secretion, anion transport, cell adhesion etc.) [6,7]. The first distinct CaCC was identified in the bovine airway, with an ion selectivity of I>Cl, and sensitive to DIDS [8]. To date, at least ten isoforms of CaCC (found from bovine, human, mouse and porcine) have been identified and published on NCBI GenBank database [8]. The electrophysiological features of CaCC are very similar in the various cell types [9]. The current-voltage relationship described here displayed obvious outward rectification characters observed also in some other cells [7]. The channel kinetics of CaCC in rat PASMC we reported here had similar properties with CaCC demonstrated in cultured rabbit pulmonary artery smooth muscle cells, portal vein smooth muscle cells, sheep lymphatic smooth muscle cells [10]. All of them had typical voltage dependence of activation and inactivation. Thus, CaCC presently tested belongs to the general CaCC family. In smooth muscle cells, the speculated roles of CaCC are regulation of membrane potential and modulation of agonist-induced store-depletion dependent intracellular calcium signaling, including regulation of calcium influx. The calcium influx sequentially regulates the contraction of vascular smooth muscle cells and plays an essential role in controlling vascular tone and other disease such as pulmonary hypertension.
Acknowledgements
This work was supported by the grants from The National Nature Science Foundation of China (30260040, 30470646), The Talent Foundation of Guangxi provinces (2001211). We express our acknowledgement for the help from Prof. Tao Xu on this work.
References
- Yuan J.X., Aldinger A.M., Juhaszova M., et al. (1998) Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation, 98:1400-6.
- Platoshyn O., Golovina V.A., Bailey C.L., et al. (2000) Sustained membrane depolarization and pulmonary artery smooth muscle cell proliferation. Am J Physiol, 279:C1540-9.
- Pacaud P., Loirand G., Lavie J.L., et al. (1989) Calcium-activated chloride current in rat vascular smooth muscle cells in short-term primary culture. Pflügers Arch, 413:629-36.
- Piper A.S., Greenwood I.A. (2003) Anomalous effect of anthracene-9-carboxylic acid on calcium-activated chloride currents in rabbit pulmonary artery smooth muscle cells. British Journal of Pharmacology, 138:31-38.
- Greenwood I.A., Ledoux J., Leblanc N. (2001) Differential regulation of Ca2+-activated Cl- currents in rabbit arterial and portal vein smooth muscle cells by Ca2+-calmodulin-dependent kinase. J Physiol, 543:395-408.
- Fuller C.M., Ji H.L., Tousson A., et al. (2001) Ca2+ activated Cl- channels: a newly emerging anion transport family. Pflügers Arch, 443(Suppl 1):S107-110.
- Nilius B., Droogmans G. (2003) Amazing Chloride channels: an overview. Acta Physiol Scand, 177:119-47.
- Pauli B.U., Abdel-Ghany M., Cheng H.C., et al. (2000)Molecular characteristics and functional diversity of CLCA family members. Clin Exp Phamacol Physiol, 27:901-5.
- Fuller C.M., Benos D.J. (2000) Electrophysiological characteristics of the Ca2+-activated Cl- channel family of anion transport proteins. Clin Exp Pharmacol Physiol, 27:906-910.
- Toland H.M., Mccloskey K.D., Thornbury K.D., et al. (2000) Ca2+-activated Cl- current in sheep lymphatic smooth muscle. Am J Physiol Cell Physiol, 279:C1327-35.
- Hartzell C., Putaier I., Arreola J. (2005) Calcium-activated chloride channels. Annu Rev Physiol, 67:719-58.
- Yuan X.J. (1997) Role of calcium-activated chloride current in regulating pulmonary vasomotor tone. Am J Physiol, 272:L959-68.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
