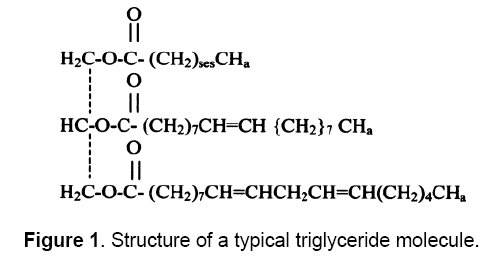Biodiesel: an Alternative fuel Produced From Vegetable Oils by Transesterification
K.M. Shereena, T.Thangaraj
Department of Zoology, Kongunadu arts and Science College (Autonomous), Coimbatore-641 029, Tamil Nadu, India
- Corresponding Author:
- E-mail: sherin.moidu@gmail.com.
Abstract
Due to the concern on the availability of recoverable fossil fuel reserves and the environmental problems caused by the use those fossil fuels, considerable attention has been given to biodiesel production as an alternative to petrodiesel. Biodiesel is an ecofriendly, alternative diesel fuel prepared from domestic renewable resources i.e. produced from vegetable oils and animal fats. It is a renewable source of energy seems to be an ideal solution for global energy demands including India as well. The general way to produce biodiesel fuel is by transesterification of vegetable oil with methanol in the presence of either alkaline or strong acid catalysts. Transesterification reaction is quite sensitive to various parameters. An ideal transesterification reaction differs on the basis of variables such as fatty acid composition and the free fatty acid content of the oil. Other variables include reaction temperature, ratio of alcohol to vegetable oil, catalyst, mixing intensity, purity of reactants. This review paper describes the chemical composition of vegetable oils, fuel properties vegetable oils and biodiesel, transesterification process, the most important variables that influence the transesterification reaction, environmental consideration and economic feasibility of biodiesel.
Keywords
Biodiesel; Transesterification; Catalyst; Alcohol; Alkaline.
1. Introduction
Recent petroleum crisis, increasing cost and unavailability of petroleum diesel gave impetus to scientists to work on alternative fuel [1]. The growth in consumption of petroleum oil throughout the world has caused urgent economic, security, and environmental problems [2]. As the fossil fuels are limited and it takes million of years for their formation, their availability may be prolonged by decreasing overall consumption. Various renewable sources of energy have successfully been tried and used by different nations to limit the use of fossil fuels. This renewable source of energy includes solar energy, wind energy, geothermal energy, tidal energy, ocean thermal energy, hydropower and others. Use of the renewable energy has made the nation self-dependent to some extent but still it is far behind to make a significant difference in import of crude oil which is the need of present day [3]. In the year 2004–2005, India imported 75 % of crude oil from other countries to meet the energy requirements. The demand for diesel and gasoline increased drastically in the year 2008 - 2009. It has been estimated that the demand for diesel will be 66.90 Mt for the year 2011-2012. Hence, government of India has taken necessary steps to fulfil future diesel and gasoline demand and to meet the stringent emission norms. Biodiesel and alcohol are being considered to be supplementary fuels to the petroleum fuels in India [4].
A new technology, i.e. transesterification reaction has been applied to produce a renewable fuel ‘‘biodiesel’’ derived from various raw materials. These raw materials include the edible and nonedible oils, algae, waste cooking oil, etc. It is named biodiesel because it is derived from biological products and matches petrodiesel in performance. The biodiesel so produced has lesser exhaust emissions in terms of unburnt hydrocarbon, carbon monoxide and particulate matter [3]. Biodiesel can be termed clean fuel as it does not contain carcinogens and its sulphur content is also lesser than the mineral diesel. It possesses high biodegradability and lubricating property which makes it even better fuel. Hence, being a renewable fuel and characteristics similar to petrodiesel, it has the potential to be an alternate for petrodiesel in long run. However, few other properties of biodiesel are of concern and have to be improved to make it usable in neat form (i.e. 100% biodiesel). These properties are increase in calorific value, engine power, reduced emission of NOx and improvement in low temperature properties [5]. An improvement in oxidation stability is also desired to prevent it from deterioration with time. At present it is compatible in blended form with mineral diesel in the ratio 20 (biodiesel):80 (mineral diesel). Biodiesel has been in use in countries such as United States of America, Malaysia, Indonesia, Brazil, Germany, France, Italy and other European nations. However, the potential for its production and application is much more. The feedstock available for development of biodiesel in these nations is 28% for soybean oil 22% for palm oil, 20% for animal fats, 11% for coconut oil, while rapeseed, sunflower and olive oils constitute 5% each [3].
1.1 Background of biodiesel
For diesel engines, vegetable oils are the renewable alternative fuels. The concept of using vegetable oil as a fuel is nothing new. Dr. Rudolf Diesel first developed the diesel engine in 1895 with the full intention of running it on a variety of fuels, including vegetable oil. Diesel demonstrated his engine at the World Exhibition in Paris in 1900 using peanut oil as fuel. Since Diesel's time, the design of the diesel engine has been modified so it can run on the cheapest fuel available: petroleum "diesel" fuel. However, despite the technical feasibility, vegetable oils as fuel could not get acceptance, as they were more expensive than petroleum fuels. This lead to the retardation in scientific efforts to investigate the further acceptability of vegetable oils as fuel. Later, due to numerous factors as stated earlier, created renewed interest of researchers in vegetable oil as substitute fuel for diesel engines [6].
In view of the potential properties of the vegetable oils, a large number of investigations have been carried out internationally in the area of vegetable oils as fuel. Some of the vegetable oils from farm and forest origin have been identified. Jamieson listed over 350 oil bearing crops [7]. Few researchers examined the fatty acid profiles of seed oils of 75 plant species having 30 % or more fixed oil in their seed. They reported that the fatty acid methyl esters of oils of 26 species were found most suitable for use as biodiesel and they meet the major specification of biodiesel standards of USA, Germany and Europe [8].
1.2 Vegetable oils as diesel fuels
The use of vegetable oils, such as palm, soya bean, sunflower, peanut, and olive oil, as alternative fuels for diesel engines dates back almost nine decades, but due to the rapid decline in crude oil reserves, it is again being promoted in many countries. Depending upon the climate and soil conditions, different countries are looking for different types of vegetable oils as substitutes for diesel fuels. For example, soya bean oil in the US, rapeseed and sunflower oils in Europe, palm oil in South-east Asia (mainly Malaysia and Indonesia) and coconut oil in the Philippines are being considered. Besides, some species of plants yielding non-edible oils, e.g. jatropha, karanji and pongamia may play a significant role in providing resources. Both these plants may be grown on a massive scale on agricultural / degraded / waste lands, so that the chief resource may be available to produce biodiesel on ‘farm scale’ [9].
1.3 Biodiesel
Biodiesel is defined as mono-alkyl esters of long chain fatty acids derived from vegetable oils or animal fats, which conform to ASTM D6751 specifications for use in diesel engines. Fuel-grade biodiesel must be produced to strict industry specifications in order to ensure proper performance. Biodiesel contains no petroleum, but it can be blended at any level with petroleum diesel to create a biodiesel blend [6]. Biodiesel is one of the current favourites to be the next generation fuel. It is made from renewable biological sources such as vegetable oils and animal fats [10]. It is biodegradable, non-toxic and has low emission profile. Chemically, biodiesel is fatty acid methyl esters (FAME) and they are called biodiesel only when used as fuel in diesel engines and heating systems [11,12]. Biodiesel shows the following general advantages: (1) lower dependence on crude oil, (2) renewable fuel, (3) favorable energy balance, (4) reduction in greenhouse gas emission, (5) lower harmful emission, (6) biodegradable and non-toxic, (7) the use of agricultural surplus, and (8) safer handling (higher flash point than conventional diesel fuel) [11]. Biodiesel is often used as a blend, B20 (20 vol% biodiesel and 80 vol% conventional diesel), rather than as B100 [13].
1.4 Chemical compositions of vegetable oils
Vegetable oils, also known as triglycerides, have the chemical structure given in Figure 1 comprise of 98% triglycerides and small amounts of mono- and diglycerides. Triglycerides are esters of three molecules of fatty acids and one of glycerol and contain substantial amounts of oxygen in their structure.
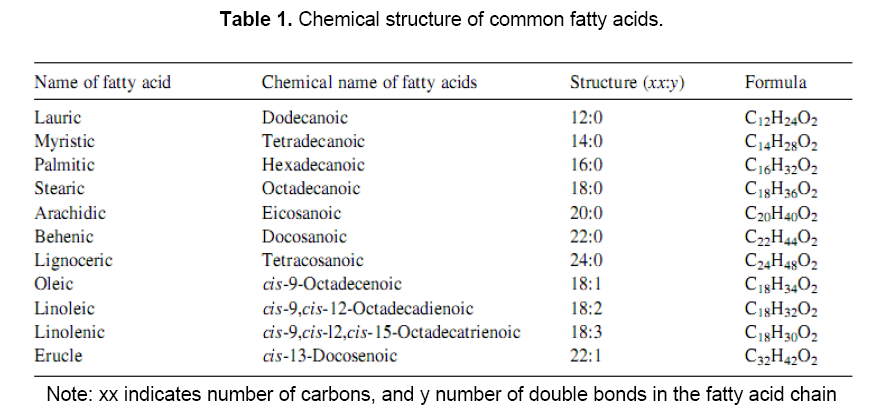
In the triglycerides alkyl chains of vegetable oil, predominate the palmitic, oleic and linoleic fatty acids. Various vegetable oils are distinguished by their fatty acid compositions. Myristic (14:0) palmitic (16:0), stearic (18:0), arachidic (20:0), Behenic (22:0), linoceric (24:0), Oleic (18:1), Erucic (22:1), Linoleic(18:2) and linoleinic (18:3) are commonly present fatty acids in vegetable oils in varying percentages. Different types of oils have different types of fatty acids. The empirical formula and structure of various fatty acids present in vegetable oils are given in Table 1 [14]. Fatty acids fully saturated with hydrogen have no double bonds[15]. Fully saturated triglycerides are solid at room temperature and thus as such can not be used as fuel. Triglyceride molecules have molecular weights between 800 and 900 and are thus nearly four times larger than typical diesel (C16H34) fuel. Due to higher molecular weights, vegetable oils have low volatility and because of their unsaturation, vegetable oils are inherently more reactive than diesel fuels. As a result, they are much more susceptible to oxidation and thermal polymerization reactions.
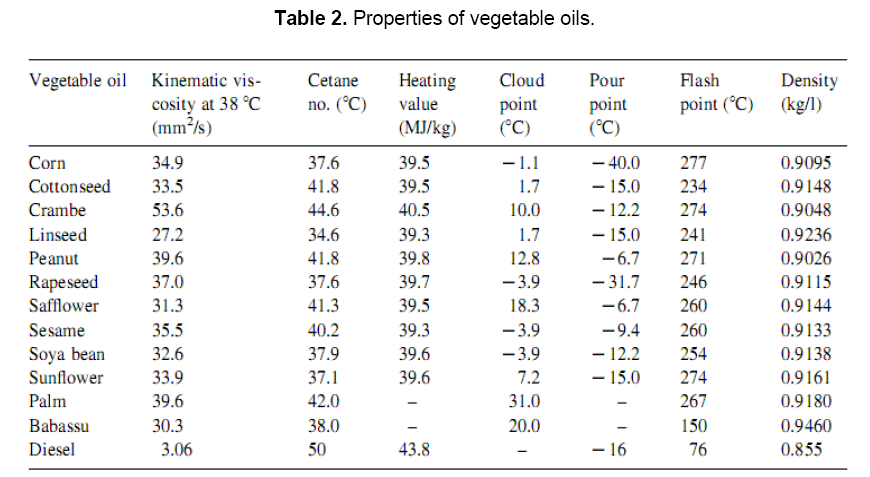
1.5 Properties of Vegetable oils as fuel
Fuel properties of vegetable oils have been studied by many researchers. Compared to the diesel, the fuel properties of vegetable oils as listed in Table 2 indicate that the kinematics viscosity of vegetable oils varies in the range of 30–40 cSt at 38°C. The high viscosity of these oils is due to their large molecular mass in the range of 600–900, which is about 20 times higher than that of diesel fuel. The flash point of vegetable oils is very high (above 200°C). The volumetric heating values are in the range of 39–40 MJ/kg, as compared to diesel fuels (about 45 MJ/kg). The presence of chemically bound oxygen in vegetable oils lowers their heating values by about 10%. The cetane numbers are in the range of 32–40[9].The major problem with the direct use of vegetable oils as fuel into compression ignition (CI) engines is their higher viscosity. It interferes the fuel injection and atomization and contributes to incomplete combustion, nozzle clogging, excessive engine deposits, ring sticking, contamination of lubricating oil etc. The problem of higher viscosity of vegetable oils can be overcome to a greater extend by various techniques, such as heating, dilution, emulsification and esterfication etc [6].
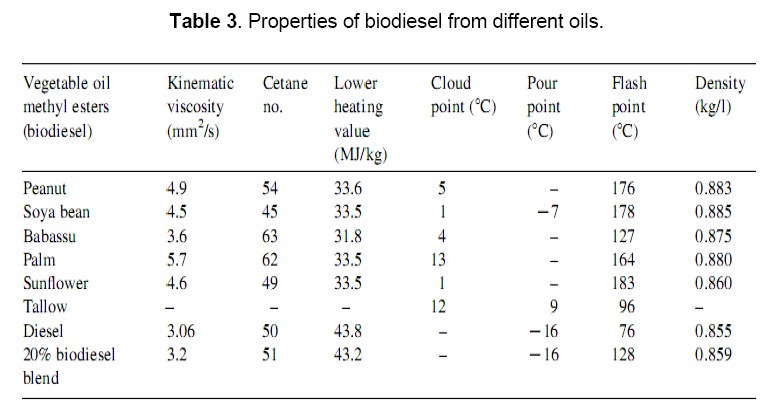
1.6 Fuel properties of biodiesel
The properties of biodiesel and diesel fuels, as given in Table 3 [16–20], show many similarities, and therefore, biodiesel is rated as a strong candidate as an alternative to diesel. This is due to the fact that the conversion of triglycerides in to methyl or ethyl esters through the transesterification process reduces the molecular weight to one-third, reduces the viscosity by about one-eighth, and increases the volatility marginally. Biodiesel contains 10–11% oxygen (w/w), thereby enhancing the combustion process in an engine. It has also been reported that the use of tertiary fatty amines and amides can be effective in enhancing the ignition quality of the biodiesel without having any negative effect on its cold flow properties. However, starting problems persist in cold conditions. Further, biodiesel has low volumetric heating values (about 12%), a high cetane number and a high flash point. The cloud points and flash points of biodiesel are 15–25°C higher than those of diesel.
2. Process of Biodiesel Production
2.1 Simple transesterification reaction
Transesterification of vegetable oils with simple alcohol has long been the preferred method for producing biodiesel. In general, there are two methods of transesterification. One method simply uses a catalyst and the other is without a catalyst. The former method has a long history of development and the biodiesel produced by this method is now available in North America, Japan and some western European countries.
2.2 Chemistry of transesterification reaction
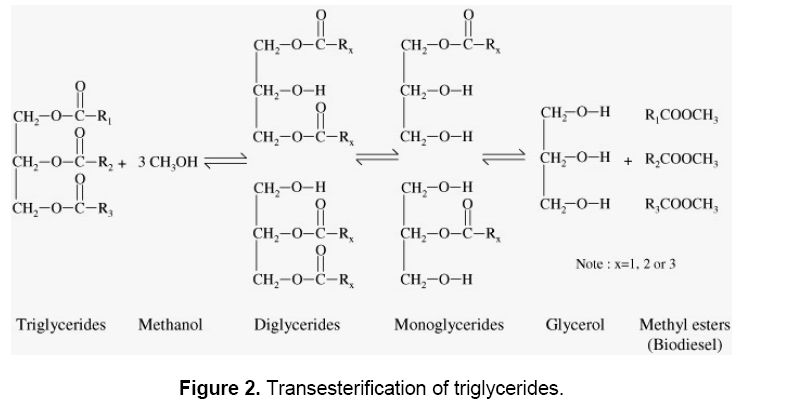
The overall transesterification reaction [21] is given by three consecutive and reversible equations:
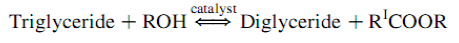
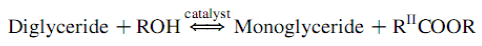

The process yields glycerol as a by-product. The stochiometry of the reaction requires 3 moles of methanol and 1 mole of triglyceride to give 3 moles of fatty acid methyl ester and 1 mole of glycerol (Figure 2). This leads to three consecutive reversible reactions in which monoglycerides and diglycerides are intermediate products. After the reaction, the glycerol is separated by settling or centrifugation and is purified to be used in traditional applications (pharmaceutical, cosmetic and food industries) or in recently developed applications in the fields of animal feed, carbon feedstock in fermentations, polymers, surfactants and lubricants [10]. The methyl ester phase is purified before being used as a diesel fuel. The fatty acid composition of biodiesel is feedstock dependent, and is affected by factors such as climatic conditions, soil type, and plant health and maturity upon harvest [22].
As seen above, the transesterification is an equilibrium reaction in which excess alcohol is required to drive the reaction close to completion. Fortunately, the equilibrium constant favors the formation of methyl esters such that only a 5:1 molar ratio of methanol: triglycerides is sufficient for 95–98% yield of ester. It might be anticipated that in such a system, glycerol would play a major role in achieving conversions close to 100%. Several catalysts were tried for the purpose of transesterification by several workers, e.g. magnesium, calcium oxides and carbonates of basic and acidic macro-reticular organic resin, alkaline alumina, phase transfer catalysts, sulphuric acids, p-toluene sulphonic acid, and dehydrating agents as co- catalysts [23]. The catalysts reported to be effective at room temperature were alkoxides and hydroxides [24].
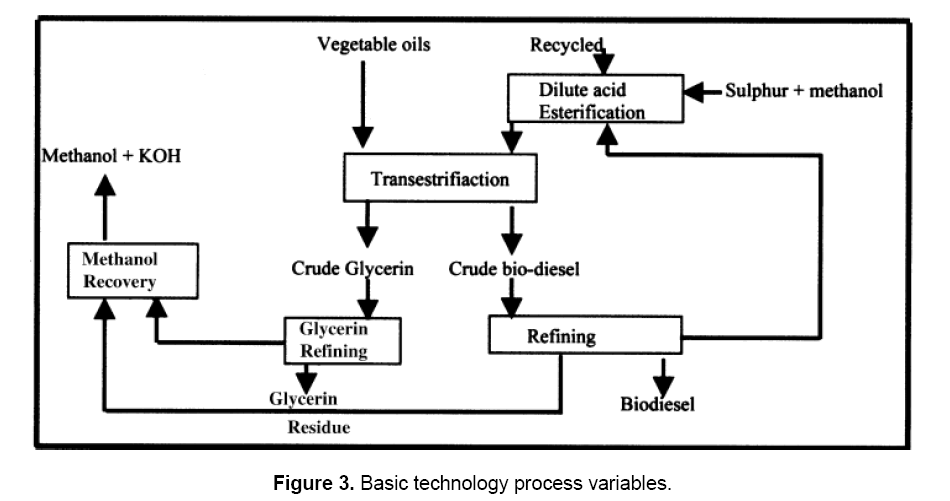
During methanolysis, two distinct phases are present as the solubility of the oil in methanol is low and the reaction mixture needs vigorous stirring. Optimum reaction conditions for the maximum yield of methyl esters have been reported to be 0.8% (based on weight of oil) potassium hydroxide catalyst and 100% excess methanol at room temperature for 2.5 h. Glycerol phase separation does not occur when < 67% of the theoretical amount of methanol is used. The excess methanol, however, is removed by distillation. Traces of methanol, KOH, free fatty acids (FFAs), chlorophyll, etc. go into the glycerin phase, which can be processed in two stages. Glycerin of 90–95% purity is obtained in the first stage and of 98% purity in the second stage. The basic process schematic of biodiesel production is given in Figure 3. The energetics have indicated that about 50 kW of electricity per ton of biodiesel is required, of which 60–70% is consumed for the production of glycerin. The process requires mixing of vegetable oil with a mixture prepared by dissolving KOH catalyst in methanol and heating at 70°C with stirring for 1 h. The mixture is allowed to settle under gravity. The glycerin, being heavier, settles down in the bottom layer and the upper layer constitutes the biodiesel (esters). The glycerin is separated and the esters are washed with water for catalyst recovery. The biodiesel layer is finally dried using silica gel and it is now ready for blending with diesel in various proportions for engine operation. The blend, for convenience, is referred to as Bxx, where XX indicates the amount of biodiesel in percentage in the blend (i.e. B-20 blend is 20% biodiesel and 80% diesel) [9].
3. Most important variables that influence the transesterification reaction
3.1 Reaction temperature
The literature has revealed that the rate of reaction is strongly influenced by the reaction temperature. However, the reaction is conducted close to the boiling point of methanol (60–70°C) at atmospheric pressure for a given time. Such mild reaction conditions require the removal of free fatty acids from the oil by refining or preesterification. Therefore, degummed and deacidified oil is used as feedstock [25]. Pretreatment is not required if the reaction is carried out under high pressure (9000 kPa) and high temperature (240°C), where simultaneous esterification and transesterification take place with maximum yield obtained at temperatures ranging from 60 to 80 Cata molar ratio of 6:1 [18,26,27].
3.2 Ratio of alcohol to oil
Another important variable is the molar ratio of alcohol to vegetable oil. As indicated earlier, the transesterification reaction requires 3 mol of alcohol per mole of triglyceride to give 3 mol of fatty esters and 1 mol of glycerol. In order to shift the reaction to the right, it is necessary to either use excess alcohol or remove one of the products from the reaction mixture. The second option is usually preferred for the reaction to proceed to completion. The reaction rate was found to be highest when 100% excess methanol was used. A molar ratio of 6:1 is normally used in industrial processes to obtain methyl ester yields higher than 98% (w/w) [25].
3.3 Catalysts
Alkali metal alkoxides are found to be more effective transesterification catalysts compared to acidic catalysts. Sodium alkoxides are the most efficient catalysts, although KOH and NaOH can also be used. Transmethylation occurs in the presence of both alkaline and acidic catalysts [29]. As they are less corrosive to industrial equipment, alkaline catalysts are preferred in industrial processes. A concentration in the range of 0.5–1% (w/w) has been found to yield 94–99% conversion to vegetable oil esters [18,28], and further increase in catalyst concentration does not affect the conversion but adds to extra cost, as the catalyst needs to be removed from the reaction mixture after completion of the reaction.
3.4 Mixing intensity.
It has been observed that during the transesterification reaction, the reactants initially form a two-phase liquid system. The mixing effect has been found to play a significant role in the slow rate of the reaction. As phase separation ceases, mixing becomes insignificant. The effect of mixing on the kinetics of the transesterification process forms the basis for process scale-up and design.
3.5 Purity of reactants
Impurities in the oil affect the conversion level considerably. It is reported that about65–84% conversion into esters using crude vegetable oils has been obtained as compared to 94–97% yields refined oil under the same reaction conditions [25]. The free fatty acids in the crude oils have been found to interfere with the catalyst. This problem can be solved if the reaction is carried out under high temperature and pressure conditions.
4. Environmental considerations
In view of environmental considerations, biodiesel is considered ‘carbon neutral’because all the carbon dioxide released during consumption had been sequestered fromthe atmosphere for the growth of vegetable oil crops. Studies have shown that the combustion of 1 - 1 of diesel fuel leads to the emission of about 2.6 kg of CO2 against 1 kg of CO2/kg of biodiesel [30], so the use of biodieselmay directly displace this amount of CO2 when used in engines. The combustion of biodiesel has been reported to emit lesser pollutants compared to diesel. The emission of SO2, CO, hydrocarbons (HC, polyaromatic hydrocarbons (PAH), and aromatics [31], which indicates that the engine exhaust contains no SO2, and shows decreasing emissions of PAH, soot, CO, HC and aromatics. The NOx emissions are reported to be in the range between G10% as compared to diesel depending on engine’s combustion characteristics.
5. Economic feasibility of biodiesel
India has rich and abundant resources of edible and non-edible oilseeds, the production of which can be stepped up manifolds if the government provides incentives to farmers for production of biodiesel. The economic feasibility of biodiesel depends on the price of crude petroleum and the cost of transporting diesel over long distances to remote areas. It is a fact that the cost of diesel will increase in future owing to the increase in its demand and limited supply. Further, the strict regulations on the aromatic and sulphur contents of diesel fuels will make diesel costlier, as the removal of aromatics from distillate fractions needs costly processing equipment and continuous high operational cost as large amounts of hydrogen are required for ring saturation. Similarly, reducing the sulphur content is also a big challenge for the industries. Currently, the production of methyl or ethyl esters from edible oils is much more expensive than that of diesel fuels due to the relatively high costs of vegetable oils (about four times the cost of diesel in India).Methyl esters produced from such oils cannot compete economically with diesel fuels unless they are granted protection from tax levies. Under such conditions, there is a need to explore alternate feedstocks for the production of biodiesel [9].
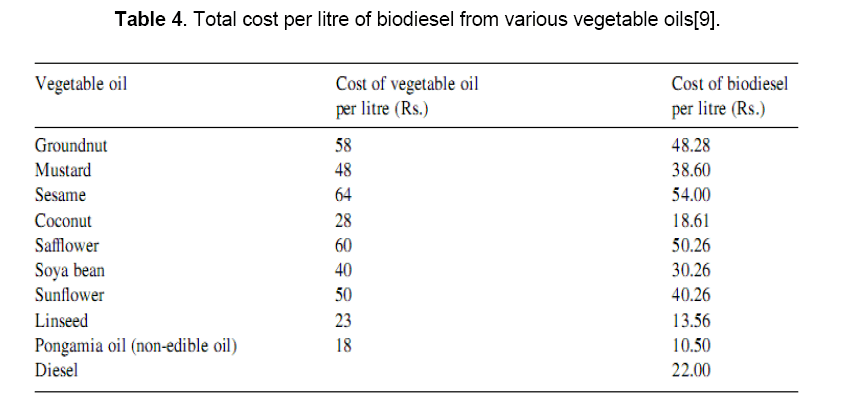
An economic analysis for the production of biodiesel using different types of edible andnonedible oils has been carried out and the results are reported in Table 4. The analysis indicates that linseed oil has the least cost (Rs. 13.56 per litre) and sesame oil has the maximum cost (Rs. 54.00 per litre). The analysis has considered the cost of a plant (Rs. 1,174,000) with an installed capacity 136,500 per year and 10% depreciation. The operating cost and by-product cost per year are Rs. 4,018,002 and 2,296,000, respectively, with a 10 year life of the plant [32]. Table 5 indicates that the cost of biodiesel produced from pongamia oil is the least, i.e. Rs.10.50, followed by linseed oil (Rs. 13.50) and coconut oil (Rs. 18.61) as compared to diesel (Rs. 22) per litre. The cost can be reduced further if we consider non-edible oils, used frying oils and acid oils instead of edible oils. Non-edible oils from sources such as neem, mahua, pongamia, karanji, babassu, jatropha, etc. are easily available in many parts of the world including India, and are very cheap compared to edible oils. With the mushrooming of fast food centers and restaurants in India, it is expected that considerable amounts of used frying oil will be discarded which can be diverted for biodiesel production, and thus may help reduce the cost of water treatment in the sewerage system and assisting in the recycling of resources. Acid oil, which is cheaper than both raw and refined oils, is a major by-product of the alkali refining industries and is a potential raw material for making biodiesel [9].
6. Conclusions
A number of studies have shown that triglycerides (vegetable oils/animal fats) hold promise as alternative fuels for diesel engines. It was observed from the reported literature that the most of the transesterification studies have been done on edible oils like rapeseed, soybean, sunflower, canola etc. by using methanol and sodium / potassium hydroxide as catalyst. There are very few studies reported on non-edible oils. From the literature review, it is observed that the biodiesel properties are close to the diesel and satisfies fuel standards of many countries. It was reported that the combustion characteristics of biodiesel are similar as diesel and the engine power output with biodiesel was found to be equivalent to that of diesel. Moreover, the use of biodiesel in diesel engine results in drastic reduction engine emissions. The oxidation of biodiesel during storage period may be reduced by the use of antioxidants. Economic feasibility study shows that the biodiesel obtained from non-edible oils is cheaper than that from edible oils. From this review, we conclude that the biodiesel is a better alternative renewable fuel for the diesel.
References
- Divya Bajpai., Tyagi.V.K. (2006) Biodiesel: source, production composition, properties and its benefits. Jounal of oleo science, 55(10): 487-502.
- GuoqingGuan, Katsuki Kusakabe, Nozomi Sakurai, Kimiko Moriyama. (2009) Transesterification of vegetable oil to biodiesel fuel using acid catalysts in the presence of dimethyl ether. Fuel, 88: 81-86.
- Sharma Y.C., Singh B. (2009) Development of biodiesel: Current scenario. Renewable and Sustainable Energy Reviews, 13:1646-1651.
- Subramanian K.A., Singal S.K., Mukesh Saxena., Sudhir Singhal. (2005) Utilization of liquid biofuels in automotive diesel engines : An Indian perspective, J. Biomass and Bioenergy, 29: 65-72.
- Kegl B. (2008) Biodiesel usage at low temperature. Fuel, 87: 1306-1317.
- Kapilan N., Ashok Babu T.P., Reddy R.P. (2009) Technical aspects of biodiesel and its oxidation stability. International Journal of Chem Tech Research, 1(2): 278-282.
- Jamieson G. S. (1943) Vegetable Fats and Oils. Reinhold Publishing Corporation, New York.
- Mohibbe Azam, Amtul Waris, Nahar N.M. (2005) Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for used as biodiesel in India. Biomas and Bioenergy, 29: 293-302.
- Barnwal B.K., Sharma M.P. (2005) Prospects of biodiesel production from vegetable oils in India. Renewable and Sustainable Energy.Reviews, 9: 363- 378.
- Ma F., Hanna MA. (1999) Biodiesel production: a review Biores.Technol, 70: 1-15.
- Vicente G., Martinez M., AracilJ. (2004) Integrated biodiesel production: a comparison of different homogeneous catalysts systems Biores. Technol, 92: 297-305.
- Meher L.C., Sagar D.V., Naik S.N. (2004) Technical aspects of biodiesel production by transesterification-a review. Renew. Sustainable Energy Rev, 3: 1-21.
- Joshi R.M., Pegg M.J. (2007) Flow properties of biodiesel fuel blends at low temperatures. Fuel, 86: 143-51.
- Marckley K.S. (1960) Fatty acids, 2nd ed. New York: Interscience.
- Bringi N.V. (1987) Non traditional Oil seed and oils of India, oxford and IBH Publishing company Pvt. Ltd., New Delhi, India, 57.
- Ali Y., Hanna M.A., Cuppett S.L. (1995) Fuel properties of tallow and soybean oil esters. J Am Oil Chem Soc, 72(12): 1557-1564.
- Rao P.S., Gopalakrishnan K.V. (1991) Vegetable oils and their methylesters as fuels for diesel engines. Indian J Technol, 29(6): 292-297.
- Feuge R.O., Gros A.T.(1949) Modification of vegetable oils. VII Alkali catalyzed interesterification of pea-nut oil with ethanol. J Am Oil Chem Soc, 26(3): 97.
- Dunn R.O., Bagby M.O. (1995) Low-temperature properties of triglyceride-based diesel fuels: transesterified methylesters and petroleum middle distillate/ester blends. J Am Oil Chem Soc, 72(8): 895- 904.
- Chang D.Y.Z., Van Gerpen J.H., Lee I., Johnson L.A., Hammond E.G., Marley S.J. (1996) Fuel properties and emissions of soybean oil esters as diesel fuel. J Am Oil Chem Soc, 73(11): 1549.
- Otera J. (1993) Transesterification.ChemRev, 93(4): 1449-1470.
- Tate R.E.,Watts K.C,Allen C.A.W., Wilkie K.I. 2006 The viscosities of three biodiesel fuels at temperature up to 300 °C. Fuel, 85: 1010-1015.
- Nye M.J., Southwell P.H. (1983) Esters from rapeseed oil as diesel fuel. Proceedings of Vegetable Oil as Diesel. Fuel—Seminar III, Peoria, IL.
- Agarwal A.K. (1988) Vegetable oils versus diesel fuel: development and use of biodiesel in a compression ignition engine. TERI Inf Dig Energy (TIDE), 8:191-203.
- Freedman B., Pryde E.H, Mounts T.L. (1984) Variables affecting the yields of fatty esters from transesterified vegetable oils. J Am Oil Chem Soc, 61(10): 1638-1643.
- Hui Y.H. (1996) editor. Bailey’s industrial oil fats: industrial and consumer non edible products from oils and fats. New York: Wiley: 5.
- Fillieres R., Benjelloun-Mlayah B., Delmas M. (1995) Ethanolysis of rapeseed oil: quantification of ethylesters,mono-, di-, and triglycerides and glycerol by high performancesize-exclusion chromotography. J Am Oil Chem Soc, 72(4): 427-432.
- Saka S, Dadan K. (2001) Biodiesel fuel, from rapeseed oil as prepared in supercritical methanol. Fuel, 80: 225.
- Formo M.W. (1954) Ester reactions of fatty materials. J Am Oil Chem Soc, 31(11): 548-559.
- Tickell J. (1999) From the fryer to the fuel tank, 2nd ed. Sarasota, FL: Green Teach Publishing.
- Rosenblum L.J. (2000) Feasibility of biodiesel for rural electrification in India [Draft]; June.
- Freedman B., Pryde E.H. (1982) Fatty esters from vegetable oils for use as a diesel fuel. Proceedings of International Conference on Plant & Vegetable oils as Fuels. ASAE, St. Joseph, MI; 17-122.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences