Antioxidant Enzyme Activities and DNA Damage as Biomarker of Copper Effect on Corbicula fluminea
Tinnapan Netpae, Chitchol Phalaraksh, Weera Wongkham
1Environmental Science Program, Faculty of Science and Technology, Nakhon Sawan Rajabhat University 60000, Thailand
2Department of Biology, Faculty of Science, Chiang Mai University, 239 Huay Kaew Road, Muang District, Chiang Mai province, Thailand
- Corresponding Author:
- Tel: +66 (0)5394 3470
Fax: +66 (0)5389 2259
E-mail: e-mail: chitchol@chiangmai.ac.th
Abstract
This research was undertaken to evaluate the potential utility of several biochemical parameters as indicators of the toxic effects of copper (Cu) in the freshwater clam Corbicula fluminea. Clams were exposed to Cu concentration between 0 to 0.5 mg l-1 in aquariums for 0 to 30 days. The antioxidative enzyme activities for superoxide dismutase, catalase, and glutathione reductase were qualified by the method of Misra and Fridovich, Lucks, and Smith respectively. DNA damage was measured by single cell gel electrophoresis (Comet Assay). Antioxidative enzyme activities in clam gills from Cu were higher than control after 30 days and suggest an increase in superoxide dismutase and catalase activities. Glutathione reductase was increased in gill of clams at 0.1 to 0.5 Cu mg l-1, but Cu concentrations at 0 and 0.05 mg l-1 were decreased. The results indicated that Cu induced a relatively high level of DNA damage in hemolymph cells comparison with the control. DNA damage was elevated in clams from the Cu exposure treatment only at 5 to 15 days; by 20 and 30 days of exposure values had declined to control levels.
Keywords
Corbicula fluminea; superoxide dismutase ; glutathione reductase; catalase; comet assay
1. Introduction
Pollution of the aquatic environment by heavy metals has received considerable attention in recent years. Elevate concentrations of metals in aquatic ecosystems can be caused by the release of sewage and industrial effluents, non-point source runoff from agricultural and urban areas, and atmospheric fallout. When heavy metals enter the aquatic ecosystems, their mechanisms can cause stress effects due to their ability to accumulate [1].
It is known that copper (Cu) is an essential trace metal for living organisms and it is present in all natural waters and sediments. This metal plays a role in many biological enzyme systems that catalyze oxidation or reduction reaction and have molecular oxygen as a cosubstrate. However, if Cu is present at relatively high concentrations in the environment, toxicity to aquatic organisms can occur. Cation of Cu (Cu2+) is biologically important molecules. In addition, high Cu levels lead to an increase in the rate of free radical formation [2]. Studies on aquatic organisms exposed to pollutant waters or sediments have implicated DNA strand breakage, which could be used as a specific indicator of genotoxic pollutant exposure [3]. Cu is an essential element for bivalve development, but at elevated concentrations it is particularly toxic [4].
Bivalve molluscs are known to accumulate high concentrations of heavy metals in their tissues and are widely used as bioindicators for pollution in freshwater, including Asian clams Corbicula fluminea [5]. The C. fluminea is a widespread genus of moderate sized clams, often tinged or colored with violet on the interior. These clams have been shown to be very good bioaccumulators of aquatic pollution able to accumulate pollutants [6].
The purpose of this study was to examine changes in antioxidative enzyme activities in gill cell suspension and DNA damage in hemolymph pellet suspension of the asian clams (C. fluminea) following in vivo treatment with Cu2+ under laboratory condition. In the present work, we also evaluated the activities of those enzymes such as superoxide dismutase, catalase, and glutathione reductase that are part of the primary defense system against reactive oxygen species and DNA damage measured using single cell gel electrophoresis method might be useful as an indicator of toxic exposure and might have applicability as a biomarker.
2. Methods
Test Organisms
C. fluminea were collected from Bung Boraphet (The largest fresh water reservoir in Thailand) and kept in water tanks at 25 – 30 °C water temperature. The specimens were transported to the laboratory where they were detoxified by maintaining them for 20 days in an aquarium containing continuously aerated well-water. During acclimation and detoxification periods, C. fluminea were fed with micro algae (Chlorella sp.) dissolved in water at a concentration of 105 algal cell ml-1 per unit [7].
Exposures and Sample Preparation
Selected living C. fluminea were sorted by length (2.0 - 2.5 cm) and distributed over the experimental treatments. An experimental treatment consisted of 60 clams placed in a aquarium (10 L), containing 6 L of Cu solution. Water temperature was remaining at 29 + 1°C. turbidity was 0 + 1 NTU and pH was 7 + 0.2 under 12 hours light (680 + 5 lux) period. The water was aerated and always air-saturated with oxygen.
The C. fluminea will be treated with Cu concentration ranging from 0 to 0.5 mg l-1. The metal concentrations selected in experiments of previous studies were very different. In the present work, the Cu concentrations were acute and sublethal levels for bivalves [8]. The stock Cu solution was prepared using copper (II) sulfate (CuSO4) for 0, 5, 10, 20, and 30 days and then sacrificed for determination of antioxidative enzyme activities and DNA damage. Antioxidative enzyme activities determined from gill cell suspension while DNA damage determined from hemolymph pellet suspension. Hemolymph was collected from the posterior adductor muscle of C. fluminea.
Antioxidative enzyme activities determination
Gill cell suspension was separated on an ice-cold surface. Gill cell suspension homogenates were prepared as described by Jebali et al. (2007). An aliquot of the homogenate and supernatant was stored at −85 °C until the determination of Superoxide dismutase (SOD), Catalase (CAT) and Glutathione reductase (GR) enzyme activities.
Superoxide dismutase is measured based on Misra and Fridovich [9], in which the activity is measured on the basis of its ability to inhibit free radical chain oxidation in which O.-2 is a chain propagating radical and the autooxidation of epinephrine (0.25mM) is induced. A SOD standard is used to calibrate such activity. This method is based on the SOD-mediated increase in the rate of autooxidation of 5, 6, 6a, 11 b-tetrahydro-3, 9, 10-trihydroxybenzo-[c] fluorine in aqueous alkaline solution to yield a chromophore with maximum absorbance at 525 nm. Catalase activity will be determined by Lucks method [10], which involves the reduction of hydrogen peroxide at 240 nm and 25°C. The study of glutathione reductase activity is carried out using the method of Smith et al. [11]. The activity of glutathione reductase is measured using oxidized glutathione (GSSG). The Oxidation of NADPH is followed at 340 nm and 25°C.
DNA damage determination
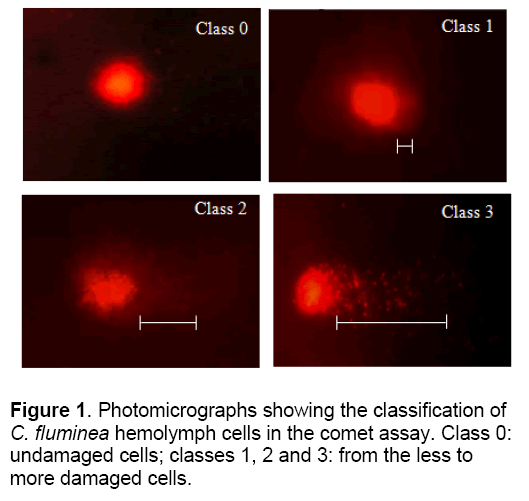
C. fluminea were placed in each solution for 0, 5, 10, 20, and 30 day, after which hemolymph was collected from the posterior adductor muscle of each mollusk using a syringe and transferred to 1.5 ml microtubes and centrifuged for 5 min at 268 g. After centrifugation the supernatant was discarded and the entire resultant pellet utilized for the comet assay. Comet assay was conducted according to the method of Speit and Hartmann [12], briefly summarized as follows: 1 h of lysis followed by 20 min denaturation in electrophoresis buffer and then electrophoresis for 20 min at 25 V and 300 mA (1.6 V/cm) followed by neutralization, fixing and staining with 0.002 mg ml-1 ethidium bromide. The cells were examined with a fluorescence microscope at 400 X, using a 420-490 nm excitation filter and a 520 nm emission filter. Fifty cells were scored per mollusk in the tissues sensitivity test and 100 cells in the DNA damage recovery test. These cells were classified according to the size of the comet tail: class 0, no tail; class 1, a small tail less than the head (nucleus) diameter; class 2, tail length equal to or up to twice the head diameter; and class 3, tail more than twice the head diameter (Figure 1). Cells were also scored visually into four classes, according to tail size (from undamaged 0, to maximally damaged 3). For each treatment damage scores were calculated by summing the number of cells in each class and then multiplying this total by the class value (0-3).
Statistical analysis
Experiments on antioxidant enzyme activities were performed in triplicate. DNA damage data (Tail of DNA from C. fluminea) is analyzed by TriTek Comet Score Free ware v1.5. Data were analyzed by two way analysis of variance or two - way ANOVA (heavy metal concentration and time), and significant differences between groups were determined by Scheffe’s multiple range test at the 5% significance level.
3. Results and Discussion
Antioxidative enzyme activities
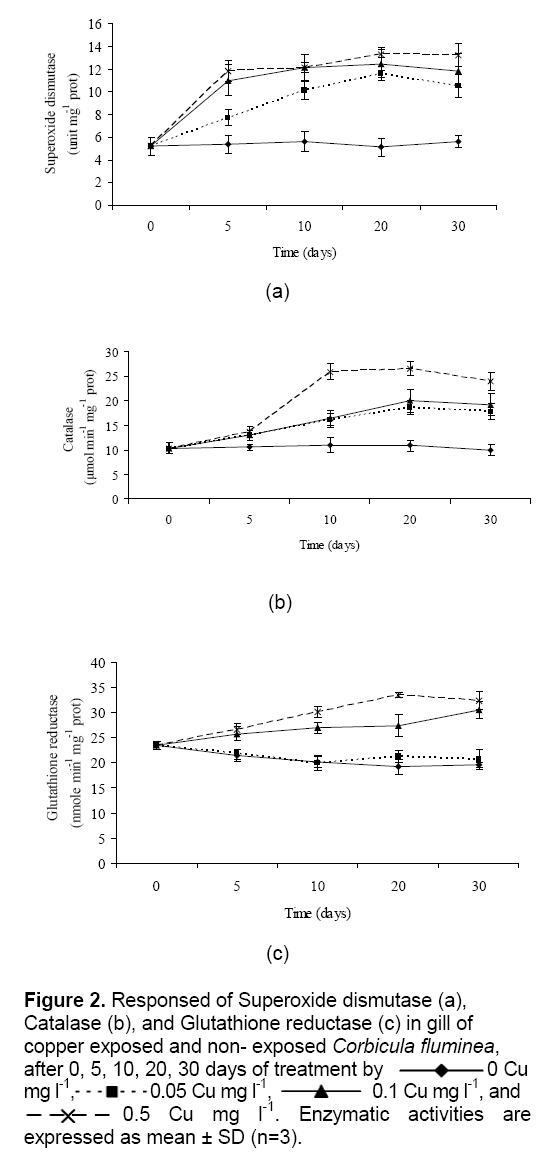
Cu exposure on antioxidant enzyme activities in gills of clams are presented in Figure 2. The superoxide dismutase, catalase and glutathione reductase activity in control C. fluminea was significantly lower than in treatment aquariums. Superoxide dismutase and catalase were elevated in clams at 0.05 to 0.5 Cu mg l-1. Figure 2a show that, the enzyme activities of superoxide dismutase of C. fluminea had rapidly increases up to 10 days and tend to remain constant afterward as well. Moreover, trends approaching significance were observed for catalase activities in clams (Figure 2b) ; the enzyme was elevated in the low exposure concentrations (0.05 and 0.1 Cu mg l-1) at 20 days, and in the high exposure concentration (0.5 Cu mg l-1) at 10 days (P < 0.05). Enzyme activities of superoxide dismutase and catalase in gill of clams have a significant (P < 0.05) relationship with the heavy metal concentration and time when the exposure time and heavy metal concentration were increased. While glutathione reductase was increase in gill of clams at 0.1 to 0.5 Cu mg l-1, but Cu concentrations at 0 and 0.05 mg l-1 were decrease (
Figure 2: Responsed of Superoxide dismutase (a), Catalase (b), and Glutathione reductase (c) in gill of copper exposed and non- exposed Corbicula fluminea, after 0, 5, 10, 20, 30 days of treatment by 0 Cu mg l-1, 0.05 Cu mg l-1, 0.1 Cu mg l-1, and 0.5 Cu mg l-1. Enzymatic activities are expressed as mean ± SD (n=3).
Cu is an integral part of many important enzymes involved in a number of vital biological processes. Cu is a cofactor of the pervasive intracellular enzymes, such as Cu/Zn superoxide dismutase (Cu/Zn SOD), which catalyzes the dismutation of O2•- by H2O2 producing [13]. Therefore, it plays an important role in antioxidant defense. In cells, Cu is capable of participating in Fenton’s reaction to produce the highly reactive OH•. Contrary to zinc, there is little evidence that Cu is directly involved in promoting either DNA synthesis or repair other than indirectly in DNA damage. Cu ion (Cu2+) react with H2O2 to form OH•, a highly reactive species that damages DNA. This DNA damage is an underlying cause of neurodegenerative and many cancers. Thus, DNA damage has been linked to chronic Cu-overload and/or exposure to excess Cu caused by accidents and environmental contamination [14].
In the present study, antioxidative enzyme activities in the gill of C. fluminea were increase. Result of antioxidative enzyme activities were consistent with those reported by Isani et al. [15] and [13] for clam Scapharca inaequivalvis, and oyster Pinctada fucata [4] while this observation were opposition with reported by Vutukuru [16] for fish Esomus danricus and mussel Bathymodiolus azoricus [17].
Invertebrates as mussels possess antioxidant systems, which have been well characterized [18] and often considered as sensitive parameters that could be useful biomarkers for the evaluation of contaminated aquatic ecosystems. Particularly, peroxisomal structures and related enzyme activities (as catalase activity) have been detected and studied in Mytilus galloprovincialis [19].
Superoxide dismutase, glutathione reductase, and catalase activities are biochemical parameters frequently used as pollution biomarkers in many biomonitoring programs [16].
DNA damage
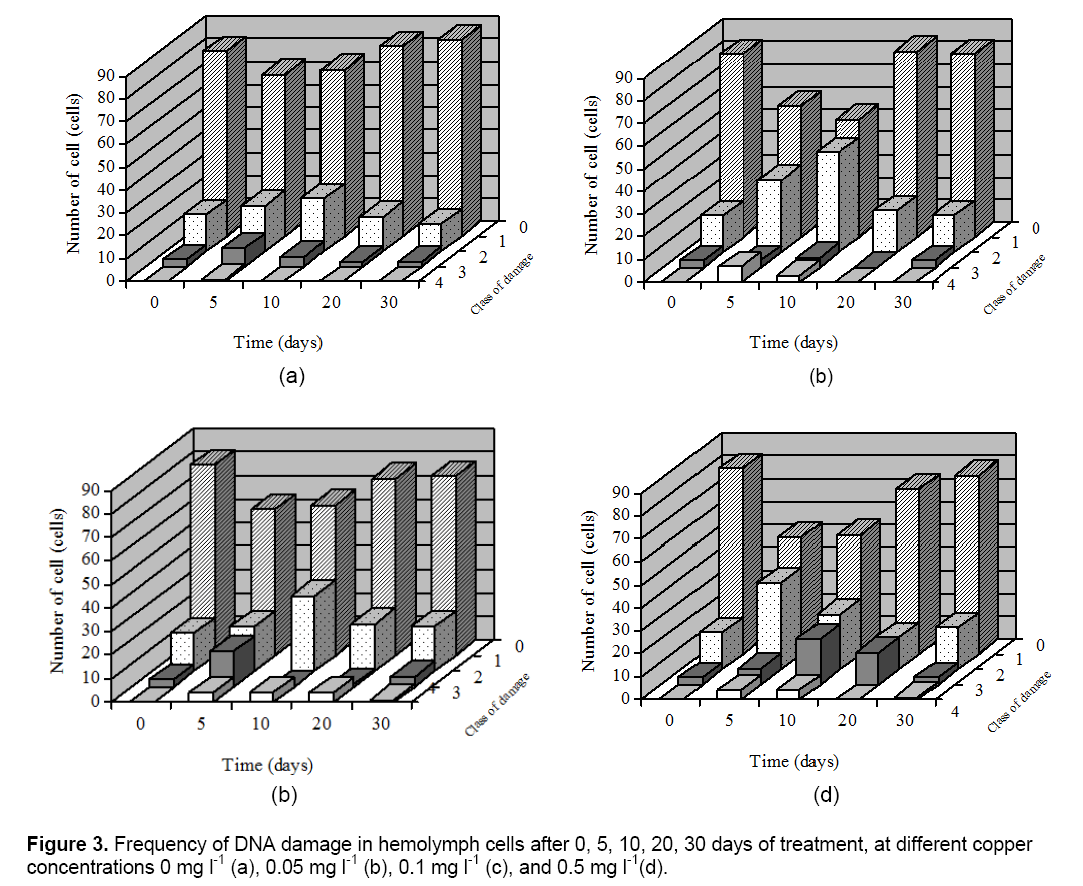
The Comet assay was used to detect the DNA damage in hemolymph cells of C. fluminea. Cells were also scored visually into four classes, according to tail size. The data, obtained by the comet assay, clearly indicate that only some parameters increased significantly with the Cu treatment (which correlates with a higher DNA damage). Cu exposures on DNA damage in hemolymph of clams are presented in Figure 3. For figure also indicates that for recovering cells there was a time-related shift in the type and frequency of damaged cells from the more damaged class 3 and 2 cells to less damaged class 1 or undamaged class 0 cells. In this work was found that C. fluminea is a good bioindicator of genotoxic agents, with the hemolymph studied showing a good correlation and a very clear dose-response effect between increased Cu concentration and increased.
DNA damage. Hemolymph which had not been exposed to Cu showed low background damage which made it easy to evaluate the response of the cells to Cu. DNA damage were elevated in clams from the Cu exposure treatment only at 5 to 15 days; by 20 and 30 days of exposure values had declined to control levels. Transient increases in DNA damage were also observed in clams from the Cu exposure treatment.
The results indicated that Cu induced a relatively high level of DNA damage in comparison with the control. This result suggest that Cu treatment is associated with oxidative stress leading to oxidative DNA damage. Therefore, DNA damage in the hemolymph of C. fluminea could be a suitable early bio-marker to monitor the environmental or occupational Cu toxicity. Result of DNA damage of in this research were consistent with those reported by other authors in aquatic animals, including fish [13], clams and aquatic animals collected in polluted sites [20].
Acknowledgements
Financial support from Science Center, Faculty of Science and Technology, Nakhon Sawan Rajabhat University, Center for Environmental Health, Toxicology and Management of Chemical (ETM) are gratefully acknowledged.
References
- Hudson A.R., Nicholas S.F. (1999). Rates and routes of trace element uptake in zebra mussels. Amrican Society of Limnology and Oceanography. 44: 1730-1749.
- Bogdanova A. Y., Gassmann M., Nikinmaa M. (2002). Copper ion redox state is critical for Its effects on ion transport pathways and met-hemoglobin formation in trout erythrocytes. Chemico-Biological Interaction. 139: 43-59.
- Mithelmore C. L., Chipman J. K. (1998). DNA strand breakage in aquatic organisms and the potential value of the comet assay in environmental monitoring. Mutation Research. 399: 135-147.
- Jing G., Li Y., Xie L., Zhang R. (2007). Different effects of Pb2+ and Cu2+ on immune and antioxidant enzyme activities in the mantle of Pinctada fucata. Environmental Toxicology and Pharmacology. 24: 122-28.
- Sun, P., Wang, B. (2004). Effects of seasonal variation and individual size on heavy Metals accumulation in Corbicula fluminea. Marine Science Bulletin/Haiyang Tongbao. 23: 19-24.
- Belanger S.E., Farris J.L., Cherry D.S., Cairns J. (1990). Validation of Corbicula fluminea growth reductions induced by copper in artificial streams and river systems. Canadian Journal of Fisheries and Aquatic Sciences. 47: 904-914.
- Marie V., Gonzalez P., Baudrimont M., Bourdineaud J. P., Boudou, A. (2006). Metallothionein response to cadmium and zinc exposures compared in two freshwater bivalves, Dreissena polymorpha and Corbicula fluminea. Bio Metals. 19: 399-407.
- Harrison F.L., Knezovich J.P., Rice D.W.Jr. (1981). Effect of Copper on adult and early life stages of the freshwater clam, Corbicula manilensis. U.S. Nuclear Regulatory Commission NUREG/CR-1379. p 34.
- Misra H. P., Fridovich I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dimutase. Journal of Biological Chemistry. 247: 3170-3175.
- Luck H. (1974). Catalase. In: Bergmayer MV (ed) Method of enzymatic analysis. Academic Press. New York.
- Smith I., Vierheller T., Thorne C. (1988). Assay of glutathione reductase in crude tissue homogenates using 5,5’- dithiobis(2-nitrobenzoic acid). Analytical Biochemistry. 75: 408-413.
- Speit G., Hartmann A. (1999). The comet assay (single-cell gel test). In HendersonDS (ed) Methods in Molecular Biology. DNA Repair Protocols: Eukaryotic Systems. Humana Press. 113: 203-212.
- Gabbianelli R., Lupidi G., Villarini M., Falcioni G. (2003). DNA damage induced by copper on erythrocytes of gilthead sea bream Sparus aurata and mollusk Scapharca inaequivalvis. Archives of Environmental Contamination and Toxicology. 45: 350-356.
- Gaetke L.M., Chow C.K. (2003). Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 189: 147-163.
- Isani G., Monari M., Andreani G., Fabbri M., Carpene, E. (2003). Effect of Copper Exposure on the Antioxidant Enzymes in Bivalve Mollusc Scapharca inaequivalvis. Veterinary Research Communications. 27: 691-693.
- Vutukuru S.S., Chintada S., Madhavi K.R., Rao J., Anjaneyulu Y. (2006). Acute effects of copper on superoxide dismutase, catalase and lipid peroxidation in the freshwater teleost fish, Esomus danricus. Fish Physiology and Biochemistry. 32: 221-229.
- Company R., Serafim A., Bebianno M. J., Cosson R., Shillito B., Medioni A. F. (2004). Effect of cadmium, copper and mercury on antioxidant enzyme activities and lipid peroxidation in the gills of the hydrothermal vent mussel Bathymodiolus azoricus. Marine Environmental Research. 58: 377-381.
- Soldatov A. A., Gostyukhina O. L., Golovina I. V. (2007). Antioxidant enzyme complex of tissues of the bivalve Mytilus galloprovincialis lam. under normal and oxidative-stress conditions. A Review Applied Biochemistry and Microbiology. 43: 556-562.
- Cancio I., Orbea A., Völkl A., Fahimi H. D., Cajaraville M. P. (1998). Induction of peroxisomal oxidases in mussels: comparison of effects of lubricant oil and benzo(a)pyrene with two typical peroxisome proliferators on peroxisome structure and function in Mytilus galloprovincialis. Toxicology and Applied Pharmacology. 149: 64-72.
- Jebali J., Banni M., Alves de Almeida E., Boussetta H. (2007). Oxidative DNA damage levels and catalase activity in the clam Ruditapes decussatus as pollution biomarkers of Tunisian marine environment. Environmental Monitoring Assessment. 124: 195-200.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences