An Insightful Overview on Microbial Pigment, Prodigiosin
Chidambaram Kulandaisamy Venil, Perumalsamy Lakshmanaperumalsamy
1Division of Environmental Microbiology, Department of Environmental Sciences, Bharathiar University, Coimbatore – 641 046, Tamil Nadu, India
2Karpagam University, Coimbatore – 641 021, Tamil Nadu, India
- Corresponding Author:
- Chidambaram Kulandaisamy Venil
Division of Environmental Microbiology
Department of Environmental Sciences, Bharathiar University
Coimbatore – 641 046, Tamil Nadu, India
Tel: +91 98426 99688
E-mail: ckvenil@gmail.com - Perumalsamy Lakshmanaperumalsamy
Karpagam University
Coimbatore – 641 021, Tamil Nadu, India.
Tel: +91 98943 11776
E-mail: drplpsamy@gmail.com
Abstract
The toxicity problems caused by those of synthetic origin pigments to the environment have created a mounting interest towards natural pigments. Among natural pigments, pigments from microbial sources are potentially good alternative ones to synthetic pigments. Prodigiosin, the bright red pigment produced by organisms of the genus Serratia, is among the more conspicuous pigments extant in the microbial world. The chemical nature of prodigiosin continues to be the subject of extensive study and it has been defined as a tri-pyrrylmethene. The rather rapid production of a flashy red pigment, which did not escape the observation of men before they had any inkling of the nature of microbial growth, can now is understood in terms of prodigiosin production. The prodigiosin pigments have intrigued organic chemists and pharmacologists and may yet play roles in the treatment of infectious diseases such as malaria and as immunosuppressant agents. However, a major reason for much of the continuing curiosity in the prodigiosin / Serratia story is the theory that these viscous, crimson bacterial colonies provide a naturalistic explanation. This review article highlights the characteristics and potential of prodigiosin pigment from Serratia.
Keywords
Prodigiosin; Serratia marcescens; tripyrrylmethane; microbial pigments
Introduction
Many artificial synthetic colorants, which have widely been used in foodstuff, dyestuff, cosmetic and pharmaceutical manufacturing processes, comprise various hazardous effects. To counter the ill effect of synthetic colorants, there is worldwide interest in process development for the production of pigments from natural sources. The utilization of natural pigments in foodstuff, dyestuff, cosmetic and pharmaceutical manufacturing processes has been increasing in recent years thanks to the apprehension about the harmful effects of synthetic pigments and their industrial byproducts on humans and the environment [1].
Natural pigments can be obtained from two major sources, plants [2,3] and microorganisms [4-9]. The accessible authorized natural pigments from plants have numerous drawbacks such as instability against light, heat or adverse pH, low water solubility and are often non-availability throughout the year. The latter are of great interest owing to the stability of the pigments produced [10] and the availability of cultivation technology [11,12].
The advantages of pigment production from microorganisms include easy and fast growth in the cheap culture medium, independence from weather conditions and colors of different shades. Hence, microbial pigment production is now one of the emerging fields of research to demonstrate its potential for various industrial applications.
Prospects and potential of microbial pigments
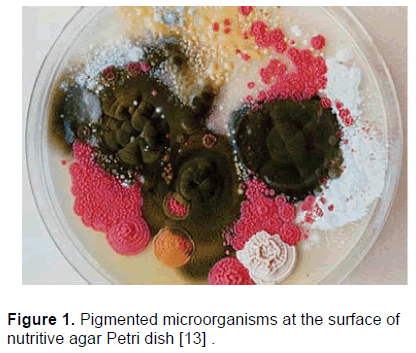
Microorganisms have been used for a long time for production of molecules as diverse as antibiotics, enzymes, vitamins, texturizing agents and so on. There is growing interest in the food industry in the use of natural ingredients. Ingredients, such as colors, are considered natural when derived from biological sources like plants or microorganisms. Microbial colors are in use in the fish industry already, for example to enhance the pink color of farmed salmon. Further, some natural colorants have commercial potential for use as antioxidants. The industry is now able to produce some microbial pigments for applications in food, cosmetics or textiles. In nature, color rich and pigment producing microorganisms (fungi, yeasts, and bacteria) are quite common, vide Figure 1 [13]. Microorganisms produce various pigments like carotenoids, melanins, flavins, quinones, prodigiosins and more specifically monascins, violacein or indigo [13].
Figure 1: Pigmented microorganisms at the surface of nutritive agar Petri dish [13] .
Carotenoids such as β-carotene and xanthophylls like astaxanthin play central roles in the metabolism of the eye's macula and retina and in maintaining healthy vision. β-carotene play a role in the prevention of cancer. The xanthophyll pigment astaxanthin widely distributed in nature is the predominant pigment in shrimp, crab, lobster, and salmonids. Additionally, it produces the red coloration of some birds such as flamingos and the scarlet ibis. There is evidence that xanthophyll function as chemo-protectives. In addition, other xanthophylls, such as adonirubin and astaxanthin, may also act as nutraceuticals that prevent carcinogenesis through anti-oxidative, anti-free radical or other mechanisms. The beneficial nutraceutical functions of the carotenes and xanthophylls extend to the prevention of heart attacks and strokes [14].
The microbial production of carotenoids, when compared with extraction from vegetables or chemical synthesis [15], seems to be of paramount interest mainly because of the problems of seasonal and geographic variability in the production and marketing of several of the colorants of plant origin [16] and because of the economic advantages of microbial processes using natural low cost substrates as carbohydrate source [15].
Monascus species, fungi which produce monascus pigments, have long been used in production of traditional East Asian foods, such as red rice wine, red bean curd [17]. The different colors of monascus pigments are classified into three types, yellow, orange and red and more than ten compounds have been reported to be produced from fermentation using Monascus species [18]. Monascorubrin [19] and monascoavin [20] are orange and yellow pigments, respectively, isolated in 1926 by the late Eijiro Nishikawa (Tottori University) from Monascus purpureus mentii. They are widely used for coloring foodstuffs in Asia and also are the main pigments in the common wine Shoko-Shu in Chinese restaurants [21].
Diversified microorganisms have demonstrated their pigment potential; of which some are already in industrial production, some in developmental stage and in research projects as illustrated in Table 1.
| Molecule | Color | Microorganism | Status |
|---|---|---|---|
| Ankaflavin | Yellow | Monascus spp. | IP |
| Anthraquinone | Red | Penicillium oxalicum | IP |
| Astaxanthin | Pink-red | Xanthophyllomyces dendrorhous | DS |
| Agrobacterium aurantiacum | RP | ||
| Paracoccus carotinifaciens | RP | ||
| Canthaxanthin | Dark red | Bradyrhizobium spp. | RP |
| Haloferax alexandrinus | RP | ||
| Gordonia jacobea | DS | ||
| Lycopene | Red | Blakeslea trispora | DS |
| Fusarium sporotrichioides | RP | ||
| Melanin | Black | Saccharomyces neoformans | RP |
| Monascorubramin | Red | Monascus spp. | IP |
| Naphtoquinone | Deep blood red | Cordyceps unilateralis | RP |
| Riboflavin | Yellow | Ashbya gossypi | IP |
| Rubrolone | Red | Streptomyces echinoruber | DS |
| Rubropunctatin | Orange | Monascus spp. | IP |
| Torularhodin | Orange-red | Rhodotorula spp. | DS |
| Zeaxanthin | Yellow | Flavobacterium spp. | DS |
| Paracoccus zeaxanthinifaciens | RP | ||
| Sphingobacterium multivorum | RP | ||
| β-carotene | Yellow-orange | B. trispora | IP |
| F. sporotrichioides | RP | ||
| Mucor circinelloides | DS | ||
| Neurospora crassa | RP | ||
| Phycomyces blakesleeanus | RP | ||
| Unknown | Red | Penicillium purpurogenum | DS |
| Paecilomyces sinclairii | RP |
RP: Research project.
Table 1. Microbial production of pigments (already in use as natural colorants or with high potential in this field) [13].
Expression of pigments in bacteria
Some groups of bacteria express various pigments at different stages of growth. Cryptococcus neoformans produces a melanin pigment that plays an important antioxidant function, with melanized cryptococcal cells being more resistant to oxygen and nitrogen derived oxidants than nonmelanized cells [22]. Azotobacter chroococcum produces melanin that has been associated with protection against reactive oxygen species [23], and iron binding by melanin in Azotobacter salinestris may protect the organism from damage caused by hydrogen peroxide. Production of pigment by group B Streptococcus also confers resistance to oxidative stresses, including H2O2 and superoxide [24].
The bacterium Clavibacter michiganensis subsp. insidiosus has been found to contain iodinine (a derivative of phenazine) [25]. Carotenoids (compounds of an isoprenoid nature) detected in Agromyces ramosus [26] and two Leifsonia sensulato species [27], are phylogenetically close to Rhodoglobus. Some Alteromonas species show pigmentation from lemon - yellow to violet. Most members of the Microbacteriaceae family exhibit a yellow, orange or red color of varying intensity and tint, the color being determined by isoprenoids (carotenoids), quinones, prodigiosin and anthracyclinone derivatives, phenazines, and other heterocyclic compounds [25]. Isoprenoids are synthesized via the nonmevalonate or mevalonate pathways, which are inhibited by fosmidomycin and mevinolin, respectively [28,29]. The suppression of pigmentation by these inhibitors at concentrations lower than those inhibitory to growth may serve as an indication (along with the results of chemical analysis) that the pigmentation is due to isoprenoids.
A red pigment and a yellow pigment were also reported in B. megaterium KM spores [30]. Spores of Bacillus megaterium QM B1551 contain a red pigment that is associated with the spore membranes [31,32]. Microbial mats are often comprised of consortia of procaryotic and eucaryotic phototrophic microorganisms [33] and thus contain complex mixtures of lipophilic pigments [34,35]. Some of the important pigment producing organisms and their pigments reported in literatures are specified in Table 2.
| No. | Organism | Pigment | Pigment | Ref |
|---|---|---|---|---|
| 1 | Serratia marcescens | Prodigiosin | Red | [36] |
| 2 | Corynebacteriu m insidiosum | Indigoidine | Blue | [37] |
| 3 | Monascus roseus | Canthaxanthin | Orange Pink | [38] |
| 4 | Staphylococcus aureus | Zeaxanthin | Yellow | [39] |
| 5 | Rugamonas rubra | Prodigiosin like pigment | Red | [40] |
| 6 | Streptoverticilliu m rubrireticuli | Prodigiosin like pigment | Red | [40] |
| 7 | Pseudomonas aeruginosa | Pyocyanin | Blue green | [41] |
| 8 | Haematococcus pluvialis | Astaxanthin | Red | [42] |
| 9 | Dunaliella salina | ß carotene | Orange | [43] |
| 10 | Bradyrhizobium | Canthaxanthin | Orange | [44] |
| 11 | Xanthomonas oryzae | Xanthomonadin | Yellow | [45] |
| 12 | Phaffia rhodozyma | Astaxanthin | Red | [46] |
| 13 | Serratia rubidaea | Prodigiosin like pigment | Red | [47] |
| 14 | Vibrio gaogenes | Prodigiosin like pigment | Red | [47] |
| 15 | Alteromonas rubra | Prodigiosin like pigment | Red | [47] |
| 16 | Janthinobacteriu m lividum | Violacein | Purple | [48] |
| 17 | Pacilomyces farinosus | Anthraquinone | Red | [49] |
Table 2. Highlights of some pigment producing microorganisms.
Prodigiosin potential of Serratia marcescens
For several decades, prodigiosin has been known to be a natural compound showing a broad range of cytotoxic activity [50], and is also produced by Vibrio psychroerythrus [51], S. marcescens, Pseudomonas magnesiorubra, and other eubacteria [52]. Prodigiosin is a tripyrrole, first characterized from S. marcescens which forms beautiful pillarbox red colonies. S. marcescens, a Gram negative bacterium characterized by production of a nondiffusible red pigment, prodigiosin [53-55], is an opportunistic pathogen, with the nonchromogenic biotypes posing a public health threat [55]. Chromogenic biotypes from the natural environment have only rarely implicated in infections. Interestingly, the water insoluble red pigment produced by S. marcescens has been reported to have antibiotic activity [56-59]. S. marcescens also produces a water-soluble, reddish-violet pigment with superoxidase dismutase mimetic activity [60].
In S. marcescens, very small and widely spaced colonies are initially non-pigmented; coloration first develops near a colony diameter of about 1 millimeter [61]. S. marcescens expresses pigments under certain conditions. Bizio and Sette isolated the deep red pigment produced by Serratia strains and tried to use it as a dye for commercial purposes. However in pure form, it turned out to be too sensitive to light to be of practical use [50].
Serratia, like other Enterobacteriaceae, grow well on ordinary media under anaerobic and aerobic conditions. They grow well on synthetic media using various compounds as a single carbon source. S. marcescens are the major producers of prodigiosin [50]. The production of prodigiosin in S. marcescens is susceptible to temperature and is substantially inhibited at temperatures higher than 37°C [62]. Conventional media used for the biosynthesis of prodigiosin by S. marcescens strains are complex media that are rich in a variety of nutrients [50,62,63]. Certain nutrients such as thiamine and ferric acid [64] are particularly crucial for prodigiosin production, whereas phosphate [65], adenosine triphosphate and ribose [66] have inhibitory effects on prodigiosin yield. Giri et al. [62] tested the performance of a series of media and discovered that a novel peanut seed broth give rise to a significant enhancement of prodigiosin production (Table 3).
| No. | Media used | Temperature / mg mL-1 | Ref. | ||
|---|---|---|---|---|---|
| 28°C | 30°C | 37°C | |||
| 1 | Dextrose broth with casein | 13.0 | - | - | [67] |
| 2 | Ethanol and carbon source | 3.0 | - | - | [68] |
| 3 | Nutrient broth | 0.52 | 0.354 | 0.111 | [62] |
| 4 | Peptone glycerol broth | 0.302 | 0.569 | 0.111 | |
| 5 | Sesame seed broth | 16.68 | 9.3 | 0.319 | |
| 6 | Nutrient broth with 0.5% maltose | 1.836 | 0.79 | 0.104 | |
| 7 | Nutrient broth with 0.5% glucose | 1.689 | 0.29 | 0.104 | |
| 8 | Sesame seed broth with 0.5% maltose | 9.43 | 8.56 | 1.63 | |
| 9 | Sesame seed broth with 0.5% glucose | 1.47 | 1.16 | 0.42 | |
| 10 | Sesame oil broth | 0.767 | 1.006 | 0.107 | |
| 11 | Peanut seed broth | 38.75 | 25.98 | 1.49 | |
| 12 | Peanut oil broth | 2.89 | 0.559 | 0.111 | |
| 13 | Copra seed broth | 1.94 | 1.39 | 0.1736 | |
| 14 | Coconut oil broth | 1.42 | 0.05 | 0.177 | |
Table 3. Comparative analysis of prodigiosin production by S. marcescens in different media at different temperatures.
Moreover, it was reported that the addition of silica gel carriers to a liquid culture of S. marcescens also leads to marked increases in cell growth and the production of prodigiosin and serrawettin [63]. In addition, since prodigiosin is often located on the cell envelope, the addition of surfactants, such as sodium dodecyl sulphate (SDS), could also enhance the recovery efficiency for prodigiosins [64].
Hardjito et al. [60] reported that the production of pigment by a non-clinical strain was meaningful and scientifically important that there might be a relationship, between pigment productions to the absence of plasmid. Thus, pigment production by non clinical strains of S. marcescens was of interest for further studies to determine a relationship, if any, of loss of pigment production to pathogenicity. S. marcescens also produces pigment fractions of various characteristics [60], influenced by both genetic and environmental factors. L-proline serves as a sole source of carbon and nitrogen for growth and prodigiosin production. Production of the watersoluble pigment is enhanced by addition of polymyxin B [69], gramicidin, and valinomycin to the growth medium [60].
No doubt, pigment production by S. marcescens, will continue to be intrigued to microbiologist, clinician and bioprocess engineer [60]. Recently, prodigiosin has been considered effective as a biological control agent against harmful algae in natural marine environments; therefore, prodigiosin should be produced in large quantities to be able to meet future needs [70].
Prodigiosin – a multifaceted secondary metabolite
A multifaceted secondary metabolite produced by S. marcescens, Pseudomonas magneslorubra, Vibrio psychroerythrous, S. rubidaea, Vibrio gazogenes, Alteromonas rubra, Rugamonas rubra and Gram positive actinomycetes, such as Streptoverticillium rubrireticuli and Streptomyces longisporus ruber forms prodigiosin and / or derivatives of this molecule [71]. The actinomycete Streptomyces coelicolor A3(2) produces a closely related linear tripyrrole, undecylprodigiosin, and a cyclic derivative, butylmeta- cycloheptylprodiginine in a 2 : 1 ratio [72].
The prodigiosin tripyrrole was first characterized from S. marcescens and was shown to be localized in extracellular and cell-associated vesicles and in intracellular granules [73]. The red pigment of S. marcescens was isolated and named “prodigiosine” by Kraft [36]. The best known prodigiosin, is a nondiffusible red pigment attached to the inner membrane [74].
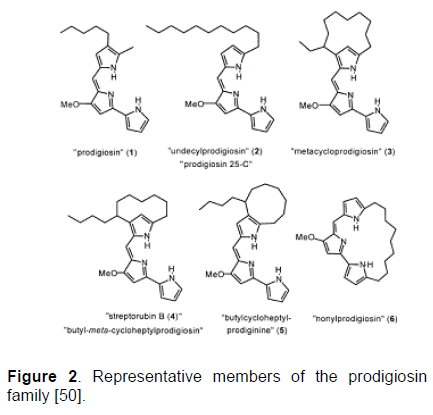
It was not until the 1960s, however, that the correct chemical constitution of “prodigiosin” as the major secondary metabolite of S. marcescens could be established beyond doubt, even then only by total synthesis [75]. Owing to the rapid advances in separation science and spectroscopy in the subsequent years, it became clear that “prodigiosin” [76] has a series of close relatives bearing the same pyrrolylpyrromethene (“prodiginine”) core with different alkyl substituents [77]. These substituents are often tied back to form medium-sized rings or macrocycles, as shown in Figure 2. Furstner [50] suggested that the traditional nomenclature was highly inconsistent and therefore strongly recommended to use the expression ‘prodigiosin’ only in a generic sense.
Figure 2: Representative members of the prodigiosin family [50].
The biosynthesis of the pigment is a bifurcated process in which mono and bipyrrole precursors are synthesized separately and then assembled to form prodigiosin [78]. Kobayashi and Ichikawa [73] and Matsuyama et al. [79] reported that prodigiosin is associated in extracellular vesicles, cell associated or present in intracellular granules. Most pigments absorb light at some defined wavelength, and pigment expression may be easily monitored spectrophotometrically. This promising pigment, having antifungal, immunosuppressive and antiproliferate activity is considered as a protein. This particular protein of assigned functions are homologues of those involved in the biosynthesis of a diverse array of natural products including fatty acid synthases, type I modular polyketide synthases, no ribosomal peptide synthetases [80].
Environmental factors influencing prodigiosin production
Prodigiosin, a typical secondary metabolite is appearing only in the later stages of bacterial growth [72]. The bright red nature of prodigiosin has made it a facile system for the study of secondary metabolite regulation. The production of prodigiosin has been shown to be influenced by numerous environmental factors including: inorganic phosphate availability, media composition, temperature and pH [81].
Optimum conditions for prodigiosin biosynthesis by Serratia
Many types of differential and selective media have been developed for the isolation and presumptive testing of Serratia spp. Capryllate Thallous (CT) agar contains caprylate as a carbon source for Serratia spp. and thallous salts as inhibitors for other organisms [82] and CT is the best at selecting for Serratia spp. The regular liquid media currently being used for prodigiosin biosynthesis are nutrient broth (0.52 mg mL-1) [83] peptone glycerol broth (0.302 mg mL-1) [84] and production medium [82]. Nakamura [85] used sodium oleate 2% in the medium and also studied oleic acid substitution instead of sodium oleate and had used only triolein as substrate and reported a yield of 0.69 mg mL-1 prodigiosin. Having an insight on the composition of already published media, the idea of designing a new, nutritious and economically cheap medium was thought of for the prodigiosin biosynthesis [62].
Giri et al. [62] reported that the initial comparative work was done using powdered sesame seed in water, nutrient broth and peptone glycerol broth as a growth medium for S. marcescens. After having observed sesame seed to give a better yield in terms of prodigiosin biosynthesis further comparison was done with readily available cheaper sources like peanut and coconut. Sesame oil, peanut oil and coconut oil were also compared with the rest of the media (Table 3). The media were also compared for growth at three different temperatures in terms of prodigiosin production. This led to the observation that fatty acids play a role as the substrate for enhanced prodigiosin production. The various components in the seeds as substrate could have stimulated cell density which in turn could have resulted in higher accumulation of the positive regulator inside the cell thereby triggering excessive pigment production. The powdered peanut seed medium supported the prodigiosin biosynthesis even at 37°C which was not so in the case of nutrient or peptone glycerol broth with and without sugars.
The crushed sesame seed broth gave the maximum yield of prodigiosin at 28°C, 30°C and 37°C when compared to nutrient broth and peptone glycerol broth. The maximum prodigiosin production was seen at 28°C and 30°C in nutrient broth. At 37°C S. marcescens did not show any pigment production in nutrient broth and the culture broth was white in color. In case of the powdered peanut broth, even at 37°C, pigment production was observed and in fact it was equal to the amount of pigment production seen in nutrient broth at 30°C [62]. In the bioreactor study with an internal adsorbent for prodigiosin the final yield was 13 mg mL-1 [85] and the media used had dextrose in the culture broth and casein in production medium. Cang et al. [86] have quoted a medium containing ethanol and carbon source but the yield was 3 mg mL-1.
Nutrient broth with glucose showed a two fold increase of pigment at 28°C. The pigment production was more in sesame seed broth even without the addition of any sugars, when compared to sesame seed broth with glucose or maltose. The pigment production was reduced in sesame seed medium with maltose at 28°C when compared to only powdered sesame seed broth. Glucose in powdered sesame seed medium showed a complete decrease of prodigiosin production at both 28°C and 30°C. Amongst the two sugars substituted, maltose acts as a better source of substrate in enhancing pigment production in nutrient broth. This clearly showed that in sesame medium the addition of maltose or glucose does not significantly enhance the pigment production. In fact the addition of glucose or maltose caused a reduction in prodigiosin production which could be due to catabolite repression. The pigment production in nutrient broth with sugars was not more than what was observed in sesame seed medium. Sesame oil broth, peanut oil broth and coconut oil broth as substrate were more efficient in inducing pigment production of 0.76, 2.89 and 1.42 mg mL-1 respectively when compared to the use of nutrient broth (0.52 mg mL-1) or peptone glycerol broth (0.302 mg mL-1) [62].
The reduction in prodigiosin production by S. marcescens mediated by glucose and other metabolizable sugar was due to a decrease in pH observed in the cell suspensions [71]. Taking into consideration the basic role of carbon source in enhancing pigment production, two justifications can be made. The first point is that in nutrient broth, which is basically devoid of carbon source, the addition of maltose or glucose enhanced the pigment production but not so in the case of sesame broth which already has carbon in the form of fatty acids. Maltose and glucose added in nutrient broth gave a two fold increase in yield over nutrient broth or peptone glycerol broth alone. The second point is that a slight enhanced pigment production was seen in the case of peptone glycerol broth at 30°C over nutrient broth at 28°C and this could be attributed to the glycerol present as carbon source. This clearly justifies the fact that carbon does support cell growth and thereby prodigiosin production [62].
Pure saturated and unsaturated fatty acids substituted in the medium and triolein an unsaturated fatty acid gave the maximum of 0.69 mg mL-1 yield of the pigment [62]. Fatty acids as a carbon source also play a role in enhanced cell density thereby an enhanced pigment production. The role of saturated fatty acids as a better carbon source in terms of pigment yield can be discussed with the following points. The overall saturated fatty acid composition is highest in copra, followed by peanut and then sesame. The reason for this could be that 50% lauric and 7% capric acid known for their antibacterial activity present in coconut could have inhibited the growth of S. marcescens in the medium thereby giving a very low yield. The second point validating the role of saturated fatty acid is that as per literature peanut has a higher concentration than sesame and the yield of prodigiosin is also higher in powdered peanut broth than in powdered sesame broth. According to Kim et al. [87] oil gave a better yield over the various carbon (not fatty acid containing seeds) and nitrogen sources tested. The bonded fatty acids as carbon source are less accessible by S. marcescens [62].
Prodigiosin is the only pigment of Serratia that has been subjected to chemical analysis. It has been claimed [88] that the chemically prepared pigment from the ether extract as obtained by Wrede can be further resolved into two components by column chromatography. The absorption spectrum of the O3 extract in the visible is similar to the spectrum of one of the components of the normal pigment separated by chromatography and has a peak at 500 mμ [89]. If such a component is separable from an induced pigment such as that obtained from the combination of P2 and W1, neither of which has an absorption peak in the visible range, then the synthesis of the pigment of O3 and the red pigment prodigiosin may be related by a common step [90]. Prodigiosin reported from Serratia spp. KH-95 had a maximum absorption spectrum at 535 nm [91].
Chromatographic separation of prodigiosin
By column chromatography, various fractions of prodigiosin were separated and identified. Williams et al. [92] reported that prodigiosin, the red pigment of S. marcescens, contains a maximum of four fractions. Weiss [88] noted three red fractions upon column chromatography with magnesium oxide and acetone petroleum ether mixtures. By further separation procedures, a total of six fractions, readily separable by column chromatography (If), then an orange-red fraction (la), which subsequent rechromatography showed to be comprised of two readily separable fractions; a pink fraction (Id and Ie) remained at the top of the column. After moving 5 to 10 cm down the column, the purple fraction (If) became stationary while the orange-red fraction (la) moved steadily down the column [93].
Someya et al. [94] tested the amount of prodigiosin by scrapping 1 g of bacterial cells from LB agar plates and suspending it in 9 ml of ethanol. Prodigiosin was then extracted from the cells by shaking this suspension for 1 h followed by centrifugation. The supernatant was then filtered through a 0.20 μm filter (Toyo Roshi, Tokyo, Japan) and concentrated under reduced pressure at room temperature in the dark. The ethanol extracts were then further extracted using chloroform. The chloroform extracts were fractionated using LK5 silica gel thin-layer chromatography (Whatman International, Maidstone, UK), and the pigment spots were scraped from the plates and extracted with ethanol. The red pigment having an Rf value in the range of 0.90 to 0.95 was defined as prodigiosin [70,95].
Prodigiosin biosynthetic clusters
The pigment gene cluster from Serratia spp. ATCC39006 was expressed in Erwinia carotovora subsp. carotovora, though it was not expressed in several other members of the Enterobacteriaceae, including E. coli [96]. In Serratia ATCC39006 the production of prodigiosin is regulated by multiple factors, including a quorum sensing system, via the LuxIR homologues, SmaI and SmaR [96,97].
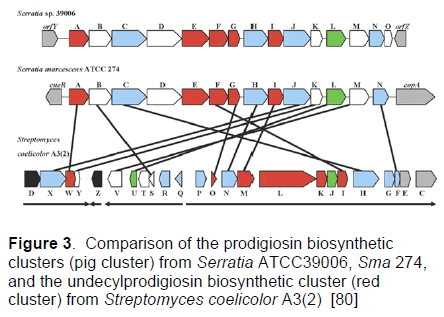
The Serratia pigment clusters contain 14 candidate genes common to Serratia and Streptomyces coelicolor and are arranged pigA through to pigN (Figure 3). The putatively assigned products of five genes to the biosynthesis of MBC (red) and four to the MAP (blue) pathway were seen. Four are unassigned, with the remaining protein (PigL) proposed to be involved in the posttranslational modification of some of the proteins in the cluster. The order of the genes is conserved between the two different Serratia species and the corresponding 14 predicted proteins are similar in size and share significant amino acid identities between the species. However, the relative genomic contexts of the clusters and their regulation are very different [80].
Figure 3: Comparison of the prodigiosin biosynthetic clusters (pig cluster) from Serratia ATCC39006, Sma 274, and the undecylprodigiosin biosynthetic cluster (red cluster) from Streptomyces coelicolor A3(2) [80]
The Serratia ATCC39006 pig gene cluster contains an additional gene, tentatively designated PigO. RTPCR and primer extension has confirmed that there is transcriptional read through between pigN and pigO [97], consistent with pigO being part of the pig operon in that strain. The Serratia ATCC39006 pig cluster (pigA - O) is transcribed as a polycistronic message from a promoter upstream of pigA [97] and so the presumption that the pigment cluster (pigA - pigN) of Sma 274 is also transcribed as a polycistronic message. The only substantial gap in both pigment clusters is between pigC and pigD. There is a 184 bp gap between pigC and pigD in Serratia ATCC39006, suggesting a possibility of two transcriptional units. However, transcription levels of the genes either side of this gap were shown to be similar in studies using lacZ reporter fusions to pigA and pigH [98]. The Sma 274 pig cluster has a 64 bp gap between pigC and pigD, smaller than that in Serratia ATCC39006, with no other significant intergenic gaps. The red cluster is larger (23 genes) than the pigment clusters (14 to 15 genes), possibly reflecting the greater complexity of the undecylprodigiosin products made by the former [72].
Twelve genes are conserved between all three clusters, as might be expected of pathways making such similar products. However, their orientation and regulation seem to have undergone pronounced variation. The Serratia pigment clusters are orientated and transcribed unidirectionally [97]. In contrast, the Red cluster is arranged in four transcriptional units and is regulated by RedZ and RedD (Figure 3). The RedD-dependent promoter region lies between RedQ and RedP and contains two divergent promoters directing transcription in opposite directions. RedZ, a response regulator, is thought to bind upstream of the RedD gene [72]. The dissimilar arrangement of these homologous genes, in clusters that produce chemically similar products, raises questions about the origins and evolution of prodigiosin biosynthetic gene clusters in both Gram positive and Gram negative bacteria. It is unclear whether there is a species related functional significance to the dissimilar arrangement of the genes or whether the differences have arisen by random events.
Differences between the other genes present in these operons might explain differences in the final molecules produced, or the biochemical routes to their production. pigD and pigE are the only pig genes that do not have homologues in the red cluster. Their respective homologues, pdtorfL and pdtorfM, are part of a gene cluster encoding the synthesis of the carbon tetrachloride dechlorination agent pyridine-2,6-bis (thiocarboxylic acid) by Pseudomonas stutzeri [99]. pdtorfL and pdtorfM are contiguous, as are pigD and pigE in the pig cluster. PigE is also similar to a putative aminotransferase (SC6A5.18, 461 aa) from Streptomyces coelicolor A3(2), encoded by a gene unlinked to the red gene cluster (38% identical to PigE from Serratia ATCC39006 and 39% identical to PigE from Sma 274; both are 50% similar over 460 aa). Indeed, it is possible that genes encoding proteins that take part in the synthesis of prodigiosin and undecylprodigiosin may not be associated with the gene clusters. RedR, RedQ and RedP do not have homologues encoded by the pig cluster, yet are thought to direct the condensation of acetate to form three of the carbon atoms of the pyrrole ring and the undecyl side chain of undecylprodigiosin [80]. The genes encoding the corresponding homologues could be present at another location on the Serratia chromosome, or other enzymes may perform the necessary catalytic roles in prodigiosin biosynthesis in Serratia. However, prodigiosin can be made in recombinant E. coli and Erwinia, this would necessitate functional activity of the corresponding E. coli and Erwinia enzymes to supplement the activity of the enzymes encoded by the pigment cluster [72].
The twelve Red proteins with homologues encoded in the pig cluster (the ‘core’ prodigiosin enzymes) are similar in size to their Pig counterparts. An exception to this is PigB (670 aa), whose homologue RedS (Sc3f7.05c) is a much smaller protein (146 aa) and is similar to only the N terminal region of PigB. The rest of PigB shows sequence identity with amine oxidases. The PigI and PigJ proteins are also significantly smaller than their respective RedM and RedX homologues [72].
PigA has good sequence identity with a wide range of acyl-CoA dehydrogenases. Sequence alignment with human isovaleryl-CoA dehydrogenase, the crystal structure of which has been determined, showed that the majority of amino acids around the flavin and the phosphopantetheinyl moiety of CoA are conserved but the amino acids involved in binding the adenosyl group of CoA are not conserved [100]. This supports the suggestion that the substrate for PigA is a prolyl PCP rather than prolyl-CoA.
Toxigenic effect of prodigiosin
The effects of prodigiosin and its fractions PE-1, C-2, A- 3, E-4, extracted in five organic solvents, petroleum ether, chloroform, acetone, ethanol and methanol, on embryogenesis showed the whole pigment and C- 2 fraction to be highly toxigenic while other fractions demonstrated toxicities approaching LD50 values of 26 to 30 μg egg-1 when dissolved in 100% dimethyl sulfoxide. The E-4 fraction in DMSO was least toxic. 95% ethanol proved to be highly toxic at a dose level of 0.1 ml egg-1 indicating that it was an unsuitable solvent for studies of this nature. Disc agar diffusion sensitivity studies were performed against E. coli, E. aerogenes, S. aureus, B. subtilis and P. aeruginosa with prodigiosin and fractions dissolved in 100% DMSO. The solvent was found to have no diffusible bacteriostatic activity in vitro. However, prodigiosin and the ethanol (E-4) and methanol (M-5) fractions produced inhibition zones with every organism tested. Data indicate that prodigiosin extracts have toxigenic effects on chick embryos and inhibit the growth of several species of bacteria [71].
Biological bustle of prodigiosin
Ecological functions
As typical secondary metabolite, prodigiosin and related materials have no clearly defined physiological functions in the producing organisms. However, it is possible that pigmented S. marcescens may have an advantage in ecological dispersion. In studies of the drops produced by bursting air bubbles rising through bacterial suspensions, pigmented strains were enriched in the drops. The pigmented cells appeared to have increased hydrophobicity, possibly due to the presence of prodigiosin. It was acknowledged, however, that the cell enrichment was a complex chain of events and was influenced by cultural conditions [101]. Other researches indicated that clinical S. marcescens strains had hydrophobic properties in the absence of prodigiosin and that hydrophobicity was only shown by growth at 30°C. Pigment is not synthesized at higher temperature. It seems clear that the cell surface hydrophobicity of S. marcescens is not totally due to surface pigment. Non pigmented cells contained an additional protein that may be responsible for higher surface hydrophobicity of some nonpigmented mutants [102].
Van der Mei et al. [103] reported that S. marcescens strains were characterized by contact angle and zeta potential measurements, X-ray photoelectron spectroscopy and infrared spectroscopy. Again it appeared that the presence of prodigiosin did not influence hydrophobicity. It was suggested that the pigment was confined in deeper layers than those probed at contact angles. Other results indicated that both pigmented and non pigmented strains produced extracellular vesicles and had wetting activity when grown at 30°C, but not at 37°C. The wetting activity was probably important for spreading cells on the surfaces of porous or fibrous materials, especially those with hydrophobic properties. Finally, the presence of O antigen may be important in adhesion of S. marcescens to plastic and glass and to human uroepithelial cells [102].
Pharmacological activity
Anticancer Activity of Prodigiosin
Since cell proliferation is a common event between function of immune system and the development of cancer, many anticancer agents are immunosuppressive and vice versa. The cell cycle inhibitory and apoptosis inducing activity of prodigiosin makes them an attractive candidate for anti-cancer therapy. The cytotoxic potency of prodigiosin has also been investigated in the standard 60 cell line panels of human tumor cells derived from lung, colon, renal, ovarian, brain cancers, melanoma and leukemia. Inhibition of cell proliferation as well as induction of cell death has been observed in these cell lines. In vitro anti cancer activity has also been reported for different prodigiosin analogues and synthetic indole derivative of prodigiosin [104]. The antiproliferative and cytotoxic effects of prodigiosin have been observed not only in cultured tumor cell lines but also in human primary cancer cells from B-cell chronic lymphocytic leukemia patients [105]. In addition, prodigiosin have also been found cytotoxic to human small cell lung carcinoma cells resistant to doxorubicin and overexpressing multi-drug resistance related protein [106].
Immunosuppressive activity of prodigiosins
Immunosuppressive activity of prodigiosins was first described by Nakamura and co-workers. They showed the presence of prodigiosin and metacycloprodigiosin in culture broth of Serratia and observed selective inhibition of polyclonal proliferation of T cells as compared to that of B cells [107]. Subsequently, immunosuppressive activity has been demonstrated for other prodigiosin analogue such as undecylprodigiosin, cPrG, MAMPDM, nonylprodigiosin and synthetic analogue PNU156804. cPrG.HCl, MAMPDM and prodigiosin show preferential suppression of polyclonal T cell proliferation as compared to that of B cells in vitro in mouse spleen cells. Prodigiosin suppressed the proliferation of lymphocytes stimulated with concanavalin A, anti CD3 and anti CD28, or phorbol myrisate acetate and ionomycin. These results show that prodigiosin inhibit both T cell receptor dependent and independent proliferation of T cells [104].
Dyeing potential of prodigiosin
Biosynthesis of colorants for textile applications has attracted increased interests in recent years. Nature produces many biocolorants from various resources including plants, animals, and microorganisms, which are possible alternatives to synthetic dyes and pigments currently employed [108]. The currently used colorants are almost exclusively made from nonrenewable resources such as fossil oil. The production of the synthetic colorants is economically efficient and technically advanced with colors covering the whole color spectrum. However, synthetic colorants are facing the following challenges: dependence on non-renewable oil resources and sustainability of current operation, environmental toxicity, and human health concerns of some synthetic dyes. Thus, searching renewable and environmentally friendly resources for production of colorants is an urgent need. Plants could produce and had been employed in production of natural colorants before synthetic dyes were invented, but in very low yields and low ecoefficiency [10]. In fact, using plants in producing colorants is not environmentally friendly and sustainable due to the large amount of biomasses produced.
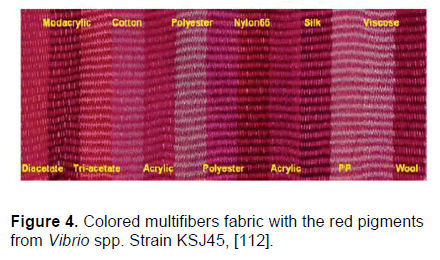
Practically, fermentation of microorganisms such as fungi and bacteria could be a valuable source of manufacturing colorants. Microorganisms produce a large variety of stable pigments such as carotenoids, flavonoids, quinones, and rubramines, and the fermentation has higher yields in pigments and lower residues compared to the use of plants and animals [109]. Thus, biosynthesis of dyes and pigments via fermentation processes has attracted more attention in recent years [109,110]. Technically speaking, biosynthesized pigments can serve as major chromophores for further chemical modifications, which could lead to colorants with a broad spectrum of colors [110]. Besides, some natural colorants, especially anthraquinone type compounds, have shown remarkable antibacterial activity in addition to providing bright colors [111], which could serve as functional dyes in producing colored antimicrobial textiles. Alihosseini et al. [112] characterized the bright red pigment prodigiosin from Vibrio spp. and suggested that it could be used to dye many fibers including wool, nylon, acrylics and silk (Figure 4).
Figure 4: Colored multifibers fabric with the red pigments from Vibrio spp. Strain KSJ45, [112].
Conclusions
While the field of pyrrole natural products is largely dominated by the tetrapyrrolic “pigments of life” such as heme, chlorophyll, vitamin B12, and the bile pigments, [113] a rapidly growing number of pyrrole alkaloids isolated from various sources deserves attention for their attractive biological properties [114]. Among them, the prodigiosin family is particularly remarkable for their diverse range of biological effects, although much deeper insight into the mode of action of these compounds is required before a fully consistent and conclusive picture of prodigiosin family is drawn.
Acknowledgements
The authors are thankful to Bharathiar University, Coimbatore, Tamil Nadu, India for providing the infrastructure facilities for this study.
References
- Unagul P., Wongsa P., Kittakoop P., Intamas S., Srikiti-Kulchai P., Tanticharoen M. (2005) Production of red pigments by the insect pathogenic fungus Cordyceps unilateralis BCC 1869. J Ind Microbiol Biotechnol, 32: 135-140.
- Mizukami H., Konoshima M., Tabata M. (1978) Variation in pigment production in Lithospermum erythrorhizon callus cultures. Phytochem, 17: 95-97.
- Papageorgiou V.P., Winkler A., Sagredos A.N., Digenis G.A. (1979) Studies on the relationship of structure to antimicrobial properties of naphthoquinones and other constituents of Alkanna tinctoria. Planta Med, 35: 56-60.
- Cross B.E., Edinberry M.N. (1972) Pigments of Gnomonia erythrostoma. Part I. The structures of erythrostominone, deoxyerythrostominone, and deoxyerythrostominol. J Chem Soc Perkin I, 3: 380-390.
- Ryu B.H., Park B.G., Chi Y.E., Lee J.H. (1989) Production of purplish-red pigment in mixed culture of Streptomyces propurpuratus ATCC 21630 and Bacillus sp R-89. Korean J Appl Microbiol Bioeng, 17: 327-333.
- Parisot D., Devys M., Barbier M. (1990) Naphthoquinone pigments related to fusarubin from the fungus Fusarium solani (Mart.) Sacc. Microbios, 64: 31- 47.
- Yongsmith B., Krairak S., Bavavoda R. (1994) Production of yellow pigments in submerged culture of a mutant of Monascus sp. J Ferment Bioeng, 78: 223-228.
- Kim C.H., Kim S.W., Hong S.I. (1998a). Production of red pigment by Serratia sp. KH-95 and its cultural properties. Korean J Biotechnol Bioeng, 13: 431-437.
- Cho Y.J., Park J.P., Hwang H.J., Kim S.W., Choi J.W., Yun J.W. (2002) Production of red pigment by submerged culture of Paecilomyces sinclairii. Lett Appl Microbiol, 35: 195-202.
- Raisainen R., Nousiainen P., Hynninen P.H. (2002) Dermorubin and 5-chlorodermorubin natural anthraquinone carboxylic acids as dyes for wool. Textile Res J, 72: 973-976.
- Kim C.H., Kim S.W., Hong S.I. (1999) An integrated fermentation separation process for the production of red pigment by Serratia sp. KH-95. Process Biochem, 35: 485-490.
- Parekh S., Vinci V.A., Strobel R.J. (2000) Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol, 54: 287-301.
- Dufosse L. (2009) Pigments, Microbial. Encyclopedia Microbiol, 4: 457-471.
- Long T.V. (2004) Process for production of carotenoids, xanthophylls and apo carotenoids utilizing eukaryotic microorganisms. US Patent 6783951.
- Simova E.D., Frengova G.I., Beshkova D.M. (2003) Effect of aeration on the production of carotenoid pigments by Rhodotorula rubra - Lactobacillus casei Subsp. casei co-cultures in whey ultrafiltrate. Z Naturforsch, 58: 225-229.
- De Haan A., Burke R.M., De Bont J.A.A. (1991) Microbial production of food colorants. Med Fac Landbouww Rijksuniv Gent, 56: 1655-1660.
- Mohankumari H.P., Akhilender Naidu K., Vishwanatha S., Narasimhamurthy K., Vijayalakshmi G. (2009) Safety evaluation of Monascus purpureus red mould rice in albino rats. Food Chem Toxicol, 47: 1739-1746.
- Jung H., Kim C., Kim K., Shin C.S. (2003) Color characteristics of Monascus pigments derived from fermentation with various amino acids. J Agr Food Chem, 51: 1302-1306.
- Carels M., Shepherd D. (1977) The effect of different nitrogen sources on pigment production and sporulation of Monascus sp., in submerged shaken culture. Can J Microbiol, 23: 1360-1372.
- Kim J.K. (1990) Development of high pigment producing mutant of Monascus anka and optimization of the culture condition. M.Sc. thesis, Seoul National University, Seoul, Korea.
- Nakanishi K. (2006) Studies in microbial and insect natural product chemistry. J Nat Med, 60: 2-20.
- Wang Y., Casadevall A. (1994) Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen and oxygen derived oxidants. Infect Immun, 62: 3004-3007.
- Shivprasad S., Page W.J. (1989) Catechol formation and melanization by Na-dependent Azotobacter chroococcum: a protective mechanism for aeroadaptation. Appl Environ Microbiol, 55: 1811- 1817.
- Keith K.E., Killip L., He P., Moran G.R., Valvano M.A. (2007) Burkholderia cenocepacia C5424 produces a pigment with antioxidant properties using a homogentisate intermediate. J Bacteriol, 189: 9057- 9065.
- Trutko S., Dorofeeva L., Evtushenko L., Ostrovskii D., Hintz M., Wiesner J., Jomaa H., Baskunov B., Akimenko V. (2005) Isoprenoid pigments in representatives of the family Microbacteriaceae Microbiol, 74: 284-289.
- Collins M.D., Bradbury J.F. (1991) The Genera Agromyces, Aureobacterium, Clavibacter, Curtobacterium and Microbacterium. In: The Prokaryotes, Balows, A., H.G. Trueper, M. Dworkin, W. Harder and K.H. Schleifer, Eds., Springer - Verlag, Berlin, Germany, pp. 1355-1368.
- Reddy N.S., Nimmagadda A., Sambasiva Rao K.R.S. (2003) An overview of the microbial α amylase family. African J Biotechnol, 2: 645-648.
- Lichtenthaler H.K. (2000) Nonmevalonate isoprenoid biosynthesis: enzymes, genes and inhibitors. Biochem Soc Trans, 28: 785-789.
- Kuzuyama T. (2002) Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units. Biosci Biotechnol Biochem, 66: 1619-1627.
- Ellar D.J., Postgate J.A. (1974) Characterization of forespores isolated from Bacillus megaterium at different stages of development into mature spores, In A.N. Barker, G.W. Gould, and J. Wolf (ed.), Spore research. Academic Press, Inc., New York, pp. 21-40.
- Racine F.M., Vary J.C. (1980) Isolation and properties of membranes from Bacillus megaterium spores. J Bacteriol 143: 1208 – 1214.
- Swerdlow R.D., Setlow P. (1984) Isolation and characterization of two distinct fractions from the inner membrane of dormant Bacillus megaterium spores. J Bacteriol, 158: 9-15.
- Cohen Y., Castenholz R.W., Halvorson H.O. (1984) Microbial Mats: Stromatolites 1982. Alan R. Liss, New York, pp. 498.
- Edmunds K.L.H., Eglinton G. (1984) Microbial lipids and carotenoids and their early diagenesis in the Solar Lake laminated microbial mat sequence. In: Microbial Mats: Stromatolites 1982 (Cohen, Y., R.W. Castenholz and H.O. Halvorson, eds.), Alan R. Liss, New York, pp. 343-389.
- Stal L.J., Van Gemerden H., Krumbein W.E. (1984) The simultaneous assay of chlorophyll and bacteriochlorophyll in natural microbial communities.J Microbiol Method, 2: 295-306.
- Kraft E. (1902) Thesis, Wurzburg from Wrede, F. and O. Hettche, Ber., (1929). 22: 2678.
- Starr M.P. (1958) The blue pigment of Corynebacterium insidiosum. Arch Mikrobiol, 30: 325- 334.
- Cooney J.J., Marks Jr H.W., Smith A.M. (1966) Isolation and identification of canthaxanthin from Micrococcus roseus. J Bacteriol, 92: 342-345.
- Hammond R.K., White D.C. (1970) Inhibition of carotenoid hydroxylation in Staphylococcus aureus by mixed-function oxidase inhibitors. J Bacteriol, 103: 607-610.
- Gerber N.N. (1975) A new prodiginine (prodigiosin like) pigment from Streptomyces. Antimalarial activity of several prodiginines. J Antibiot, 28: 194-199.
- Baron S.S., Rowe J.J. (1981) Antibiotic action of pyocyanin. Antimicrob Agents Ch, 20: 814-820.
- Kobayashi M., Kakizono T., Nagai S. (1993) Enhanced carotenoid biosynthesis by oxidative stress in acetate induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol, 59: 867-873.
- Jacobson G., Wasileski J. (1994) Production of food colorants by fermentation. In Bioprocess Production of Flavor, Fragrance, and Color Ingredients. Ed. A. Gabelman, John Wiley and Sons, Inc, pp. 205-237.
- Lorquin J., Molouba F., Dreyfus B.L. (1997) Identification of the carotenoid pigment canthaxanthin from photosynthetic Bradyrhizobium strains. Appl Environ Microbiol. 63: 1151-1154.
- Rajagopal L., Sundari C.S., Balasubramanian D., Sonti R.V. (1997) The bacterial pigment Xanthomonadin offers protection against photodamage. FEBS Lett, 415: 125-128.
- Florencio J.A., Soccol C.R., Furlanetto L.F., Bonfim T.M.B., Krieger N., Baron M., Fontana J.D. (1998) A factorial approach for a sugarcane juice-based low cost culture medium: increasing the astaxanthin production by the red yeast Phaffia rhodozyma. Bioprocess Eng, 19: 161-164.
- Moss M. (2002) Bacterial pigments. Micobiologist, 3: 10-12.
- Matz C., Deines P., Boenigk J., Arndt H., Eberl L., Kjelleberg S., Jurgens K. (2004) Impact of violacein- producing bacteria on survival and feeding of bacteriovorans nanoflagellates. Appl Environ Microbiol, 70: 1593-1599.
- Velmurugan P. (2008) Studies on the production and dyeing properties of water soluble pigments from filamentous fungi, Ph.D Thesis, Bharathiar University.
- Furstner A. (2003) Chemistry and biology of roseophilin and the prodigiosin alkaloids: A survey of the last 2500 years. Chem Int Ed Engl, 42: 3582-3603.
- D’Aoust J.Y., Gerber N.N. (1974) Isolation and purification of prodigiosin from Vibrio psychroerythrus. J Bacteriol, 118: 756-757.
- Lewis S.M., Corpe W.A. (1964) Prodigiosin producing bacteria from marine sources. Appl Microbiol. 12: 13- 17.
- Kalesperis G.S., Prahlad K.V., Lynch D.L. (1975) Toxigenic studies with the antibiotic pigments from Serratia marcescens. Can J Microbiol. 21: 213-220.
- Gargallo D., Loren J.G., Guinea J., Vinas M. (1987) Glucose-6-phosphate dehydrogenase alloenzymes and their relationship to pigmentation in Serratia marcescens. Appl Environ Microbiol, 53: 1983-1986.
- Gargallo-Viola D. (1989) Enzyme polymosphism, prodigiosin production and plasmid fingerprints in clinical and naturally occurring isolates of Serratia marcescens. J Clin Microbiol, 27: 860-868.
- Tsuji R.F., Yamamoto M., Nakamura A., Kataoka T., Magae J., Nagai K., Yamasaki M. (1990) Selective immunosuppression of prodigiosin 25-C and FK506 in the murine immune system. J Antibiot, 43: 1293- 1301.
- Kataoka T., Magae J., Kasamo K., Yamanishi H., Endo A., Yamasaki M., Nagai K. (1992) Effects of prodigiosin 25-c on cultured cell lines: Its similarity to monovalent polyether ionophores and vacuolar type H+ -ATP ase inhibitor. J Antibiot, 45: 1618-1625.
- Tsuji R.F., Magae J., Yamashita M., Nagai K., Yamasaki M. (1992). Immunomodulating properties of prodigiosin 25-C, an antibiotic which preferentially suppresses induction of cytotoxic T cells. J Antibiot, 45: 1295-1302.
- Songia S., Mortellaro A., Taverna S., Fornasiero C., Scheiber E.A., Erba E., Colotta F., Mantovani A., Isetta A.M., Golay J. (1997) Characterization of the new immunosuppressive drug undecylprodigiosin in human lymphocytes. J Immunol, 158: 3987-3995.
- Hardjito L., Huq A., Colwell R.R. (2002) The influence of environmental conditions on the production of pigment by Serratia marcescens. Biotechnol Bioprocess Eng, 7: 100-104.
- Haddix P.L., Werner T.F. (2000). Spectrophotometric assay of gene expression: Serratia marcescens pigmentation. Biosciene, 26: 3-13.
- Giri A.V., Anandkumar N., Muthukumaran G., Pennathur G. (2004) A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol, 4: 1-10.
- Yamashita M., Nakagawa Y., Li H., Matsuama T. (2001) Silica gel dependent production of prodigiosin and serrawettins by Serratia marcescens in liquid culture. Microb Environ, 16: 250-254.
- Wei Y.H., Chen W.C. (2005) Enhanced production of prodigiosin-like pigment from Serratia marcescens SMΔR by medium improvement and oil supplementation strategies. J Biosci Bioeng, 99: 616- 622.
- Witney F.R., Failia M.L., Weinberg E.D. (1977) Phosphate inhibition of secondary metabolism in Serratia marcescens. Appl Environ Microbiol, 33: 1042-1046.
- Lawanson A.O., Sholeye F.O. (1975) Inhibition of prodigiosin formation in Serratia marcescens by adenosine triphosphate. Experientia, 32: 439-440.
- Pruss B.M., Matsumura P. (1996) A regulator of the flagellar regulon of Escherichia coli, flhD, also affects cell division. J Bacteriol, 178: 668-674.
- Mandarville R.A. (2001) Synthesis, Proton affinity and Anticancer properties of the prodigiosin group of natural product. Curr Med Chem, 1: 195-218.
- Suzuki T., Nakayama T., Kurihara T., Nishino T., Esaki N. (2001) Cold active lipolytic activity of psychrotrophic Acinetobacter sp. strain No. 6. J Biosci Bioeng, 92: 144-148.
- Someya N., Nakajima M., Hirayae K., Hibi T., Akutsu K. (2001) Synergistic antifungal activity of chitinolytic enzymes and prodigiosin produced by biocontrol bacterium, Serratia marcescens strain B2 against gray mold pathogen, Botrytis cinerea. J Gen Plant Pathol, 67: 312-317.
- Khanafari A., Assadi M.M., Fakhr F.A. (2006) Review of prodigiosin, pigmentation in Serratia marcescens. Online J Biol Sci, 6: 1-13.
- Harris K.P., Williamson R., Slater H., Cox A., Abbasi S., Foulds I., Simonsen T., Leeper J., Salmond P.C. (2004) The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species and strain dependent genome context variation. Microbiol, 150: 3547-3560.
- Kobayashi N., Ichikawa Y. (1991) Separation of the prodigiosin localizing crude vesicles which retain the activity of protease and nuclease in Serratia marcescens. Microbiol Immunol, 35: 607-614.
- Iranshahi M., Shahverdi A.R., Mirjani R., Amin G., Shafiee A. (2004) Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratia marcescens. Z Naturforsch, 59: 506-508.
- Rapoport H., Holden K. G. (1962) The synthesis of prodigiosin. J Am Chem So., 84: 635-642.
- Hesse M. (2000) Alkaloide. Fluch oder Segen der Natur?, Helvetica Chimica Acta, ZQrich.
- Wasserman H.H., Rodgers G.C., Keith D.D. (1969) Metacycloprodigiosin, a tripyrrole pigment from Streptomyces longisporus ruber. J Am Chem Soc, 91: 1263-1264.
- Boger D.L., Patel M. (1988) Total synthesis of prodigiosin, prodigiosene and desmethoxyprodigiosin: Diels-Alder reactions of hetero cyclic azidenes and development of an effective palladium (II)-promoted 2'2'-bipyrrole coupling procedure. J Org Chem, 53: 1405-1415.
- Matsuyama T., Murakami T., Fujita M., Fujita S., Yano I. (1986) Extracellular vesicle formation and biosurfactant production by Serratia marcescens. J Gen Microbiol, 132: 865-875.
- Cerdeno A.M., Bibb M.J., Challis G.L. (2001) Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3(2): new mechanisms for chain initiation and termination in modular multienzymes. Chem Biol, 8: 817-829.
- Williamson N.R., Simonsen H.T., Ahmed R.A., Goldet G., Slater H., Woodley L., Leeper F.J., Salmond P.C. (2005) Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. Mol Microbiol, 56: 971-989.
- Staunton J., Wilkinson B. (1997) Biosynthesis of erythromycin and rapamycin. Chem Rev, 97: 2611- 2629.
- Pryce L.H., Terry F.W. (2000) Spectrophotometric assay of gene expression: Serratia marcescens pigmentation. Bioscience, 26: 3-13.
- Hiroaki M., Hiroyuki A., Masakatsu F., Takeji S., Teisuya T. (1996) Industrial production of optically active intermediate in the synthesis of dialtizem with lipase. Seibutsu kogaku, 74: 273-288.
- Jones D., Schultheis B., Klas C., Krammer P.H., Bhakdi S. (1993) Cytocidal effects of Escherichia coli hemolysin on human T lymphocytes. Infect Immun, 61: 1715-1721.
- Cang S., Sanada M., Johdo O., Ohta S., Nagamatsu Y., Yoshimoto A. (2000) High production of prodigiosin by Serratia marcescens grown on ethanol. Biotechnol Lett, 22: 1761-1765.
- Kim C.H., Sung-Ho K., Suk-In K. (1998b). Isolation and Characteristics of prodigiosin like red pigment produced by Serratia sp. KH-95. Korean J Appl Microbiol Biotechnol, 26: 283-289.
- Weiss C.M. (1949) Spectrophotometric and chromatographic analyses of the pigment produced by members of the genus Serratia. J Cellular Comp Physiol, 34: 467-492.
- Bunting M.I. (1940) A description of some color variants produced by Serratia marcescens, strain 274. J Bacteriol, 40: 57-68.
- Hubbard R., Rimington C. (1950) The biosynthesis of prodigiosin, the tripyrrylmethane pigment from Bacillus prodigiosus (Serratia marcescens). Biochem J, 46: 220-225.
- Song M.J, Bae J., Lee D.S., Kim C.H., Kim J.S., Kim S.W., Hong S.I. (2006) Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. J Biosci Bioeng, 101: 157-161.
- Williams R.P., Green J.A., Rappoport D.A. (1956) Studies on pigmentation of Serratia marcescens. I. Spectral and paper chromatographic properties of prodigiosin. J Bacteriol, 71: 115-120.
- Lynch D.L., Worthy T.E., Kresheck G.C. (1968) Chromatographic separation of the pigment fractions from a Serratia marcescens strain. Appl Microbiol, 16: 13-20.
- Someya N., Nakajima M., Hamamoto H., Yamaguchi I., Akutsu K. (2004) Effects of light conditions on prodigiosin stability in the biocontrol bacterium Serratia marcescens strain B2. J Gen Plant Pathol, 70: 367-370.
- Okamoto H., Sato Z., Sato M., Koiso Y., Iwasaki S., Isaka M. (1998) Identification of antibiotic red pigments of Serratia marcescens F-1-1, a biocontrol agent of damping off of cucumber, and antimicrobial activity against other plant pathogens. Ann Phytopathol Soc Jpn, 64: 294-298.
- Thomson N.R., Crow M.A., McGowan S.J., Cox A., Salmond G.P. (2000) Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol Microbiol, 36: 539-556.
- Slater H., Crow M., Everson L., Salmond G.P. (2003) Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum sensing dependent and independent pathways. Mol Microbiol, 47: 303-320.
- Crow M.A. (2001) The genetic regulation of pigment and antibiotic biosynthesis in Serratia sp. Ph.D thesis, University of Cambridge.
- Lewis T.A., Cortese M.S., Sebat J.L., Green T.L., Lee C.H., Crawford R.L. (2000) A Pseudomonas stutzeri gene cluster encoding the biosynthesis of the CCl4- dechlorination agent pyridine-2,6-bis(thiocarboxylic acid). Environ Microbiol, 2: 407-416.
- Tiffany K.A., Roberts D.L., Wang M., Paschke R., Mohsen A.W., Vockley J. and Kim J.J. (1997) Structure of human isovaleryl-CoA dehydrogenase at 2.6A resolution: structural basis for substrate specificity. Biochem, 36: 8455- 8464.
- Syzdek L.D. (1985) Influence of Serratia marcescens pigmentation on cell concentrations in aerosols produced by bursting bubbles. Appl Environ Microbiol, 49: 173-178.
- Bennett J.W., Bentley R. (2000) Seeing red: the story of prodigiosin. Adv Appl Microbiol, 47: 1-32.
- Van der Mei H.G., Cowan M.M., Genet M.J., Rouxhet P.G., Busscher H.J. (1992) Structural and physic-chemical surface properties of Serratia marcescens strains. Can J Microbiol, 38: 1033- 1041.
- Pandey R., Chander R., Sainis K.B. (2007) Prodigiosins: A novel family of immunosuppressants with anticancer activity. Indian J Biochem Biophy, 44: 295-302.
- Campas C., Dalmau M., Montaner B., Barragan M., Bellosillo B., Colomer D. (2003) Prodigiosin induces apoptosis of B and T cells from B-cell chronic lymphocytic leukemia. Leukemia, 17: 746-750.
- Llagostera E., Soto-Cerrato V., Joshi R., Montaner B., Gimenez-Bonafe P., Perez Tomas R. (2005) High cytotoxic sensitivity of the human small cell lung doxorubicin resistant carcinoma (GLC4/ADR) cell line to prodigiosin through apoptosis activation. Anticancer Drugs, 16: 393-399.
- Han S.B., Park S.H., Jeon Y.J., Kim Y.K., Kim H.M., Yang K.H. (2001) Prodigiosin blocks T cell activation by inhibiting interleukin - 2Rα expression and delays progression of autoimmune diabetes and collagen induced arthritis. J Pharm Exp Ther, 299: 415-425.
- Mapari S.A.S., Nielsen K.F., Larsen T.O., Frisvad J.C., Meyer A.S., Thrane U. (2005) Exploring fungal biodiversity for the production of water soluble pigments as potential natural food colorants. Curr Opin Biotechnol, 16: 109-238.
- Duran N., Teixeira M.F.S., Conti R., Esposito E. (2002) Ecological-friendly pigments from fungi. Crit Rev Food Sci, 42: 53-66.
- Hobson D.K., Wales D.S. (1998) Green colorants. J Soc Dyers Colour, 114: 42- 44.
- Frandsen R.J.N., Nielsen N.J., Maolanon N., Sorensen J.C., Olsson S., Nielsen J., Giese H. (2006) The biosynthetic pathway for aurofusarin in Fusarium graminearum reveals a close link between the naphthoquinones and naphthopyrones. Mol Microbiol, 61: 1069-1080.
- Alihosseini F., Ju K.S., Lango J., Hammock B.D., Sun G. (2008) Antibacterial colorants: Characterization of prodiginines and their applications on textile materials. Biotechnol Prog, 24: 742-747.
- Falk H. (1989) The Chemistry of Linear Oligopyrroles and Bile Pigments, Springer, Berlin.
- Furstner A., Weintritt H., Hupperts A. (1995) A New, Titanium-Mediated Approach to Pyrroles: First Syntheses of Lukianol A and of Lamellarin O Dimethyl Ether. J Org Chem, 60: 6637-6641.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences