Purpureocillium lilacinum Strain BP13 Produces Flavoglaucin
Thachat Jayakrishnan, Sailas Benjamin
Thachat Jayakrishnan and Sailas Benjamin*
Enzyme Technology Laboratory, Biotechnology Division, Department of Botany, University of Calicut, Kerala - 673635, India.
Received date: September 26, 2016; Accepted date: October 25, 2016; Published date: November 01, 2016
Citation: Jayakrishnan T, Benjamin S. Purpureocillium lilacinum Strain BP13 Produces Flavoglaucin. Electronic J Biol, 12:4
Abstract
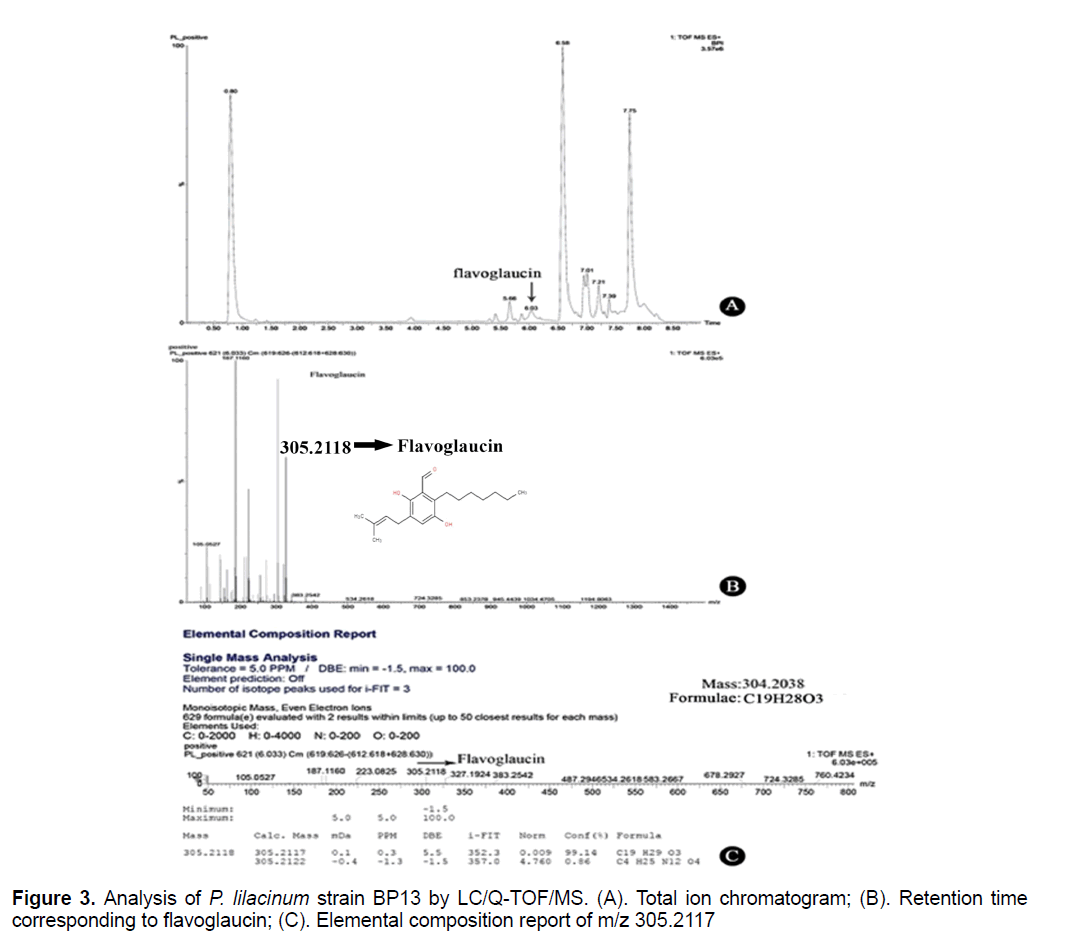
Liquid chromatograph/quardapole-time of flight/mass spectrometry (LC/Q-TOF/MS) is a modern analytical approach in the rapid screening of crude extracts of biological sample for the characterization of secondary metabolites. LC/Q-TOF/MS offers new possibilities for studying fungal metabolites in its crude form, as it requires no sample cleaning. Furthermore, targeted analysis is also needless as the instrument can provide accurate mass measurement of molecular ions of any components detected in an LC run, and these can be identified by searching a database of exact masses of relevant secondary metabolites, which is linked to the analytical system. In the present study, Purpureocillium lilacinum strain BP13 (a novel isolate from this laboratory) was grown in potato dextrose medium for 14 days and the supernatant was extracted in ethyl acetate and subjected to LC-Q-TOF-MS analysis. The peak at 6.03 min had a mass of m/z 305.2118, exactly corresponding to flavoglaucin with chemical formulae C19H28O3, based on the available fungal database search. Literature shows that flavoglaucin is an excellent antioxidant and a synergizing agent, and also shown to have antibacterial and anticancer properties. This is the first report on the production of flavoglaucin by P. lilacinum.
Keywords
Purpureocilluim lilacinum BP13; Flavoglaucin; LC/Q-TOF/MS; Crude extract.
1. Introduction
Secondary metabolites have a major role in human life by providing foods, leads of novel drugs or as therapeutic agents, colorants and biocontrol agents in agriculture. Since the discovery of penicillin by Alexander Fleming in 1929, swift developments took place in the field of biological research, including the characterization of primary and secondary metabolites from various living sources including plants, bacteria and fungi. Many fungal secondary metabolites are shown to have antibacterial, antifungal and anti-tumor activities [1]. Production of secondary metabolites often occurs after the microbial growth has ceased as a result of nutrient depletion but with an excess carbon source available, making it possible to manipulate their formation [2]. Therefore, production of novel biomolecules requires identification of novel microbial strains, and to bioaugment for producing newer metabolites so as to meet the increasing human needs.
The field of metabolomics has found a significant place in biological research; which gives an insight on the small-molecule basis of biological processes such as those associated with pathogenesis, interactions of microbial community, microbial biochemistry, plant physiology, drug target and metabolism. In general, there are two technical areas in performing metabolomics, which involve either NMR spectroscopy or mass spectrometry. By using liquid chromatography quadrupole time-offlight mass spectrometry (LC/Q-TOF/MS), hundreds to thousands of peaks with a specific m/z ratio and retention time are routinely detected from crude biological samples. Each peak can be interpreted on the basis of its accurate mass, retention time and tandem mass spectral fragmentation pattern for the identification of unknown molecule. Using the information provided by LC-Q-TOF analysis, the set of known secondary metabolites could be identified from the available metabolites database.
Among the known fungal strains like Penicillium chrisogenum, P. notatum, Fusarium subglutinens, P. veruculosm, Purpureocillium lilacinum, etc., P. lilacinum is a least studied species. P. lilacinum is a metabolite-rich fungal species and many of its secondary metabolites yet to be identified and characterized. P. lilacinum (formerly Paecilomyces lilacinus) is a common saprobic and filamentous fungus showing cosmopolitan distribution, as it was reported from various habitats such as rhizosphere of crops, esturine sediments, soils, forests, grassland, disserts, sewage sludge, and from the body of insects and nematodes [3]; and P. lilacinum also showed promising results for use as a biocontrol agent in controling the growth of destructive root-knot nematodes [3-5]. Various secondary metabolites including paecilotoxin, a mycotoxin and nematicidal metabolite, leucinostatins are reported from P. lilacinum [4-7]. Considering these facts, the focus of the present study is screening of this fungus, P. lilacinum strain BP13 for the production of novel secondary metabolites using LC/Q-TOF/MS technique, and subsequent analysis using available fungal metabolite database.
2. Materials and Methods
2.1. Fungal culture
Pure fungal culture (Purpureocillium lilacinum strain BP13) reported from the Enzyme Technology Lab, Department of Botany, University of Calicut, was used as the mother culture for the present study [8]. Stock cultures were maintained on potato dextrose (PDA)-agar slants at 28°C.
2.2. Chemicals
Analytical grade chemicals from HiMedia (India) and Nice (India) were used for the study. The fungus was grown in potato dextrose (PD) medium. Methanol, dichloromethane, ethyl acetate are the reagents used for the extraction; and silica gel, toluene, formic acid, iodine reagent were used for the biophysical analysis.
2.3. Medium and innoculum
P. lilacinum strain BP13 was maintained at 4°C on PDA medium. Seed culture (4 days old) was prepared in the PD broth by inoculating a loopful of fungal spore in 100 mL flask containing the medium, which was incubated at 27°C and 150 rpm.
2.4. Medium for the production of secondary metabolites
Spores of P. lilacinum BP13 collected from seed culture were amended into the PD liquid medium in 100 mL flasks, and incubated at 27°C for 2 weeks on a rotary shaker at 150 rpm.
2.5. Fungal culture extraction
Metabolites were extracted from 14 days old P. lilacinum culture in PD broth. The culture broth was centrifuged (9440 g) and the pH was adjusted at 3 by adding HCl. The filtrate was extracted with ethyl acetate (1:1; v/v) and phase separated using a separating funnel, subsequently concentrated using a rotary evaporator at 77°C; and 100 μg of dried extract was dissolved in 1 mL methanol as stock for further analysis.
2.6. Preliminary screening by TLC
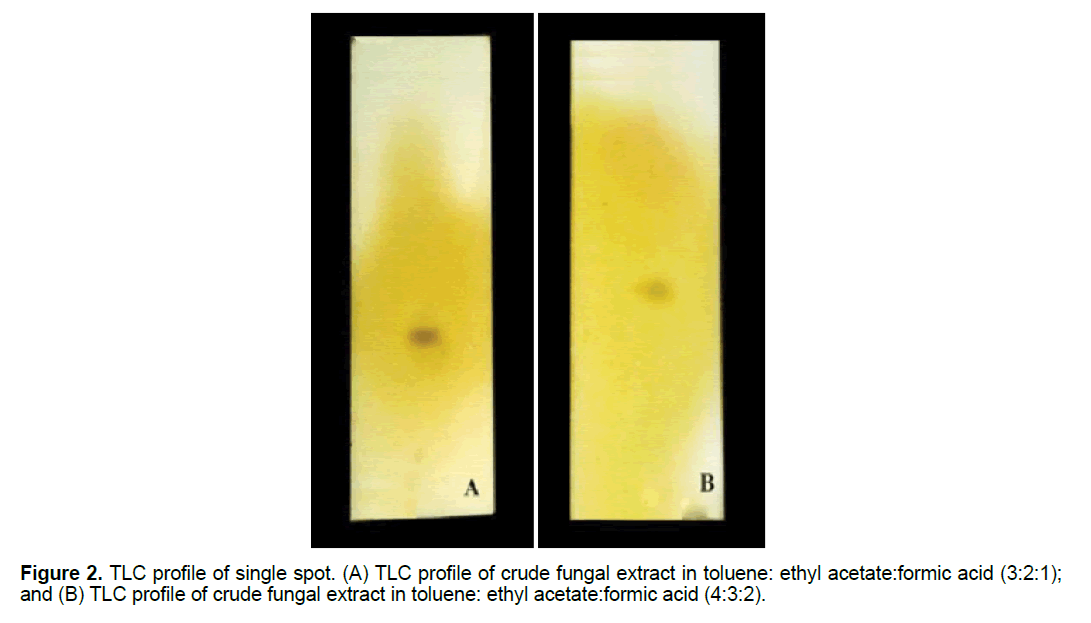
The crude sample was loaded on silica gel coated plate. The solvent system was toluene, ethyl acetate and formic acid in ratio 3:2:1 and 4:3:2, respectively. The chromatograms on the TLC plates were developed by spraying iodine reagent on the dried plate.
2.7. LC-Q-TOF MS analysis
The crude extract was used for LC/Q-TOF/MS analysis using the facility available at the Inter- University Instrumentation Center, MG University in Kottayam. All analytical works were performed using a Xevo G2 (Waters) Quadrapole–Time-of-Flight (Q-TOF) coupled to an Acquity H class (Waters) Ultra Performance Liquid Chromatograph (Agilent), equipped with BEH C18 column (50 mm × 2.1 mm × 1.7 μm) at a flow rate of 0.3 mL/min. The LC/Q-TOF/ MS was equipped with a dual nebulizer electrospray source. The instrument scanned the probable peaks from m/z 100 to 1,000 in the crude sample. The total run time was 8 min. The source type was Electro- Spray Ionization (ESI) with the capillary temperature of 135°C. Capillary voltage of positive mode of ESI was 3.50 KV.
3. Results and Discussion
3.1. Effects of culture media
P. lilacinum strain BP13 grown in PD medium showed significant growth within one week of incubation at 25°C; the fungal mycelia were white and formed small globular cottony masses (Figure 1). During the second week of growth, the medium turned into pale yellow, an indication of the production of extracellular secondary metabolites [9].
3.1. Preliminary screening by TLC
Preliminary screening for fungal secondary metabolites was carried out using TLC method. The solvent mixture contained toluene, ethyl acetate and formic acid in ratios 3:2:1 and 4:3:2, respectively (Figure 2). Iodine was used as developing reagent. Clear spot was observed on the chromatograms developed under both solvent systems, i.e., the Rf of the former system was 0.266 cm and that of the latter system was 0.316 cm.
3.2. LC/Q-TOF/MS analysis
For LC/Q-TOF/MS analysis, the facility available at the Inter-University Instrumentation Center, Mahatma Gandhi University, Kottayam was used. The analysis provided data based on mass to charge ratio, chemical formula and molecular mass of the metabolite from the crude sample. Using the data provided by LC/Q-TOF/MS analysis, the secondary metabolites were identified from the fungal secondary metabolites database stated earlier [10].
The crude extracts were directly analyzed by LC/QTOF/ MS. Figure 3A shows the total ion chromatogram of the extract of P. lilacinum, indicating the presence of about 8 components. From the database, the peak at 6.03 min showed an exact mass of m/z 305.2118. Based on the M+H+ ions, this mass corresponds to flavoglaucin with a 0.3 ppm mass match, as compared to the exact mass of flavoglaucin in the database. The software uses an algorithm that rejects all ions that are not actual peaks and align the real ions into molecular features. The Molecular features and accurate mass measurement of P. lilacinum for the peak at a retention time of 6.03 min is typical of flavoglaucin, as seen in Figure 3. Using the molecular features, the software will calculate the probable chemical formula, and score the isotopes for the “fit” to the proposed formula. The formula with the score of 100 is also that of flavoglaucin. The search results for fungal secondary metabolites database as stated earlier clearly indicated the formula and structure of flavoglaucin (Figure 3) [10].
Flavoglaucin is found as an excellent antioxidant and synergist. It is a phenolic compound first isolated from Eurotium chevalieri [11]; E. herbariorum and E. repens are other two prominent species producing the compound. Of them, the latter two are the excellent producers of flavoglacin, i.e., up to 126 μg/mL [12]. Incorporation studies with (13C) acetate have established the regular polyketide nature of flavoglaucin, modified by isoprenylation; and the aromatic isoprenylation occurs without any change in stereochemistry of the olefin in the dimethylallyl moiety [13]. Under autoxidation conditions, flavoglaucin remarkably synergized with tocopherol and stabilized many oils and fats. It was found that the vegetable oils retained their original stabilities even after thermal treatment at 180°C for 25 h with the addition of 0.05% flavoglaucin [10]. Flavoglaucin also showed some antimicrobial and antitumor properties; hence, production of flavoglaucin from P. lilacinum strain BP13 is a promising step in the field of pharmacology.
4. Conclusion
Strains of P. lilacinum are popularly used as biocontol agents, especially to control nematodes; therefore, most of the studies on the metabolites from this fungus are confined to those responsible for the nematicidal activity. This study for the first time showed that P. lilacinum strain BP13 is an efficient producer of flavoglaucin, an excellent antioxidant and synergent known to exhibit antitumor and antimicrobial properties. Further studies required for the optimization of nutrient as well as chemical and physical parameters involved in the production of flavoglaucin, coupled with its purification and quantification and also mining for more secondary metabolites not only in the culture supernatant but also from the mycelial extract at various stages of the growth of this valuable fungus.
5. Acknowledgement
The financial assistance (Grant No.50/SPS54/2015/ CSTE) from Kerala State Council for Science, Technology and Environment (KSCSTE), Government of Kerala, is gratefully acknowledged.
References
- Demain AL. (1999). Pharmaceutically active secondary metabolites of microorganisms.Applied Microbiology and Biotechnology.52: 455-463.
- Gunatilaka AA, Leslie. (2010). Fungal secondary metabolites. In Access Science. McGraw Hill Education.YB100063.
- Spatafora JW, Quandt CA, Kepler RM, et al. (2015). New 1F1N species combinations in Ophiocordycipitaceae (Hypocreales).IMA Fungus.6: 357-362.
- Park JO, Hargreaves JR, McConville EJ, et al. (2004). Production of leucinostatins and nematicidal activity of Australian isolates of Paecilomyces lilacinus (Thom) Samson.Letters in Applied Microbiology.38: 271-276.
- Sharma A and Sharma S. (2016). Production of Secondary nematicidal metabolites from Purpureocillium lilacinum using Karanja Deoiled Cake. IJTA. 34: 1.
- Mikami Y, Yazawa K, Fukushima K, et al. (1989). Paecilotoxin production in clinical or terrestrial isolates ofPaecilomyces lilacinus strains.Mycopathologia.108: 195–199.
- Khan A, Williams K, Nevalainen H. (2003). Testing the nematophagous biological control strain Paecilomyces lilacinus 251 for paecilotoxin production.FEMS Microbiology Letters.227: 107-111.
- Pradeep S, Faseela P, Josh MS, et al. (2013). Fungal biodegradation of phthalate plasticizer in situ. Biodegradation.24: 257-267.
- Senyuva HZ, Gilbert J, Hutton S. Rapid analysis of crude fungal extracts for secondary metabolites by LC/TOF-MS – A new approach to fungal characterization. Agilent Technologies. 1-8.
- Nielsen KF, Mansson M, Rank C, et al. (2011). Dereplication of microbial natural products by LC-DAD-TOFMS.Journal of Natural Products.74: 2338-2348.
- Ishikawa Y, Morimoto K, Hamasaki T. (1984). Flavoglaucin, a metabolite of Eurotium chevalieri, its anti-oxidation and synergism with tocopherol. Journal of the American Oil Chemists’ Society. 61: 1864-1868.
- Miyake Y, Ito C, Itoigawa M, et al. (2009). Antioxidants produced by Eurotium herbariorum of filamentous fungi used for the manufacture of Karebushi, dried bonito (Katsuobushi).Bioscience, Biotechnology and Biochemistry.73: 1323-1327.
- Allen JK, Barrow KD, Jones AJ, et al. (1978). Biosynthesis of flavoglaucin. Stereochemistry of aromatic isoprenylation.Journal of the Chemical Society, Perkin Transactions. 1: 152-154.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences