Microbial Quality Analysis of Milk and Flavoured Milk Products from Local Vendors in Vellore
Aditya Sood, Ridhi Sood, Abhijit Kumar, Gaganjot Kaur, Candy Sidhu
Aditya Sood*, Ridhi Sood, Abhijit Kumar, Gaganjot Kaur, Candy Sidhu
Assistant Professor, Chandigarh University, Punjab.
- Corresponding Author:
- Aditya Sood
Assistant Professor
Chandigarh University, Punjab
Tel: +919329844585
E-mail: er.adityasood@gmail.com
Received date: January 27, 2015 Accepted date: February 01, 2016 Published date: February 07, 2016
Citation: Sood A, Sood R, Kumar A, et al., Microbial Quality Analysis of Milk and Flavoured Milk Products from Local Vendors in Vellore. Electronic J Biol, 12:1
Abstract
Milk is one of the utmost reasonable foundations of much nutrition like proteins and vitamins. The quality of milk is resolute by facets of composition and hygiene. Due to its compound biochemical structure and high water activity milk aids as an outstanding culture medium for the growth and multiplication of numerous kinds of microorganisms. Consequently in the processing of milk, some of them may produce unwanted effects and some microorganisms yield food infections which either transmits pathogens that will upsurge the likelihood of infection of the consumer’s food. Milk is a foremost part of human food and plays protuberant role in one’s diet.
The adulteration of milk is mainly due to human feature and unhygienic conditions. Typically milk is polluted with diverse types of microorganisms at milk gathering places. The microbial superiority of raw milk is vital for the fabrication of excellence dairy products. There can be deterioration in milk’s quality, colour, odour or flavour to a point where it is improper for human consumption. Pathogenic micro-organism in milk comprises E. coli, Staphylococcus aureus, Listeria monocytogenes, Clostridium, Microbacterium, Micrococcus and Streptococcus. The samples were collected from Vellore area. All the samples positive for altered microbial contaminations were established using biochemical examinations.
The cultural features of the isolates were established by inoculating the pure colonies on nutrient agar, mannitol salt agar and using methylene blue agar and other biochemical tests such as Simmon’s citrate agar, indole production, methyl red, VP test and coagulase test were made to confirm the occurrence of diverse microorganisms. In this way the occurrence and absence of numerous microorganisms was recognized. However a large volume of these products were produced in unorganised sector with little precautions of food safety and quality.
Keywords
Milk; E. coli; Staphylococcus aureus; Listeria monocytogenes; Flavoured milk; Total plate count; PFA rules 1956; FSSAI.
1. Introduction
Prevention of food adulteration (PFA) rules, 1956 specifies microbial supplies for pathogens such as E. coli, Staphylococcus aureus, Listeria monocytogenes in foods frequently involved in food-borne diseases. According to standards given by PFA these microorganisms must be absent in one gram of milk and milk products (flavoured also). Hence we checked if the microorganisms were present or absent in one gram of milk and milk products representing whether there was contamination or not. This implied the use of selective and differential media.
• EMB (Eosine Methylene Blue) agar – for E. coli
• MSA (Mannitol Salt Agar) – for Staphylococcus aureus
• Listeria selective agar base – for Listeria monocytogenes
The microbial excellence of raw milk is vital for the fabrication of excellence dairy products. Spoilage is the term used to define the worsening of food’s texture, colour, odour or flavour to a point where it is inappropriate for human feeding. Pathogenic microorganism in milk comprises E. coli, Staphylococcus aureus, Listeria monocytogenes, Clostridium, Microbacterium and Streptococcus, etc. Bacteria proliferate during fabrication and holding of milk, depending on storage time and conditions. The variations take place in the physic-chemical properties of milk is result of the activities of the individual microorganisms during their period of growth and reproduction or of substances produced during such activities [1].
1.1 Objectives
• To isolate diverse micro-organisms from milk and flavoured milk collected from diverse localities in Vellore.
• Examining the microbial superiority of milk and flavoured milk by detecting the presence or absence in a specific amount.
• Total plate count of isolated micro-organisms.
1.2 Need of the project
According to the new food safety rules that came into effect, the FSSAI (Food Safety Standards Authority of India) has made it compulsory for milk to be tested for E. coli, staphylococcus aureus and Listeria monocytogenes. According to a recent report FSSAI has found that nearly 70% of the 1791 samples picked from 33 states failed to approve the FSSAI’s standards. E. coli and S. aureus are the most common contaminants and are important from public health point of view as they have been connected with the onset of food poisioning in human beings. Adulteration of milk leads to many human diseases like tuberculosis, gastroenteritis, brucellosis, salmonellosis and staphylococcal food poisoning.
2. Methodology
2.1 Sample collection
• 17 samples of milk were collected from local vendors in Vellore area.
• These samples were carried in ice bag so that the action of the microorganism cease and to reduce the bottle effect.
2.2 Chemicals used
• 0.85% NaCl used
• EMB agar
• MSA
• Nutrient broth
• Methyl Red
• VP reagent
• Simmon’s citrate agar
• Barritt’s A and B reagent
2.3 Procedure followed
• 1 ml of each sample was added to each test tube containing 9 ml NaCl. This is 10-1 dilution.
• 20 ml EMB agar and MSA was poured in each petriplate and the media was allowed to solidify.
• 100 μl of sample was spread properly onto the solidified plates.
• The plates were kept in incubator at 37ºC for 2 days.
• After 2 days the plates were checked for the growth, if any.
• 1.25 gm nutrient broth in 100 ml distilled water was used to make pure culture.
• Nutrient broth was then separately inoculated with the colonies obtained from EMB agar plate and MSA plate.
• The inoculated NB media is then kept in shaking incubator at 37ºC.
• After the growth it was kept at 4ºC for further use.
2.4 Biochemical tests
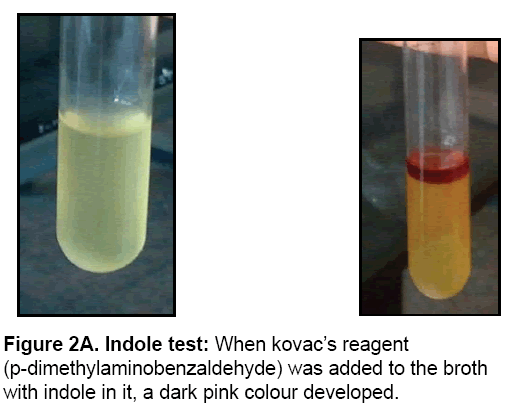
• Indole test
E. coli was inoculated into tryptone broth, a rich source of the amino acid tryptophan. It produced tryptophanase, an enzyme that cleaves tryptophan, producing indole and other products. Then Kovac’s reagent was added to the broth.
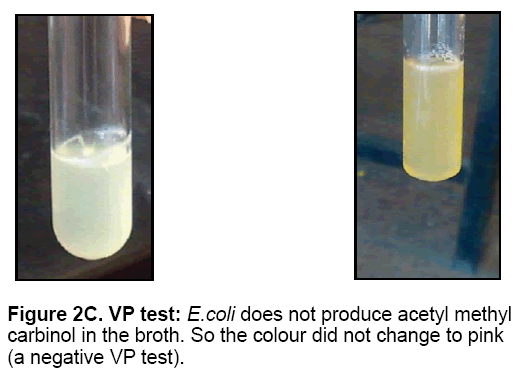
• The Methyl red and voges-proskauer (MR-VP) test
The MR-VP tests were read from a single inoculated tube of MR-VP broth. After 48 hours of incubation the MR-VP broth was split into two tubes. One tube was used for MR test and other was for VP test. MR-VP media comprises glucose and peptone. E. coli oxidized glucose for energy. Both the MR and VP tests were used to determine what end products result when E. coli reduces glucose. E. coli produced acids causing the pH to be acidic. The pH indicator methyl red was added to this acidic broth. The reagents used for the VP test were Baritt’s A and Baritt’s B. These reagents were added to the broth.
• The citrate test
The citrate test utilizes Simmon’s citrate media to determine if a bacterium can grow using citrate as its sole carbon and energy source. Simmon’s media contains bromothymol blue. A pH indicator with a range of 6.0 to 7.6, bromothymol blue is yellow at acidic pH (around 6.0) and progressively variations to blue at more alkaline pH (around 7.6).
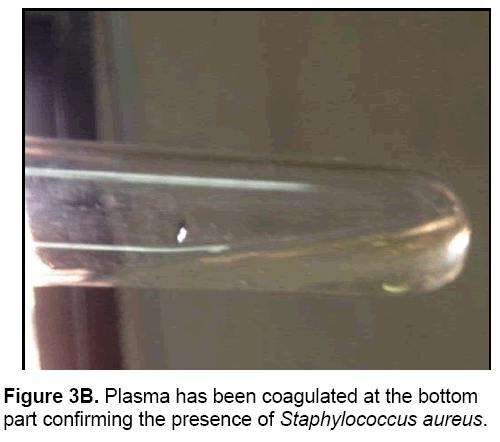
• For Staphylococcus aureus, 0.8 ml of the pure culture was occupied and was mixed with the blood plasma which was found by the centrifugation of blood. It was then kept in water bath for 37ºC for 4 hours.
2.5 Total plate count
• NB was prepared and inoculated with E. coli and S. aureus which was isolated from milk samples.
• The culture was kept in incubation for overnight.
• Respective dilutions were prepared with NaCl for both E. coli and S. aureus.
• Spread plating of these dilutions was performed in NB agar plates.
• Plates were then incubated overnight to grow colonies.
• Colonies were counted and Colony Forming Unit (cfu/ml) was calculated by given formula
Cfu/ml= (no. of colonies × dilution factor) / volume inoculated
3. Observations and Results
3.1 For E. coli
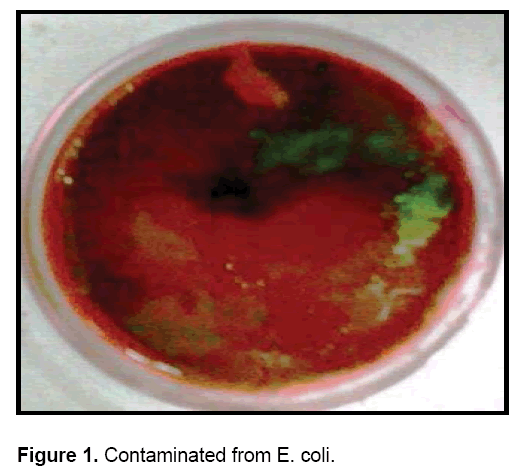
• On spreading the milk samples on EMB agar petriplates, a distinctive metallic green sheen was observed indicating the presence of E. coli.
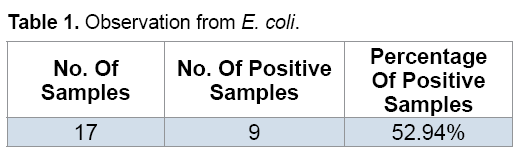
Out of 17 samples of milk 9 samples gave positive results, i.e., they were contaminated from E. coli. (Figure 1 and Table 1).
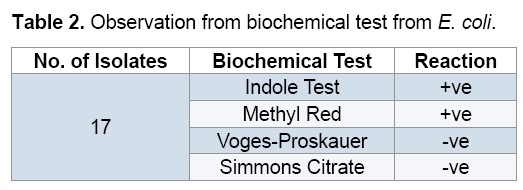
Biochemical reactions of E. coli
(Figures 2A-2D and Table 2).
3.2 For S. aureus
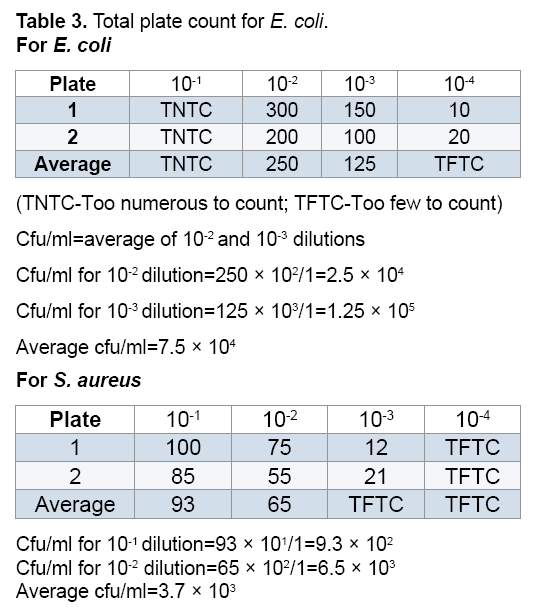
The bacterial waste products generated, organic acid metabolites change the pH indicator in MSA from red to bright yellow (Table 3 and Figures 3A and 3B).
4. Discussion
Coliforms chiefly E. coli is commonly used in the microbiological analysis of food as an indicator of poor hygienic condition. S. aureus results in food poisoning. Microbiological examination of milk is vital to find the degree of adulteration. The coliform bacteria are capable to grow well in a diversity of substrates and to use a number of carbohydrates and some other organic mixtures as food for energy.
(TNTC-Too numerous to count; TFTC-Too few to count)
Cfu/ml=average of 10-2 and 10-3 dilutions
Cfu/ml for 10-2 dilution=250 × 102/1=2.5 × 104
Cfu/ml for 10-3 dilution=125 × 103/1=1.25 × 105
Average cfu/ml=7.5 ×104
For S. aureus
So with the microbiological quality analysis of milk in Vellore area people can be made aware of the fact that how much polluted milk they consume and what can be the hazards of consuming them. On understanding this, now there will be more careful in future regarding numerous features such as cleaning their hands and udders of cow, utensils in which milk are to be transported etc. [2,3].
5. Conclusion
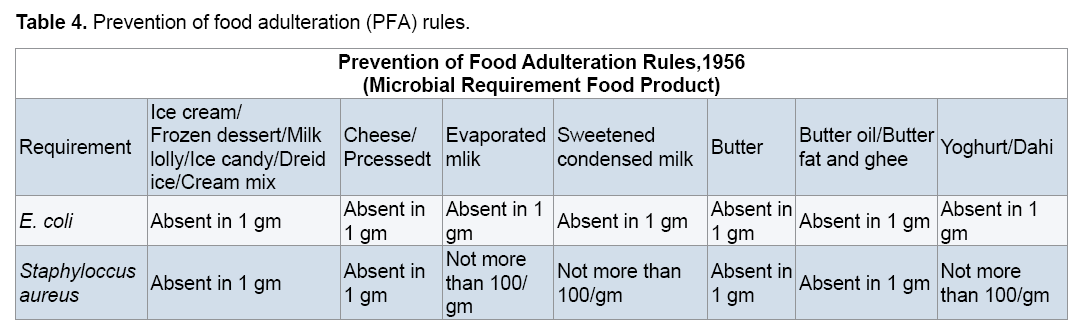
Prevention of Food Adulteration (PFA) rules, 1956 specifies microbiological necessities for pathogens such as E. coli, S. aureus in foods usually involved in food-borne diseases such as sea food, milk and milk products, fruits and vegetable products, packaged drinking water, mineral water, etc. The below mentioned are the standards given by Prevention of Food Adulteration Rules, 1956. According to these E. coli and S. aureus should be absent in 1 gram of milk and milk products. But according to the results found after doing the microbiological analysis of milk and milk products in Vellore area. It was seen that these microorganisms were present in 1 gram of samples of milk and milk products. This means that these samples are polluted and unfit for consumption (Table 4).
References
- Soomro AH, Arain MA, Khaskheli M, et al. (2002). Isolation of Escherichia Coli from Raw Milk and Milk Products in Relation to Public Health Sold under Market Conditions at Tandojam. Department of Dairy Technology, Department of Parasitology, Faculty of Animal Husbandry and Veterinary Sciences, Sindh Agriculture University, Tandojam, Pakistan.
- Kumbhar SB, Ghosh JS, Samudre SP. (2009). Microbiological Analysis of Pathogenic Organisms in Indigenous Fermented Milk Products. Department of Microbiology, Shivaji University, Kolhapur, MS,India.
- Kumar R,Prasad A. (2010). Detection of E.coli and Staphylococcusin Milk and Milk Products in and around Pantnagar, College of Veterinary and Animal Sciences, G.B. Pant. University of Agriculture &Technology, Pantnagar, Uttarakhand, India.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences