Efficiency of Fermented Fish Offal Meal on Growth and Fatty Acid Profile of Tilapia (Oreochromis niloticus)
Mukti Pada Bag, Subhash Chandra Mahapatra
Mukti Pada Bag*,Subhash Chandra Mahapatra
Rural Development Centre,Indian Institute of Technology,Kharagpur,West Bengal,India
Abstract
The growth performance of O. niloticus fingerlings fed with diets having fermented fish offal was investigated for a 90 days. One reference (RFM) diet (Mustard oil cake + wheat flour + rice bran + egg shell dust) and one experimental diet containing fermented fish offal (39%) were (FOM)formulated. The diets were fed twice (06.00 and 17.00 hours) daily ad libitum in two replicates. The growth experiment with these diets showed that the diet with fermented fish offal rendered highest growth of the tilapia. The diet, containing fermented fish offal showed significantly higher (P<0.05) growth, feed conversion ratio (FCR), protein efficiency ratio (PER), specific growth rate (SGR), hepatosomatic index (HSI) and gonadosomatic index (GSI) than the reference diet without fermented fish offal. Fatty acid profile of FOM fed fish was also better as compared to the reference diet. It was concluded that effectiveness of fermented fish offal could be substantially increased the growth and fish quality by accumulating more poly unsaturated fatty acid (PUFA) in fish flesh of tilapia (O. niloticus).
Keywords
Fermented fish offal,FCR,PER,fatty acid profile,PUFA.
1. Introduction
Considering the importance of nutritionally balanced and cost-effective alternative diets for fish,there is a need for research effort to evaluate the nutritive value of different animal and plant by-products as feed staff in fish diet to replace fishmeal,which is expensive and gradually becoming scarce [1,2]. Use of fish (offal) wastes as alternative feedstuffs in fish and other animal diets is gaining importance because it produces environmental benefits and reduces the cost of animal production [3,4]. Fish-offal (FO),particularly the viscera of carps,has immense potentiality to be used as fish feed. The viscera of carps,which are thrown in bulk as offal in the retail fish markets,contains high amounts of crude protein (30-32%) and lipid (15-18%) [5]. However,these materials are not evaluated for fish diet earlier. However,the FO needs to be processed suitably before using it in a diet formulation. Fermentation has been proved to be a most suitable technique to improve nutritional quality of animal by-products and to make them suitable for inclusion in fish diet formulation [2,6-8]. Fermented fish-offal has been proved to be a viable source of protein for the diet of carps [5]. The freshwater catfish H. fossilis has also been found to accept a diet containing fermented fish offal [9]. The influence of fish offal on fatty acid profile of fish is very scanty. The beneficial fatty acid in fish body is synthesis from the feed materials they consumed [10]. The beneficial effects of fish lipids on human health have already been well established [11]. It is currently well established that increased fish consumption in human is associated with decreased mortality,as well as morbidity from cardiovascular disease (CVD) and coronary heart disease (CHD) [12,13]. Consequently,the links between fish as food and human health are strongly related to the fatty acid composition of the food [14]. The fatty acid profile of fish can be modified with diets containing non-conventional sources [15]. It is therefore of utmost importance to determine the influences of feed on growth as well as fatty acid profile of fish [16]. The aim of this study is to evaluate the growth of O. niloticus by using fermented FOM and also to study the qualitative changes in fish flesh.
2. Materials and Methods
Experimental Set-up
Fifteen fingerlings in triplicate groups used in two different treatments. Altogether ninety (90) Nile tilapia (male and female ratio 1:1) fingerlings were used in this experiment. The fish fingerlings were treated with potassium permanganate solution (1mg L–1) to remove any external parasites and were acclimatized in a big tank for five days. Experiments were carried out at the tanks of aquacultural engineering section of IIT-Kharagpur,Paschim Medinipur,West Bengal,India. Each group of fingerlings also were initially weighed to record the initial biomass. They were stocked in six rectangular cemented tanks (1000 L). The water system was static in nature and the bottom of the tank was filled with local agricultural soil (pH 6.4 ± 0.05). The experiment was conducted for 90 days from June to August in the year 2010.Dechlorinated well water (temperature 28 ± 3 °C,pH 7.0 ± 0.05,free CO2 0.4 ±0.01 mg L–1,available nitrogen0.5 ±0.05 mg L–1 and dissolved oxygen (DO) 6± 0.5 mg L–1)was used in the experiment
Feed Formulation and Preparation
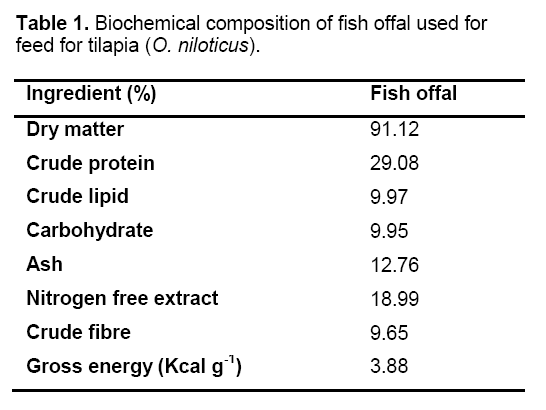
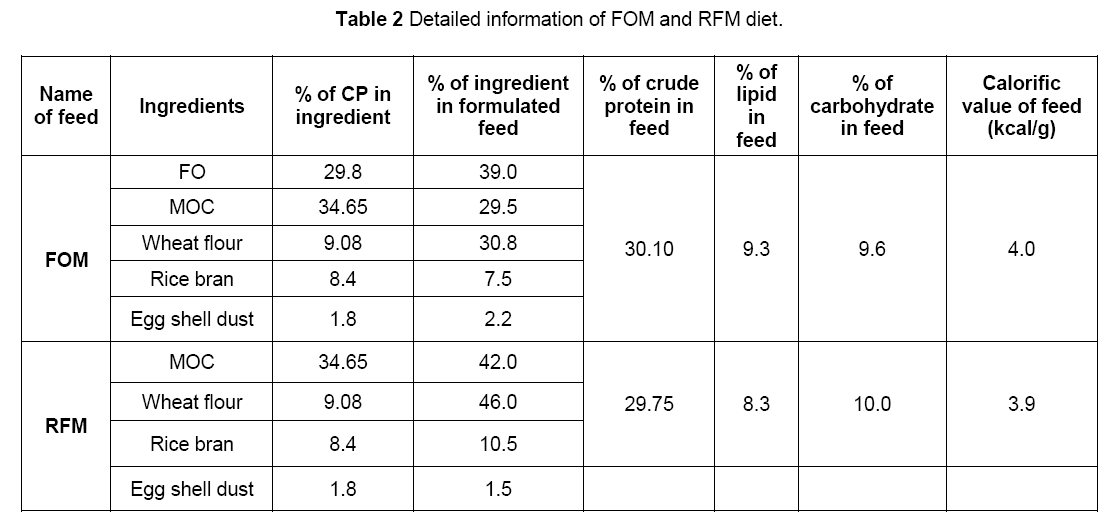
The principal feed ingredient (fish offal) was collected from local retailer fish market at very low cost. These substances were economically cheap but contained significant amount (36–40%) of crude protein [17]. Biochemical composition of fish offal used in the feed for tilapia is shown in Table 1. Diets used for growth trial were prepared that feed formulations remain almost isoproteinicious (30 g 100 g–1) and isoenergetic (4Kcal g–1) in nature. Diet formulations are presented in Table 2. Mustard oil cake,wheat flour,rice bran and egg shell dusts were used for making reference diet (RFM). In addition to these components fish offal is key ingredient in FOM diet. The proportion of different feed ingredients was determined by using Pearson’s square method for making diet isoproteinicious. The different ingredients were thoroughly mixed using a food mixer (A200 Hobart Ltd).The mixture was given the shape of pellets using a Pellet Mill (Model CL2) with a 12 mm die. The resulting pellets were dried in a hot air oven for 48 h at 50ºC,packed in polythene bags and kept in dry and cool place.
Feeding
The feed was given ad libitum through submerged hanging tray (two trays in each tank). Unconsumed feed was removed after 1hour from the beginning of feed administration and dried in a hot air oven at 50°C. Feed consumption was estimated by subtracting the weight of the unconsumed feed from the weight of the feed offered. Fish,feed samples,and unconsumed feeds were weighed on pan electric balance to an accuracy of 0.1 mg.
Growth Calculation
Growth and nutrient utilization were determined in terms of feed intake (FI),specific growth rate (SGR),feed conversion ratio (FCR),protein efficiency ratio (PER),hepatosomatic index (HSI) and gonadosomatic index (GSI) as follows [18]:
FI (g fish-1 day-1) = Total feed intake per fish/number of days
SGR (% day-1) = 100 × (ln[final body weight]- ln[initialbody weight])/no. of Days
FCR = feed intake/live weight gain
PER = live weight gain/crude protein intake
HSI (%) = 100 × (liver weight/total body weight)
GSI (%) =100 × (weight of gonad /total body weight)
Analysis
Feeds and carcass samples were analyzed following standard procedures of AOAC [19] Dry matter(DM) after drying in a hot air oven (Gallenkamp,UK) at 105°C for 24 h; crude protein (CP) by Kjeldahl method (N × 6.25) after acid hydrolysis,crude lipid (CL) after extraction with petroleum ether for 7-8 h by Soxhlet method (40–60°C boiling range),total ash by igniting at550°C for 3 h in muffle furnace (Size 2,Gallenkamp,UK). Organic matter (OM) was calculated by subtracting total ash from DM [20]. Crude fibre was determined using a moisture free defatted sample which was digested by a weak acid HCl (0.1N) followed by a weak base NaOH (0.1N) using the Fibertec System 2021 (FOSS,Denmark). Nitrogen-free extract was determined by subtracting the sum of crude protein,crude lipid,crude fibre and ash from DM [21]. Gross energy was determined using a Bomb Calorimeter Model-DFU 24 following the process as described below. The sample was combusted in a chamber pressurized with pure oxygen and resulting heat measured by increase in the temperature of the water surrounding the bomb.
Extraction of Lipids
The total lipids were extracted from all the samples (fish flesh-2,feed-2),following the method of Bligh and Dyer [22] using methanol-chloroform (2:1,v/v),methanol-chloroform-water (2:1:0.8,v/v/v),and then again with the first solvent system viz.,methanol-chloroform (2:1,v/v). Sample was ground with the solvent methanol-chloroform (2:1,v/v),filtered through Whatman No. 1 filter paper and residue was extracted with the next solvent system ,consisting of methanol-chloroform-water (2:1:0.8,v/v/v). The process was repeated once again with methanol-chloroform (2:1,v/v). Finally,the three extracts were pooled,diluted with three volumes of water (100-200 ml,depending on the volume of pooled extracts) and layer was allowed to separate in a separatory funnel made by Pyrex glassCo. The chloroform layer at the bottom of the separatory funnel was withdrawn and dried over anhydrous sodium sulphate in glass stoppered conical flasks,by Pyrex. The chloroform solution of lipid was evaporated in a rotary vacuum evaporator by Rotavapunder a pressure of 40-50 mm of Mercury,weighed on a micro-balance by Sartorius and redissolved in distilled n-hexane (10-20 ml) and kept at -20°C for future use.BHT (butylated hydroxy toluene) was added at a level of 100 mg/L to the solvent as antioxidant.
Preparation of Methyl Ester of Fatty Acids
Total lipid of various (fish flesh-2,feed-2) samples was dissolved in anhydrous methanol containing concentrated Sulfuric acid (1.0%,v/v) and the mixture was refluxed [23] for 2 hours. Methanol was evaporated to a small volume (1-3 ml) and cooled to 4°C,in a freezer. Distilled water 10–15 ml was added to the cooled mixture (1-3 ml) in hard glass test tubes by Pyrex and the methyl esters of fatty acids were extracted 3 times with aliquots (5-10 ml) of diethyl ether,vortexed in a Vortex mixer. The ethereal extracts were taken out by Pasteur pipettes,pooled and dried over anhydrous sodium sulphate,(1-2 gm) in conical flasks (25-50 ml capacity) with glass stopper,filtered through Whatman no. 1 filter paper,vacuum dried,redissolved in n-hexane ( 1-2 ml volume) and kept in a freezer at 4°C for future use.
Purification of Fatty Acid Methyl Ester (FAME) by Thin Layer Chromatography (TLC)
Fatty acid methyl esters were purified by TLC [24,25] using a solvent system of n-hexane-diethyl ether (90:10,v/v). A standard methylester was also run on the same plate in a separate lane,for identification of the methyl ester bands in the samples. The location of methyl ester bands were indicated by placing the TLC plate in an iodine vapour chamber by Pyrex glass co.. The methyl ester bands corresponding to the standard were marked and then scrapped off the plate with a sharp rajor blade. Methylesters were recovered by extracting the silica gel bands containing the methyl ester samples in a mini glass column (10 cm length x 0.8 cm internal diameter (i.d),by Pyrex) with chloroform (30-50 ml),the later was evaporated and the methyl esters were kept in n-hexane(1-2 ml) in a freezer at 4°Ctill analyzed by Gas Liquid Chromatography (GLC).
Gas Liquid Chromatography (GLC)
GLC of fatty acid methyl esters were done on a Chemito 1000 instrument,equipped with Flame Ionization Detector (FID). Quantifications were done by computer using specific Clarity Lite software.
Analysis of Fatty Acid Methyl Esters (FAME)
GLC of FAME was done on a BPX-70 megabore capillary column of 30 mt length and 0.53 mm i.d. obtained from SGE,Australia. Oven temperature was programmed from 150°C – 240°C with a rate of 8°C/min. Initial and final temperatures were kept isothermal for 1 minute and 20minutesrespectively. Injection port and detector temperatures were 250°C and 300°Crespectively.Nitrogen gas was used as carrier gas and its flow rate was 6.18ml/min.
Statistical Analysis
Data are presented as means ± SD. One-way ANOVA was used to determine the significant effects of different types of feed on growth and growth parameters and also on fatty acid profile of fish flesh. The differences between the means of treatments were examined using Duncan’s multiple range tests [26].
3. Results and Discussions
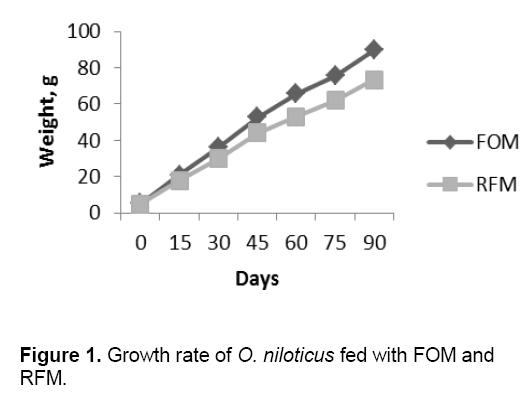
The highest weight gain (89.67 g) was observed in the FOM applied feed. The growth rate always faster in FOM fed fish than fish fed with RFM (Figure 1). This indicates that fish can consume the FOM feed well. This was possibly due to their higher palatability and preference of the fish to take it as their potential food. Sammader et al. [27] reported that M. vittatus grows better on diets supplemented by fermented mixture containing FOM as compared to the fish fed the reference diet without FOM.
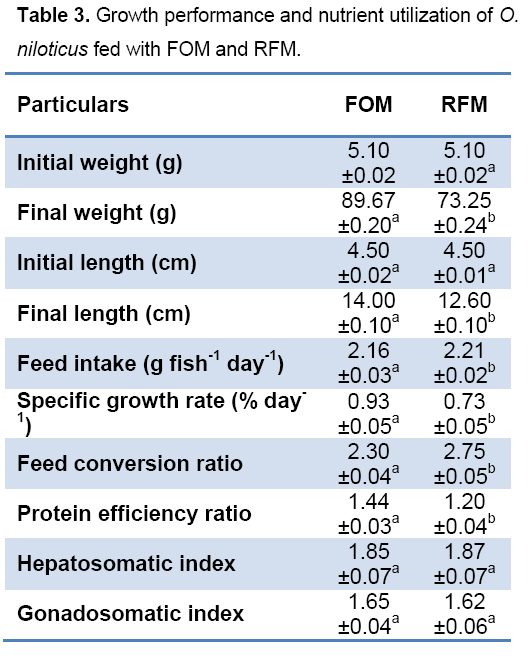
But the amount of feed intake was highest (2.21g) in RFM.The feed conversion ratio (FCR) was differed significantly and lowest value (2.30) was recorded from ETM fed fish indicating an encouraging effect on economic involvement in fish farming.
The specific growth rate (0.93) and protein efficiency ratio (1.44) were highest in ETM fed treatment. This indicates the superior quality of protein in the feed produced from fish offal (FOM). The HSI (%) was not differed significantly between two treatments (Table 3). The highest (1.65) value of GSI (%) was observed in FOM feed treatment but it was not differed significantly with RFM. The Saturated Fatty Acid (SFA) content (%) was highest (53.9) in MAF fed treatment. The Mono Unsaturated Fatty Acid MUFA content (%) was highest (25.6) in MAF fed treatment. Both the Poly Unsaturated fatty acid (PUFA) and the n-3/n-6 ratio were highest in ETM fed fish (Table 4).
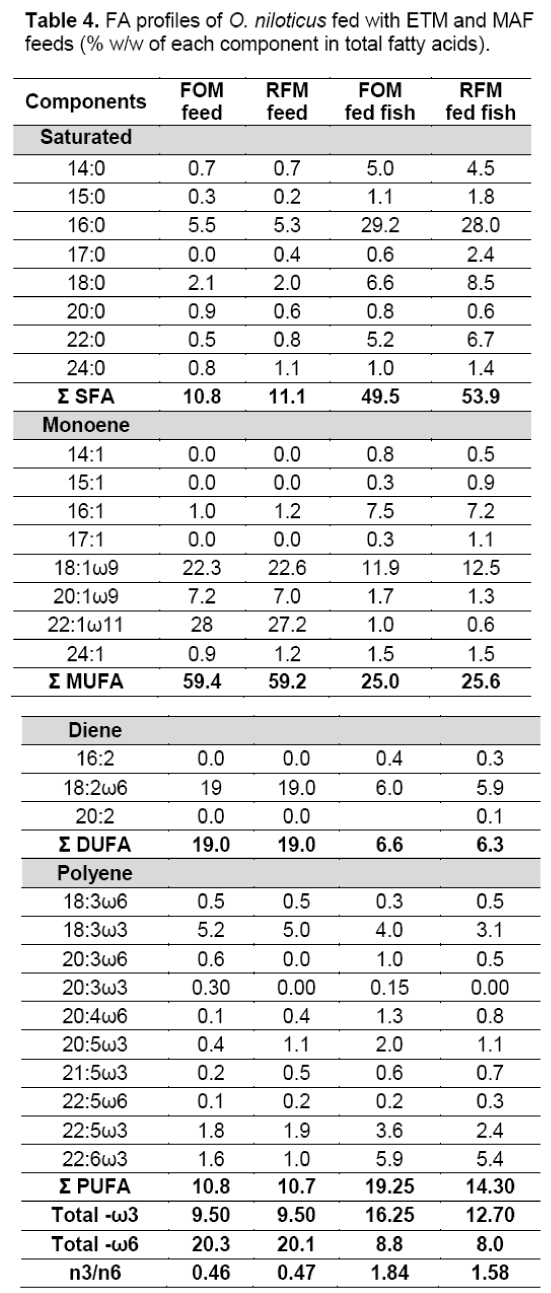
Only 14 fatty acids are really needed to describe the fatty acids of fish [28]. However,Ackman et al [29] listed 64 fatty acids from 5 fresh water fishes of West Bengal,India. The fish under discussion recorded 28 fatty acids of the total lipid (TL) and the result is more or less similar to those reported from other tropical and certain temperate zone fresh water fishes. The dominant fatty acids in lipids of all the fishes were myristic (14:0),palmitic (16:0),stearic (18:0),palmitoleic (16:1ω7),oleic (18:1ω9),linoleic (18:2ω6),linolenic (18:3ω3),arachidonic (20:4ω6),eicosapentaenoic (20:5ω3) and docosahexaenoic (22:6ω3) acids [28]. The present results corroborate with the above findings. The total SFA of the experimental fish was nearly double than the amount reported by Ackman et al [29].
Fatty acid deficiency in fish species is indicated by the presence of eicosatrienoic acid (20:3n-9) [30].Thus,the absence of eicosatrienoic acid in these fish indicates that these fish are not suffering from any fatty acid deficiency. This observation corroborates with the findings of Nematipour and Gatlin [30]for hybrid striped bass in the USA [31].
The n-3 PUFA is the chief group of components through which the beneficial effects of fish are mediated. The principal effects of n-3 PUFA are antithrombogenic and antiarrhythmic,whereas that of n-6 PUFA is antiatherogenic [32,33].The n3/n6 ratio should range 1–2 for fresh water fish [29]. The n3/n6 ratio of the experimental fish was within the same range.
4. Conclusion
The fish fed with ETM accumulates more n-3 fatty acids than n-6 fatty acids which increase n-3/n-6 ratio (1.84) in return. The feed prepared from earthworm enhance growth and thereby yield of Oreochromis niloticus. It improves quality of fish by accumulating more n-3 PUFA in the flesh of the fish as well as increasing the n3/n6 ratio which is beneficial for human health. Moreover,the feed can be formulated at local level leading to employment generation in rural areas
Acknowledgements
The authors are grateful to Kamalesh Kumar Misra,Ex. Professor,Culcutta University,India,for providing his valuable support in preparing this manuscript.
References
- Yegorov Y.E.,Terekhov S.M.,Vishnyakova K.S. et al. (2003) Telomerization as a method of obtaining immortal human cells preserving normal properties. Ontogenez,34: 183-192. [Article in Russian].
- Bairagi A.,Sarkar Ghosh K.,Sen S.K.,Ray A.K. (2002) Duckweed (Lemna polyrhiza) leaf meal as a source of feedstuff in formulated diets for rohu (Labeo rohita Ham.) fingerlings after fermentation with a fish intestine bacterium. Biores. Technol.,85: 17-24.
- Rangacharyulu R.V.,Giri S.S.,Paul,B.N.,Yashoda K.P.,JagannathaRao R.,Mahendrakar N.S.,Mohanty S.N.,Mukhopadhyay P.K. (2003) Utilization of fermented silkworm pupae silage in feed for carps. Bioresource Technology.86: 29–32.
- Hammoumi A.,Faid M.,Yachioui H.,Amarouch H. (1998) Characterization of fermented fish waste used in feeding trials with broilers. Process Biochemistry.32: 423–427.
- Esteban M.B.,Gracia A.J.,Ramos P.,Mcrquez M.C. (2007) Evaluation of fruit-vegetable and fish wastes as alternative feedstuffs in pig diets. Waste Management. 27: 193–200.
- Mondal K.,Kaviraj A.,Mukhopadhyay P.K.,Datta M.,Sengupta C.,(2007) Evaluation of fermented fish-offal in formulated diet of the Indian major carp,rohu,Labeo rohita (Hamilton). Acta Ichthyologica et Piscatoria. 37: 99–105.
- Fagbenro O.A.,Jauncey K. (1995) Growth and protein utilization by juvenile catfish(Clarias gariepinus) fed dry diets containing co-dried lactic acid-fermented fish silage and protein feedstuffs. Bioresource Technology. 51: 29–35.
- Nwanna L.C. (2003) Nutritional value and digestibility of fermented shrimp head waste meal by African catfish Clarias gariepinus. Pakistan Journal of Nutrition. 2: 339–345.
- Bertsch A.,Coello N. (2005) A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Bioresource Technology. 96: 1703–1708.
- Mondal K.,A. Kaviraj A.,Mukhopadhyay P.K. (2008) Evaluation of fermented fish-offal in the formulated diet of the freshwater catfish,Heteropneustes fossilis. Aquaculture Research.39: 1443–1449.
- Horrobin D.F.,Manku. M.S. (1990) Clinical biochemistry of essential fatty acids.In D. F. Horrobin (Ed.),Omega-6 essential fatty acids.Pathophysiology and roles in clinical medicine (pp. 21–53). New York: Wiley-Liss.
- Mukhopadhyay P.K. (2009) Fish as a nutritional source of long chain fatty acids. Science and Culture.75: 53–60.
- Connor W.E.(2000) Importance of n-3 fatty acids in health and disease. Am J Clin Nutrit.71: 1715-1755.
- Arts M.T.,Ackman,R.G. Holub,B.J. (2001) Essential Fatty Acids in Aquatic Ecosystems: A Crucial Link between Diet and Human Health and Evolution. Canadian Journal of Fisheries and Aquatic Sciences. 58: 122-137.
- Crawford M.A.,Cunnanes S.C Harbige L.S. (1993) A New Theory of Evolution: Quantum Theory,” In: A. J. Sinclair and R. Gibson,Eds.,Proceedings of the 3rd InternationalCongress on Essential fatty acids and Eicosanoids,Association of Official Analytical Chemists Press,Adelaide,Australia,pp. 87-95.
- Steffens W. (1997) Effects of Variation in Essential Fatty Acids in Fish Feeds on Nutritive Value of Freshwater Fish for Humans,” Aquaculture. 151: 97-119.
- Cengiz E.I.,Unlu E.,Bashan M. (2003) The effect of dietary fatty acids on the fatty acid composition in the phospholipids fraction of Gambusia affinis. Turkish J. Biol.27: 145–148.
- Mondal K.,Kaviraj A.,Mukhopadhyay P.K. (2006) Fish waste in urban and suburban markets of Kolkata: problems and potentials. Aquaculture Asia.11: 22–25.
- Bag M.P.,Mahapatra S.C.,Rao P.S.,Chakrabarty,D.,Pal H. (2012) Nutritive Potential of Earthworm (Eisenia foetida) Meal in the Diet for Nile Tilapia (Oreochromis niloticus) Fingerlings. Int. Res J Pharm. App Sci. 2:117-123.
- AOAC (Association of Official Analytical Chemist) (2000) Official methods of analysis,17th edition.Association of Analytical Chemists,Gaithersburg,Maryland,USA.
- Giri S.S.,Sahoo S.K.,Sahu A.K.,Mukhopadhyay P.K. (2000) Nutrient digestibility and intestinal enzyme activity of Clarias batrachus (Linn.) juveniles fed on dried fish and chicken viscera incorporated diets. Bioresource Technology.71: 97–101.
- Maynard L.,Loosil J.,Hintz H.,Warner R. (1979) In C.R. Zappa (Ed.),Animal nutrition (7th ed.,pp. 13–14). New York: McGraw-Hill.
- Bligh E.G.,Dyer W.J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol.37: 911–917.
- Christie W.W. (1982) In Lipid Analysis 2nd Edn. Pergamon Press,Oxford (England).
- Mangold H.K. (1969)In Thin Layer Chromatography (E. Stahl Ed.),Springer,New York,155pp.
- Misra S.,Ghosh A.,Dutta J. (1984) Production and composition of microbial fat from Rhodotorulaglutinis. J. Sci. Food. Agric.35: 59–65.
- Duncan D.B. (1955) Multiple range and multiple F-tests. Biometrics. 11: 1–42.
- Samaddar A.,Mondal K.,Kaviraj A. (2011) Evaluation of Fermented Mixture Containing Fish Offal Meal in Compound Diets for the Freshwater Catfish Mystus vittatus (Bloch). Proc Zool Soc. 64: 117–123.
- Ackman R.G. (2000) Fatty acids in fish and shell fish. In: Chow,C.K. (Ed.),Fatty Acids in Foods and their Healh Implication. M. Dekker,Inc,N.Y. and Basel,pp. 153–172.
- Ackman R.G.,Mcleod C.,Rakshit S.,Misra K.K. (2002) Lipids and fatty acids of five freshwater food fishes of India. J. Food Lipids.9:127–145.
- Watanabe,T. (1982) Lipid Nutrition in Fish. Comparative Biochemistry and Physiology.73: 3–15.
- Nematipour G.R.,Gatlin III D.M. (1993) Requirement of hybrid striped bass for dietary (n-3) highly unsaturated fatty acids. J. Nutr.123: 744–753.
- Dewailly E.E.,Blanchet C.,Gingras S.,Lemieux L.,Sauve L. (2001) Relations between n-3 fatty acid status and cardiovascular disease risk factors among Quebecers. Am. J. Clin. Nutr.74: 603–611.
- Christensen J.H.,Skou H.A.,Fog L.,Hansen V. (2001) Marine n-3 fatty acids,wine intake,and heart rate variability in patients referred for coronary angiography. Circulation.103: 651–657.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences