Aspect s in Early Colonization Processes by AMF Glomus intraradices of “in vitro” Sweetpot ato, Cucumber and Maize Root and Callus Cultures
Ana Rosu, Aurelia Brezeanu, Cristian Banciu, Raluca Stoiculescu, Silvana Danaila-Guidea
1University of Agronomical Sciences and Veterinary Medicine, Faculty of Biotechnologies, 59, Bd. Marasti, Sector 1, 011464, Bucharest, Romania
2Institute of Biology-Romanian Academy, 296, Spl. Independentei, Sector 6, Bucharest, Romania
- *Corresponding Author:
- Tel: +4 (0)720845885
Fax: +4 (0)21 6425801
E-mail: anabiotech@yahoo.com
Abstract
The aim of our researches was to follow the establishment of the symbiotic associations in “in vitro” dual cultures. The partners consisted in root cultures with continuous growth and calli derived from the root tissues in Ipomoea batatas, Zea mays, and Cucumis sativus and spores of the VAM fungus Glomus intraradices. Our results highlighted that following the processes of dedifferentiation in other cell types, specific to cells and tissue culturing in artificial conditions, the plant cells preserve not only their intrinsic capacity of morphogenetic capacity, but also the capacity of biochemical totipotency. This is expressed by signal exchanges and recognition between the plant cells and the VAM fungus, thus permitting the establishment of the symbiosis.
Keywords
Arbuscular mycorrhizal symbioses; Callus cells; Root cultures; VA fungus inoculum.
1. Introduction
The significant role of mycorrhizal symbiosis as an alternative strategy for a more rational sustainable agriculture is widely accepted at present. The rhizosphere is a dynamic environment and the interactions working at this level have substantial impact on plant growth and development processes. A number of mutually beneficial relationships between plants and microorganisms affect agricultural productivity and the growth of plants in general. Among these, mycorrhizae, the intricate associations that roots establish with specific fungal groups are by far the most frequent and well-known for their function in the efficient exploitation of soil mineral resources and their bioprotective role against a number of common soilborne pathogens. Thanks to mycorrhizal symbiosis and nutrient exchanges, regulated by complex molecular signals, the plant improves its vegetative growth, while the fungus accomplishes its life cycle [1;5]. The potential of mycorrhizae as bioregulators, biofertilizers and bioprotectors to enhance plant production and fitness offers new tools to land managers for regulating the ecosystem functioning. Mycorrhizae are successful in colonizing diverse environments and their ecological success reflects a high degree of diversity in the genetic and physiological abilities of the fungal endophytes. Gross changes in root morphology are not generally seen in VAM (vesicular arbuscular mycorrhizal) symbioses, although subcellular modifications are extensive, involving especially cell walls, membranes and cytoskeleton of both partners [3;4]. It is a continuous "molecular dialogue" between plant and fungus, which function through the exchange of recognition and acceptance signals [2]. Analyzing the molecular bases of the dialogue between the two partners is a challenging research topic that still remains open to future genomic projects, having in view the biotrophic status of the symbiotic fungi, their multicellular condition and an unexpected level of genetic variability [6]. Therefore it is still a need of recording imminent advances in understanding of the molecular and cellular mechanisms of the coordinated regulation of developmental and metabolic gene expression in symbiotic partners.
A thorough knowledge of specific adaptations in plant-microbial interactions of mycorrhizal type is critical for successful attempts to obtain an optimum symbiotic efficacy. In natural conditions the mycorrhizal fungi as obligate symbionts express a high degree of specificity only for the epidermal and for the root cortex cells, but their interaction with parenchymatous callus cells derived from the root tissues is a less studied aspect up to now, though the practical connotations in producing VAM fungus inoculum is of upmost importance [7].
2. Methods
2.1 Plant material
(1) Callus cultures induced from Zea mays roots
The explant sources consisted in plantlets obtained by the germination of corn kernels in aseptic conditions, on a Murashige & Skoog basal medium (MS) in the absence of growth regulators; the surf ace sterilization of the kernels was performed by using a 10% Domestos solution, for 10 minutes; the plantlet roots were detached and were used as explants for callus initiation.
The callus cultures were estsblished on MS basal medium supplemented with:
2 mg/l 2,4-D (2,4 dichlorophenoxyacetic acid)
1 mg/l 2,4,5 T (2,4,5 trichlorophenoxyacetic acid)
(2) Callus cultures induced from Cucumis sativus roots
The explant sources consisted in plantlets obtained by aseptic germination of Cucumis sativus seeds as described above; the plantlet roots were detached and cultured on a MS basal medium with the following hormonal supplement:
1.1 mg/l 2,4-D;
0.9 mg/l NAA (naphthalene acetic acid);
0.45 mg/l BAP (benzyl amino purine).
(3) Root cultures with continuous growth in Ipomoea batatas
Roots of sweetpotato micropropagated plantlets were cultured on MS basal medium supplemented with 0.2 mg/l TDZ (Thidiazuron ).
2.2 Fungal inoculum
For the co-cultivation of the plant material with mycorrhizal fungi non-sterile commercial product from BIORIZE (France)- "AMF for Basic Research" - was used, consisting in approximately 3000 Glomus intraradices spores in 50 ml suspension.
For a partial sterilization of the spore suspension the antibiotic Cephotaxime was used, in concentration of 25 mg/50 mI; aliquots of 1 mI were taken from this suspension for the co-cultivation of the fungal spores (approx. 60 spores/ml) with the 3 plant species taken into study as regards the interaction with the mycorrhizal fungus.
2.3 Histological studies
The histological studies in light microscopy were carried out by using the staining procedure with trypan blue, after a prior preparation of the sweetpotato roots, as follows:
fixation in FAA (formalin-acetic acid-ethanol);
clearing in 10% KOH solution;
staining with 0.05% trypan blue in lactic acid.
3. Results and Discussion
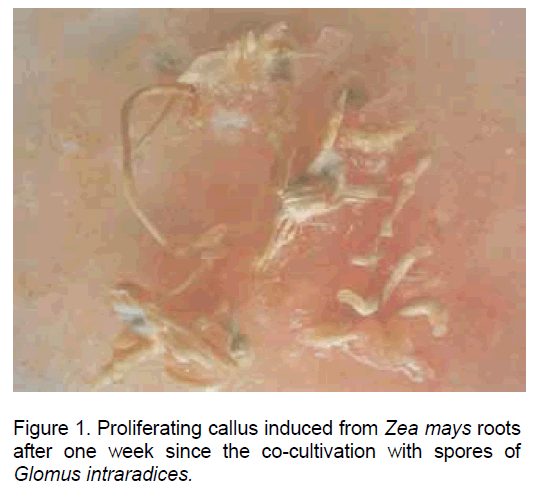
Following the inoculation of maize root explants on the culture medium mentioned above a pronounced hypertrophy and the initiation of callus development were recorded after one week since culture initiation. The auxins introduced in the culture medium stimulated both cell proliferation and the development of new root, some of them from the callus, thus making this type of "in vitro" culture especially suitable for the infection with mycorrhyzal fungi (Figure 1).
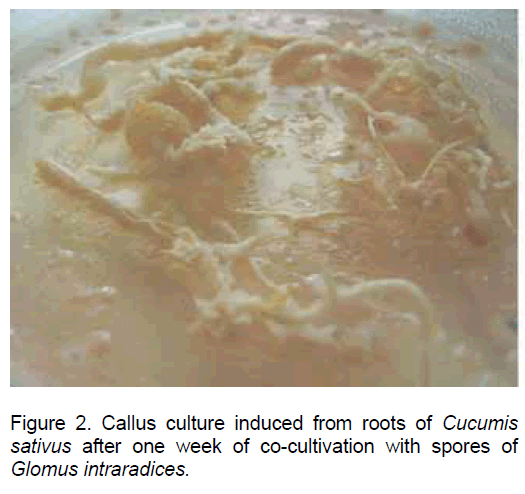
In the root explants of Cucumis sativus the process of dedifferentiation was evident after 3 days since inoculation, and later on all the root tissues were engaged in the callus development process (Figure 2).
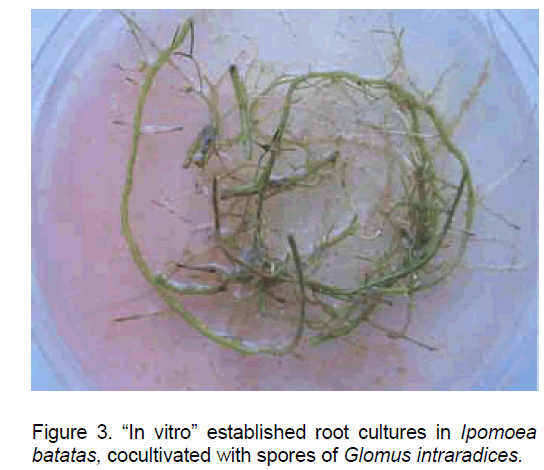
The sweetpotato root explants cultured in the presence of TDZ resulted in green root cultures with continuous growth, which actively developed new secondary roots (Figure 3).
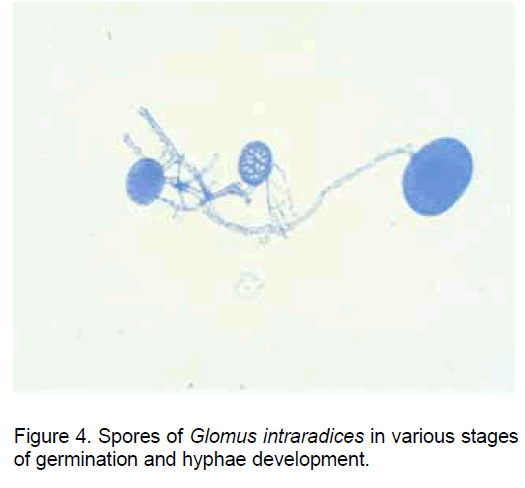
Though the spores of AM fungi have the capacity to initiate the germination in the absence ob the host plant (the non-symbiotic stage) (Figure 4), the hypha elongation stops and an apoptopic process is installed even in the pre-colonization stage if in the proximity there are not roots of a plant species compatible with the establishment of the symbiosis.
In the pre-symbiotic stage, though the fungal hypha does not reach in physical contact with the plant root surface, the signal system between the two partners starts to express and an important role from this point of view have the root exudates and the carbon dioxide [4,5].
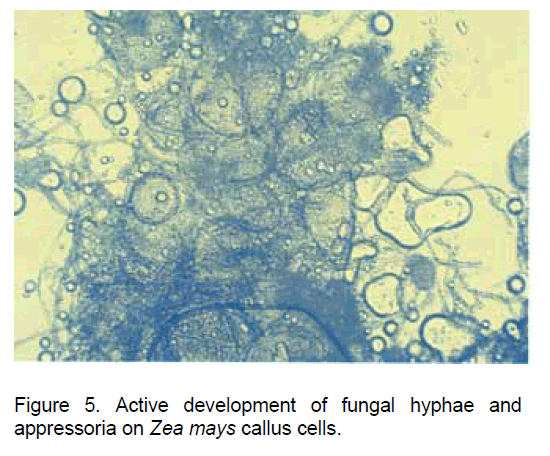
In our experimental variant having the Glomus intraradices spores co-cultivated both with the callus cells developed from Zea mays root explants and with the new root structures developed "in vitro", the spores germinated in a one week interval and developed physiologically active hyphae (Figure 5).
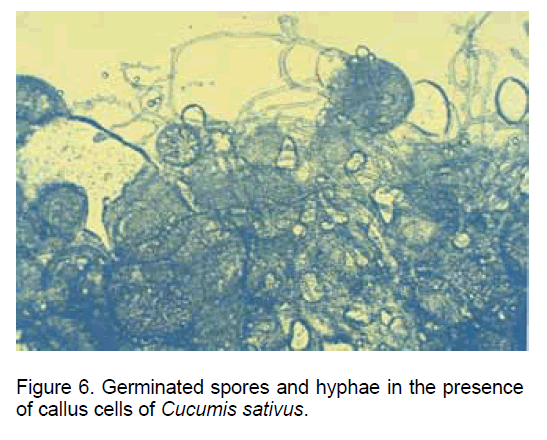
A similar situation was recorded in the case of co-cultivating the fungal spores with the callus cells derived from "in vitro" cultivated Cucumis sativus roots (Figure 6).
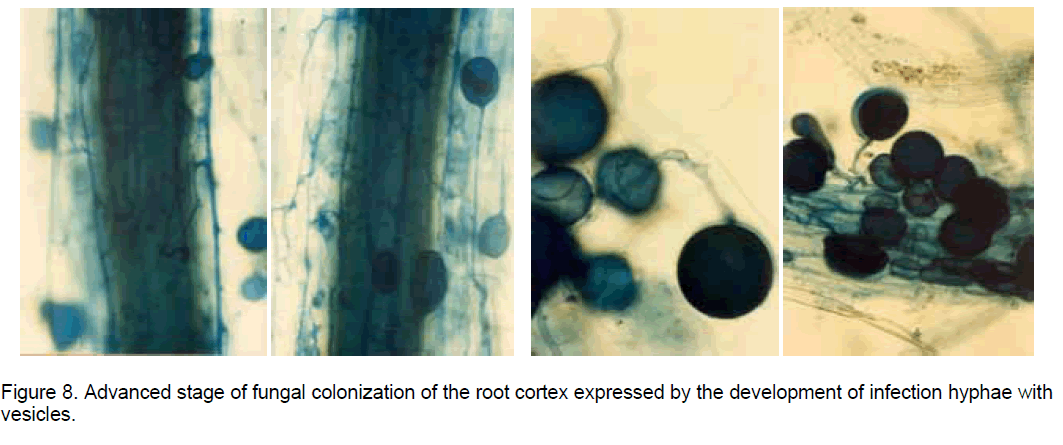
The histological observations on the "in vitro" established root cultures in Ipomoea batatas (sweetpotato) revealed that after the fungal hypha resulted by the spore germination came in contact with the root surface, the colonization stages developed similarly with the process described in natural conditions, when the propagules in the soil, represented by resistance spores or by fragments of mycorrhizal roots, meet the roots of a compatible plant [1]. Once in contact with the root epidermal cells the hypha tip develops an appressorium (Figure 7), from which an infection hypha is forming, which penetrates deep in the root cortex. Here the hyphae form vesicles which are oval-to-spherical sac-like structures with a storage function (Figure 8), coils or highly branched arbuscules (considered intracellular haustoria), playing a key role in reciprocal nutrient exchange between the plant cells and the AM fungal symbionts.
The formation of appressoria is induced by root exudates and is the first morphological sign of recognition between the plant and the fungus. Root exudates have been shown to stimulate growth and branching of the AM fungal hyphae and some flavonoid compounds were described as major components of plant root exudates which are capable of promoting hyphal growth [6,7].
4. Conclusions
The mycorrhyzal fungi demonstrated a high degree of specificity for the root epidermal and cortex cells. Consequently, the investigation of the establishment of the symbiotic associations "in vitro", in dual cultures, using as partners root cultures with continuous growth, callus cultures derived from root tissues and spores of Glomus intraradices represents a research topic less taken into study, but with important connotations of basic and practical importance. Our studies revealed that following the processes of dedifferentiation and redifferentiation in other cell types, specific to the culturing of plant cells and tissues in artificial conditions, the plant cells preserve not only the morphogenetic totipotency but the biochemical totipotency as well, expressed in this case by the signal exchanges and the recognition between the plant cell and the AM fungus, thus allowing the establishment of the symbiosis.
References
- Azcon - Aguilar C., Barea J.M. (1977) Applying mycorrhiza biotechnology to horticulture: significance and potentials. Scientia Horticulture 68: 1-24.
- Jackson A.O., Taylor C.B. (1996) Plant – microbe interactions; life and death at the interface. The Plant Cell 8:1651-1668.
- Genrea A., Chabaud M., Timmers T., et al. (2005) Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. The Plant Cell 17: 3489-3499.
- Gianinazzi-Pearson V. (1996) Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. The Plant Cell 8: 1871-1883.
- Bonfante P. (2003) Plants, mycorrhyzal fungi and endobacteria: a dialog among cells and genomes. Biol. Bull. 204: 215-220.
- Martin F. (2001) Frontiers in molecular mycorrhizal research - genes, loci, dots and spins. New Phytologist 150: 499 - 507.
- RHODES L.H. (1984) Mycorrhizae. In: Handbook of Plant Cell Culture, Eds. Evans D.A., et al., pp. 924- 940.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences