Antitrypanosomal Property of Some Extracts of Different Parts of Moringa oleifera, Lam
Sunday E. Atawodi, Hassana Shehu
Sunday E. Atawodi* and Hassana Shehu
Biochemistry Department, Ahmadu Bello University, Zaria, Nigeria
Abstract
Different extracts of Moringa oleifera parts were evaluated for their anti-trypanosomal activity and broad phytochemical classes. Alkaloids, resins and saponins were detected. In vitro, the petroleum ether extract of the root bark, chloroform extract of the stem bark, methanol extracts of the stem and the aqueous extracts of all parts were active at 4 and 2 mg/ ml doses. The chloroform and methanol extracts of the leaves, and the chloroform extract of the root also possessed strong activity. In vivo, methanol and aqueous extracts of the leaves, stem and root barks at 300mg/kg displayed significant effect on parasitaemia and survival period, indicating their anti-trypanonosomal potential.
Keywords
Medicinal plant; antitrypanosomal effect; Trypanosomiasis; Moringa oleifera; Drumstick.
1. Introduction
Sleeping sickness, now commonly referred to as Human African trypanosomiasis (HAT), is caused by Trypanosoma brucei gambiense which is responsible for chronic form of HAT in West and central Africa, and Trypanosoma brucei rhodesiense, the etiological factor for acute form of the disease in East Africa [1]. The prevalence of the disease is 300,000 – 500,000 [2]. Despite the high prevalence rate, only a few drugs, namely, melarsoprol, Suramin, pentamidine and efflornithine are available for its treatment.
Recent approaches to alternative therapeutic agents for treatment of trypanosomiasis have focused on plants and other natural products [5-9]. In this investigation, we assess the phytochemical as well as the in vitro and in vivo antitrypanosomal potential of a common Nigerian medicinal plant, Moringa oleifera.
These drugs are old, toxic, expensive and not readily available [1]. In addition, resistances to these major drugs as well multiple drug resistant populations have been described for different species of the parasite [3]. Relapses of unknown etiology have also been reported for melarsoprol in recent epidemics. Hence, there is urgent need to seek for new sources of therapeutic agents [4].
Moringa oleifera, Lam. Known in Hausa and Igala languages of Nigeria as zogale and gargedi, respectively, and drumstick in English, is one of the thirteen species belonging to the genus, Moringa, the others being, M. drouhardii, M. hildebrandtii, M. rivae, M. ovalifolia, M. stenopetale, M. concanesis, M. arborea, M. peregrina, M. borziana, M. longituba, M. pygmaea, M. ruspo. It is a short, slender , deciduous, drought resistant, perennial pan-topical tree of the family Moringaceae which grows up to about 10m in height [10]. The leaves are widely consumed as human and animal food in north and central Nigeria [11], as well as in other parts of the world [12, 13]. In Indian folk medicine, it is used as an anti-diabetic [14], while in Nigeria it is locally used as tonic and aphrodisiac, and in the treatment of intestinal worms and asthma.
Studies in the last decade have indicated that the plant possess antiplasdmodial activity [15], chemomodulatory effect in hepatic metabolizing enzymes [16], hepatoprotective and radioprotective capacities [17] and thyroid hormone regulatory properties [18]. Other biological activities associated with Moringa oleifera include hypocholestreolemic action [19], hypotensive [20] and antifungal effects [21], arbortificient [22] and antispasmodic properties [23]. The flowers, leaves and roots are used in folk medicine for treatment of tumours of different sites, and the seed for abdominal tumours [24].
Despite this widespread medicinal importance of Moringa oleifera, no report appears to exist with respect to its possible antitrypanosomal action. Therefore, this study was designed to study the major classes of phytochemicals present and the antitrypanosomal potential of different solvent extracts of wide ranging polarities of the different parts of the plant.
2. Materials and Methods
Plant materials, chemicals and instruments
The plant was collected from Samaru-Zaria in Kaduna State of Northern Nigerian in March 2004. The Crop Residue Program of the National Animal Production Research Institute (NAPRI), Ahmadu Bello University, Zaria, Nigeria confirmed the identity of the plant with Voucher No1011. All reagents and solvents used were of analytical grade.
Sample Preparation and Extraction
Appropriate parts of the plant were harvested, dried under the shade or in open air in the laboratory. Dried materials were pounded in laboratory mortar into small particles. Fifty grams (50g) of the pounded dried plants materials were weighed and extracted with 3 X 150ml methanol on Wrist Action Shaker following prior similar extraction with petroleum ether and chloroform. The extracts were dried under electric fan and stored in a refrigerator at 4oC until required. Aqueous extract was obtained by a 2 hr shaking of the methanol-extracted materials with water and drying the filtrate obtained through glass wool in water bath maintained at 45 °C. In all cases, first extraction with each solvent was conducted following soaking in the appropriate solvent and storing in the refrigerator for 18 – 24 hours.
Test Organism
Trypanosoma brucei brucei was the test organism used. It was obtained from stabilates maintained at the Nigerian Institute for Trypanosomiasis Research (NITR), Vom, Plateau State, Nigeria. The parasite was maintained in the laboratory by continuous passage in rats until required. Passage was considered necessary when parasitaemia was in the range of 16 – 32 parasites per field (usually 3 - 5 days post infection in rats and 8 – 12 days in mice). In passaging, 1 x 104 parasites were introduced intraperitoneally or intramuscularly into rats in 0.1 - 0.2ml blood / PBS solution. For several passages, approximately 90% blood solution (v/v) was obtained by cardiac puncture into 1ml syringe containing 0.1ml EDTA (1%w/v). About 0.1 - 0.2ml of the blood collected as described above or blood (diluted with PBS to contain approximately 1 x 104 Parasite/ml) was injected into clean animals acclimatized under laboratory condition for at least two weeks. For mice used in the in vivo experiment, about 0.05 ml of the inoculant was used to avoid sudden upsurge in parasitaemia [8].
Determination of Parasitaemia
Parasitaemia was monitored in blood obtained from the tail, pre-sterilized with methylated spirit. The number of parasites was determined microscopically at X 400 magnification using the “Rapid Matching” method of Herbert and Lumsden25. Briefly, the method involves microscopic counting of parasites per field in pure blood or blood appropriately diluted with buffered phosphate saline (PBS, pH 7.2). Logarithm values of these counts obtained by matching with the table of Herbert and Lumsden25 is converted to antilog to provide absolute number of trypanosomes per ml of blood [7, 8].
In Vitro Test for Antitrypanosomal Activity
Exactly 10mg of the different plant extracts were weighed into Eppendorf tubes and first dissolved in 0.1ml of 10% dimethylsulfoxide (DMSO) in PBS, before further dilution with PBS to produce extract solutions of 20.0mg/ml (stock). Extract concentration of 10.0 mg/ml was prepared from the ‘stock’ extract solution by appropriate dilution with PBS. Extract solutions were prepared just before use.
Assessment of in vitro anti-trypanosomal activity was performed as per the rapid but subjective method of Herbert and Lumsden [25] in triplicates in 96 well micro titer plates (Flow laboratories Inc., Mclean, Virginia 22101, USA). In wells of micro titre plates, 20μl of blood containing about 20-25 parasites per field obtained as described under “determination of parasitaemia” was mixed with 5 μl of extract solution of 20.0mg/ml or 10.0mg/ml to produce effective test concentrations of 4mg/ml and 2mg/ml respectively. These effective concentrations were selected to provide basis for comparison with published data [8, 26]. To ensure that the effect monitored was that of the extract alone, a set of control was included which contained the parasite suspended in 2% DMSO only. For reference, tests were also performed with the same concentrations of DiminalR (445mg diminazene diaceurate+ 555mg phenazone /g, Eagle Chemical Company LTD, Ikeja, Nigeria) - a commercial trypanocidal drug.
After 5 minutes incubation in closed Eppendorf tubes maintained at 37 °C, about 2μl of test mixtures were placed on separate microscope slides and covered with cover slips and the parasites observed every 5 minutes for a total duration of sixty minutes. It should be noted that under this in vitro system adopted, parasites survived for 3 - 4 hours when no extract was present. Cessation or drop in motility of the parasites in extract-treated blood compared to that of parasite-loaded control blood without extract was taken as a measure of anti-trypanosomal activity [8, 26]. All analyses were performed in triplicates.
In Vivo Assay
Following in vitro studies, the chloroform extract of the leaves and the stem bark, and petroleum ether, methanol and aqueous extracts of the roots which displayed strong anti-trypanosomal activity in vitro were selected for in vivo evaluation. Mice inoculated with Trypanosoma brucei brucei were intramuscularly treated with 300 mg/kg body weight of the extracts when average parasitaemia was approximately one parasite per field. Preliminary investigation indicated relatively poor efficacy with 100 and 200 mg/kg doses of the extracts. The treatment continued daily with continuous monitoring of parasitaemia for 10 days. After withdrawal of treatment, parasitaemia was also monitored daily until the 14th day, and thereafter monitoring was reduced to thrice a week, for surviving animals. Six animals were used per treatment group. An infected but untreated group was included as a negative control, while another control group in which the animals were treated with a standard drug (DiminalR ) was also included.
Reagent – based Phytochemical Evaluation
Basically, the methods outlined by Trease and Evans [27] were utilized. Alkaloids were tested with Dragendorff’s Mayers and Wagner’s reagents, while carbohydrate and steroidal rings / triterpenes were evaluated with Molisch test and Salkowski/ Lieberman Burchard’s tests respectively. Antraquinones and saponin derivatives were confirmed by Borntranger’s and Frothing tests, respectively. The presence of flavonoids were established by a battery of tests, including , sodium hydroxide test, ferric chloride test, lead acetate test and Shinod’s test, while tannins were determined by a combination of gelatinous salt block test on 5% infusion, ferric ammonium citrate test, lead sub-acetate test and bromine water test. Presence of resins was evaluated with acetic anhydride-sulphuric acid reaction. All analyses were performed in duplicates.
3. Results
3.1 Reagent – based Phytochemical Evaluation
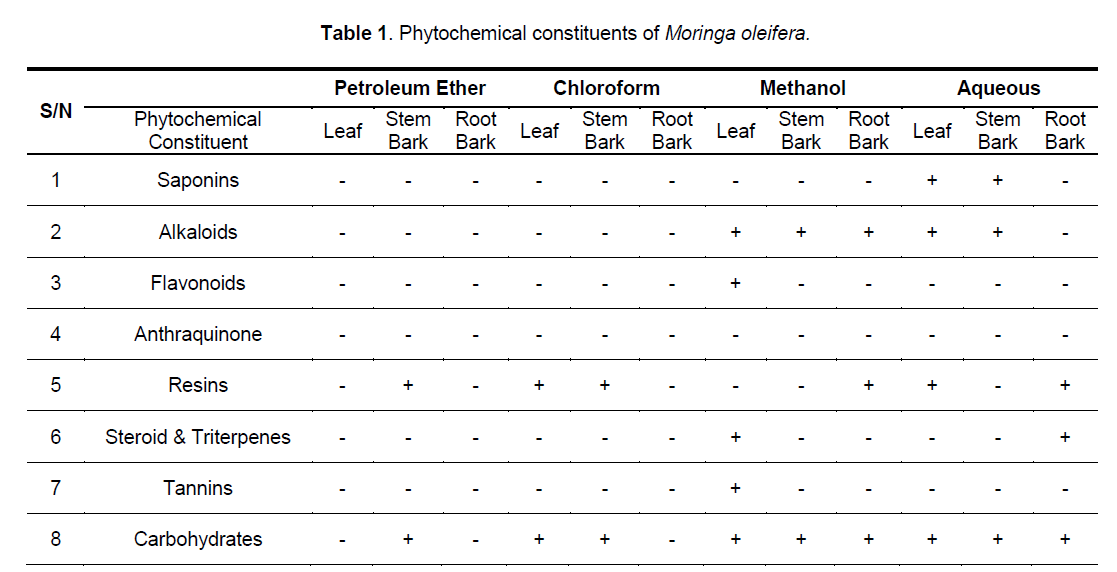
The stem bark contains anthraquinones and carbohydrate (Table 1). The chloroform extract of leaves, stem and root barks contained no detectable level of saponins, alkaloids, flavonoids, tannins and steroids / triterpenes, but carbohydrate and resins were present in the extracts of the leaves and stem bark. The methanol extract of the three parts contains alkaloids and carbohydrate, but only that of the leaves contained flavonoids, steroids/ triterpenes and tannins, while only the root methanol extracts contained resins. The aqueous extract of the leaves and stem bark are rich in saponins, alkaloids and carbohydrate, but only that of the root bark contained steroids/triterpenes, and only the roots and leaves chloroformic extract contain resins.
3.2 In vitro Anti-trypanasomaleffect of M. oleifera extracts
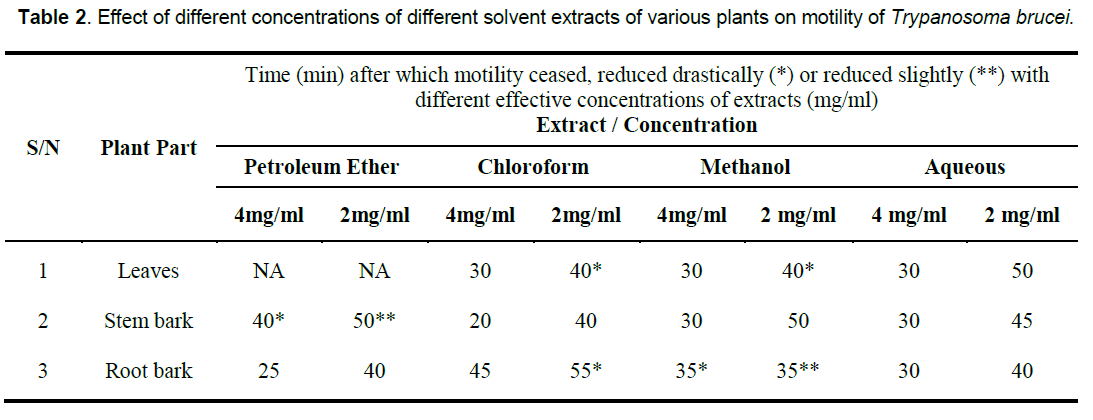
In vitro, the petroleum ether extract of the root bark, chloroform extract of the stem bark, methanol extracts of the stem as well as the aqueous extracts of all the parts were clearly active at the two concentrations tested. The chloroform, and methanol extracts of the leaves as well as the chloroform extract of the root also displayed strong in vitro anti-trypanosomal activity at 4mg/ml. However, the petroleum extract of the leaves, and to some extent, that of the stem bark, and methanol extract of the root bark showed little or no effect on the trypanosomes under the conditions of the experiment (Table 2).
4. Discussions and Conclusions
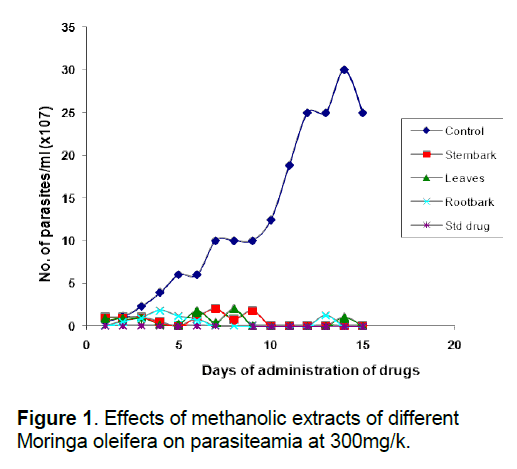
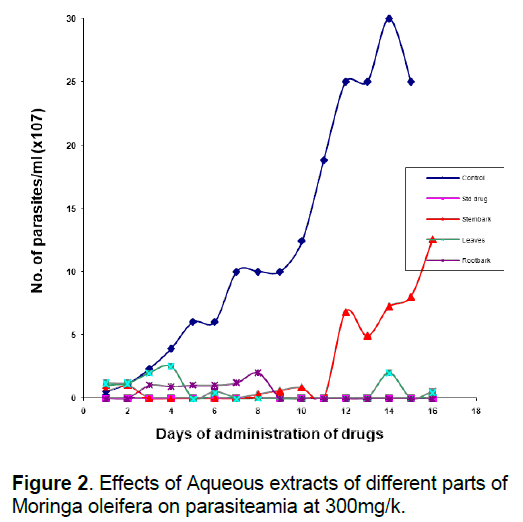
That the anti-trypanosomal effect observed under in vivo condition following administration of extracts of Moringa oleifera (Figure 1, 2) is attributable to the extracts, appears to be confirmed by the death of all members of the control group that were infected with the parasite but left untreated within 10 days of infection, while most others survived for more than 7 days after the extract administration was terminated. The in vivo results for the methanol and aqueous extracts are consistent with the in vitro results which show that both extracts of the various parts were highly active compared to the control, although the in vivo activity of the methanol extracts were a little higher than would have been predicted from the in vitro results (Table 2). This would appear to underscore our earlier observation [8, 26] that in vitro efficacy or otherwise of a natural product and indeed, all therapeutic agents, may not necessarily replicate the situation under in vivo conditions because of differences in metabolic disposition of different compounds.
That extracts which are active both under in vivo and in vitro conditions (Table 2, Figure 1, 2) contain detectable amount of alkaloids (Table 1) which is relatively absent in none-active extracts may suggest that this group of bioactive compounds may play some role in the anti-trypanosomal action observed. Similarly, resins which are consistently present in the methanol and aqueous extracts of the roots may also play a role in its anti-trypanosomal activity. Similarly, aqueous extracts, and to some extent, methanol extracts which were particularly active in vitro for all parts were rich in saponins and alkaloids for leaves and stem bark, and resins for/steroids for the root bark (Table 1). It is remarkable, that chloroformic extract of the leaves and stem bark which also contain resins showed good activity in vitro (Table 2). While it is possible that these bioactive components act in concert or synergistically towards the observed antitrypanosomal action, detailed bioassay-guided fractionation is required to establish the principal phytochemical constituents responsible. However, it is noteworthy that bioassay-guided analyses of ethanol extract of Moringa oleifera leaves and seeds have revealed the presence of fully acetylated carbamate and thiocarbamate glycosides [20, 28, 29].
The weakness observed in the mice of different groups with continuous administration of the extracts; even after parasites were eliminated from the blood stream suggest that the extracts may have some cumulative toxic effects at the high dose used. However, put together, these results suggest that Moringa oleifera possess significant anti-trypanonosomal effect to warrant further detailed studies utilizing bioassay-guided fractionations under varied pharmacological conditions in order to unequivocally establish its therapeutic efficacy, active ingredient (s) and toxicological properties. This is already underway in our laboratory.
References
- Brun R., Schumacher R., Schmid C., et al. (2001) The phenomenon of treatment failures in Human African Ttrypanosomiasis. Trop. Med. Intl. Health, 6(11): 906-914.
- Mhlanga, J D M. (1999) Sleeping sickness: perspectives in African trypanosomiasis. Sci Progress, 79: 183-187.
- Anene B.M., Onah D. N., Nawa Y. (2001) Drug Resistance in pathogenic African trypanosomes: What hopes for the future? Vet. Parasitol, 96: 83-100.
- Gutteridge NE. (1985) Existing chemotherapy and its limitations. Brit. Med. Bull, 41(2): 162-168.
- Freiburghaus F, Kaminsky R, Nkuna MHN, Brun R (1996). Evaluation of African medicinal for their in vitro trypanocidal activity. J. Ethnopharmacol, 55: 1-11.
- Freiburghaus F, Jonker SA, Nkuna MHN, et al. (1997) In vitro trypanocidal activity of some rare Tanzanian medicinal plants. Acta Trop, 67: 181-185.
- Atawodi S.E, Ameh D. A., Ibrahim S., et al. (2002) Indigenous knowledge system for treatment of trypanosomiasis in Kaduna state of Nigeria. J. Ethnopharmacol, 79(2): 279-282.
- Atawodi S.E., Bulus T. Ibrahim S., et al. (2003) In vitro trypanocidal effect of Methanol extract of some Nigerian Savannah Plants. Afri J. Biotechnol, 2(9): 317-321.
- Youan B.B.C., Coulibaly S., Miezan T.B., et al. (1997) In vivo evaluation of sixteen plant extracts on mice inoculated with Trypanosoma brucei Gambiense, WHO Bull, 75(4): 343-348.
- Keay, R.W.J. (1989) Trees of Nigeria. Clarendon Press, Oxford, pp. 137-140, 253-255.
- Lockett C.T., Calvert C.C., Grivetti L.E. (2000) Energy and micronutrient composition of dietary and medicinal wild plants consumed during drought. Study of rural Fulani, north eastern Nigeria. Int. J. Food Sci Nutr. 51(3):195-208.
- Sena L. P., Vanderjagt D. J., Rivera C., et al. (1998) Analysis of nutritional components of eight famine foods of the Republic of Niger Plant Foods Hum. Nutr, 2(1):127-130.
- Seshadri S, Nambiar VS. (2003) Kanjero (Digera arvensis) and drumstick leaves (Moringa oleifera): nutrient profile and potential for human consumption. World Rev. Nutr. Diet, 91: 41-59.
- Kar A, Choudhary B. K, Bandyopadhya R. (2003) Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J. Ethnopharmacol, 84(1): 105-108.
- Kohler I, Jenett-Siems K, Siems K. Et al. (2002) In vitro antiplasmodial investigation of medicinal plants from El Salvador. Z Naturforsch.C., 57(3-4): 277-281.
- Bharali R., Tabassum J., Azad M. R. (2003) Chemomodulatory effect of Moringa oleifera, Lam, on hepatic carcinogen metabolising enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pac J.Cancer Prev, 4(2):131-139.
- Rao AV, Devi PU, Kamath R. (2001) In vivo radioprotective effect of Moringa oleifera leaves. Indian J. Exptl. Biol, 39(9): 858-863.
- Tahiliani P, Kar A. (2000) Role of Moringa oleifera leaf extract in the regulation of thyroid hormone status in adult male and female rats. Pharmacol Res, 41(3): 319-323.
- Ghasi S, Nwobodo E, Ofili J.O. (2000) Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed wistar rats. J. Ethnopharmacol, 69(1): 21-25.
- Faizi S., Siddiqui B.S., Saleem R. et al. (1998) Hypotensive constituents from the pods of Moringa oleifera. Planta Med, 64(3): 225-228.
- Nwosu M. O., Okafor J. I. (1995) Preliminary studies of the antifungal activities of some medicinal plants against Basidiobolus and some other pathogenic fungi. Mycoses, 38(5-6): 191-195.
- Nath D, Sethi N., Singh R. K. et al. (1992) Commonly used Indian abortifacient plants with special reference to their teratologic effects in rats. J Ethnopharmacol, 36(2): 147-154.
- Caceres A Saravia A, Rizzo S et al. (1992) Pharmacologic properties of Moringa oleifera. 2: Screening for antispasmodic, antiinflammatory and diuretic activity. J. Ethnopharmacol, 36(3): 233-237.
- Guevara, A.P., Vargas C., Sakurai H. et al. (1999) An antitumor promoter from Moringa oleifera Lam. Mutat, 12: 188-194.
- Herbert W.J., Lumsden W.H.R. (1976) Trypanosoma brucei: A Rapid “Matching” method for Estimating the host parasitemia. Expt parasitol. 40: 427-431.
- Atawodi S. E. (2005) Comparative in vitro trypanocidal activities of the petroleum ether, chloroform, methanol and aqueous extracts of some Nigerian Savannah Plants. Afr. J. Biotechnol, 4: 177-182.
- Trease, G.E., Evans W.C. (1985) Introduction and General Methods In Pharmacognosy, 12th Edition, Published by Alden press, Oxford London pp. 469-474.
- Faizi S, Siddiqui BS, Saleem R, et al. (1995) Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochem, 38(4): 957-963.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences