Lactic Acid Bacteria Isolation, Phenotypic Characterization and Growth Kinetics of Lactobacillus Curvatus from Carob Pods Syrup
Bouhadi D, Raho B, Hariri A, Benattouche Z, Ould Yerou K, Sahnouni F
Hariri A1,*, Ouis N2, Bouhadi D1
1 Bioconversion Laboratory, Microbiology Engineering and Health Safety, Department of Biology, Faculty of Science the Nature and Life, University of Mascara, (UN 2901), BP.763, Sidi Said, Mascara, 29000, Algeria
2 Laboratory of Chemisty, Department of Chemistry, Faculty of Sciences, University of Oran Es- Sénia, BP 1524 El M’naouer, Oran, 31100, Algeria.
Received date: January 27, 2016; Accepted date: February 08, 2016; Published date: February 14, 2016
Citation: Hariri A, Ouis N, Bouhadi D, Lactic Acid Bacteria Isolation, Phenotypic Characterization and Growth Kinetics of Lactobacillus curvatus from Carob Pods Syrup. Electronic J Biol, 12:2
Abstract
Lactic acid is considered as a very important chemical compound with significant applications in pharmaceuticals, cosmetics and especially in the food industry. New applications, such as degradable plastics made from poly (lactic) acid, have the potential to greatly expand the market for lactic acid, if more economical processes could be developed. Industrial processes for the production of lactic acid typically use sucrose from cane and beet sugar, whey containing lactose and maltose and dextrose from hydrolyzed starch. The pulp of carob (Ceratonia siliqua L.) pods contains high contents of sugar (sucrose, fructose and glucose) and can be employed as a raw material for the production of lactic acid. The aim of the present study consists in the optimization of physicochemical parameters (temperature and pH) , and also the culture medium for producing lactic acid by Streptococcus thermophilus on the carob extract based medium. The optimum pH and temperature for bioactive metabolite production were 6.6 and 41°C respectively. Highest lactic acid production (29.95 g/L) was found when the strain was inoculated into the medium amended with Tween 80 at the concentration of 1%. Carob extract could serve as a good potential source of raw materials for the efficient production of lactic acid by Streptococcus thermophilus.
Keywords
Carob; Fermentation; Lactobacillus curvatus.
1. Introduction
Carob (Ceratonia siliqua L.), which has been widely grown in the Mediterranean region, belongs to the Caesalpinaceae subfamily of the family Leguminoseae [1,2]. Carob tree has an economic and environmental importance in Algeria. It is used in reforestation of arid and degraded areas and also as for ornamental purposes [3-5]. However, in recent years, it has been used in the food industry as bioresource and biomass substrate and thus has attracted the attention of producers because of increasing market value. The pulp content in the pod ranges from 73 to 95% [6]. When the fruits are ripe enough, they have 91-92% total dry matter and 62- 67% total soluble solids, which consist of 34-42% sucrose, 10-12% fructose, and 7-10% glucose [7]. Carob pods are also characterized by high sugar content 200-500 g/kg [8]. Moreover, carob pods contain appreciable amount of fiber (4.2-39.8%), depending on the type of the extracted fiber [6,9]. Carob pulp is a good source of polyphenols (mainly tannins 16-20%) [10], and protein (2.7-7.6%) but it is poor in lipid (0.4-0.8%). This study was designed not only to evaluate the potential of carob extract for lactic acid production but also to isolation of lactic acid bacteria and determine the effect of alternative addition of Sodium Acetate and Tween 80 on lactic acid fermentation.
2. Material and Methods
2.1 Vegetable material
The carob (Ceratonia siliqua L.) used in current experiments was harvested in the month of August 2014 from the region of ELBORDJ (Mascara, Algeria). The choice of this variety is justified by its availability and important nutritive value, especially the one of reducing fermentable sugars such as glucose and sucrose.
2.2 Isolation and characterization
A 25 g sample of carob pods sample was taken aseptically and transferred to sterile plastic bags and then homogenized in 225 mL of sterile buffered peptone water (BPW). Five 10-fold dilutions of the homogenates were then prepared and these were inoculated on plates of MRS agar (Oxoid) acidified with glacial acetic acid to pH 5.4 and M17 medium to pH 7.1 and incubated anaerobically at 30°C for MRS and 37°C for M17. Colonies with typical characteristics were randomly selected from plates and tested for Gram stain, cell morphology, catalase and oxidase reactions before further sugar fermentation and characterization tests [11]. The short term conservation of the pure isolates was done on solid media [12]. After growth at optimal temperature, the culture was maintained at 4°C and cultures were renewed every month. The long-term conservation of the purified isolates was carried out in MRS broth containing glycerol (Merck, Darmstadt, Germany) (v/v 70/30) and were maintained as frozen stocks at -80°C [13]. Strains were characterized according to the methods and criteria of Buchanan [14] and Klein [15] as follow: They were checked for gas production from glucose in MRS broth containing Durham tubes. Growth at different temperatures was observed in MRS broth after incubation for 5 days at 15, 37, 45°C then 12 days at 5 and 10°C [12]. The ability to grow at pH 3.9 and 9.6 was tested on MRS broth [16]. The tolerance to NaCl was studied by growth in MRS broth containing NaCl at concentrations 2, 4 and 6.5% [17]. Arginine dihydrolase was determined in MRS broth supplemented with 0.3% arginine, which was incubated for 3 days, followed by NH3 detection by addition of Nessler’s reagent. Hydrolysis of esculin and the effect of bile salts were observed on bile esculin agar. Heat resistance was determinated in MRS broth at 60 and 65°C for 30 min [18]. Citrate utilization was studied on the media of Kempler and Mc Kay [19]. Production of dextran was recorded on MSE agar [20] and the production of acetoine from glucose was determined by using the Voges–Proskauer test [21]. Carbohydrate fermentation patterns of LAB were determined by means of miniaturized API 50 CH biochemical tests (BioMérieux, Marcy L_Etoile, France).
2.3 Extraction and analysis of carob pods syrup
Carob pods were chopped into small particles (1-3 cm). One liter of hot water at 80-85°C was added to 200 g of carob pods, homogenized and through a cloth. The syrup obtained was centrifuged at 15000 rpm for 10 min to separate the cellulose debris. The collected supernatant was used as culture medium. The syrup is fixed in a pH 6 and sterilized during 20 min at 120°C. The extraction parameters were obtained from method advocated by Turhan et al. [22]. pH is measured using a pH meter and density was determined by density meter. Concentration of lactic acid was determined by acidity titration with NaOH 0.1 N. Total nitrogen of carob and protein content was determined by the method of Kjeldahl digestion and distillation apparatus [23]. Total and reducing sugars were determined colorimetrically at 480 nm by Dubois method [24]. The ash content of the carob was determined according to the AOAC official method 972.15 by incineration one gram of syrup at a temperature of 600°C during 3 h. Moisture and dry matter were determined by drying 10 mL of syrup at 105°C during 18 h. The mineral salts are determined according to the methods advocated by Muhamed [25].
2.4 Fermentation conditions and methods
A total of 6 isolates were isolated from carob pods, of which one isolate (Lactobacillus curvatus) was shown to produce a high amount of acidity, why it is used for studying the kinetics of growth and acidification of carob syrup. All experiments were carried out in a 2 L jar fermenter (Applikon Biocontroller ADI1030) with an initial volume of 1 L at 30°C. The agitation speed was set at 300 rpm to insure complete mixing of the fermentation medium. The inocula were incubated at 30°C for 12 h at 300 rpm before their transfert to the fermenter in a 10%. The culture pH was maintained at 6.2 by automatic addition of 25% (w/w) NH4OH solution during the 30 hours of fermentation. The samples were withdrawn at desired intervals and frozen for further analysis. The biochemical analysis applied on the carob pods syrup shows that it is poor in sodium acetate and fatty-acids, so the addition of these elements (growth factors) is necessary to the syrup in order to import the quantity of lactic acid. Four culture mediums were used: carob syrup (CS), carob syrup with 5 g of Sodium Acetate (CS+SA), carob syrup with 1 mL of Tween80 (CS+T80) and carob syrup with 5 g of Sodium Acetate and 1 mL of Tween 80 (CS+SA+T80). The biomass is determined by measurement of the optical density (OD) at 600 nm by a spectrophotometer HITACHI 4-2000. Culture samples were centrifuged (13200 g at 4°C for 5 min), diluted and filtered. Residual glucose and lactic acid concentrations were determined by Multi parameter Medical Analyzer. The enzymatic kit used for the lactic acid dosage is the PAP Ref-61 192 and for the glucose dosage it is the Elitech diagnosis ref - GPSL-0500. The various analyses carried out allow the following time evolution of the component concentrations present in the culture medium: [Biomass: (OD) =f (t), sugars: S=f (t) and the lactic acid: P=f (t)]. From these raw data it is possible to calculate the fermentation kinetic parameters in the batch culture by the calculation of the specific rate of growth (μ in h-1), of substrate consumption (Qs in g.g-1.h-1) and lactic acid production (QL.A in g.g-1.h-1).
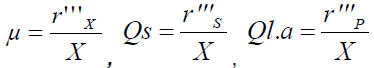
The maximal specific growth rate (μmax) was determined from the slopes of the plotted linear curve: LnX/X0=f (t). The biomass (Yx/s) and products (Yp/s) yields are defined as the mass ratios in biomass and metabolites formed per gram of consumed carbonaceous substrate.
3. Result and Discussion
3.1 Isolation and identification of lactic acid bacteria (LAB)
Colonies on MRS medium are whitish, smooth and small. These strains are Gram+ (purple) and bacillary form. The development on M17 gives white colonies, round, opaque, with smooth boundary. These strains are present in the form of cocci in pairs or chains Gram+. Strains were characterized and identified according to the methods and criteria of Buchanan [14] and Klein [15]. A total of 6 isolates were isolated from carob pods, of which 1 isolate (Lactobacillus curvatus) was shown to produce a high amount of acidity, why it is used for studying the kinetics acidification of carob syrup. From the results obtained, the strains are Lactobacillus curvatus, Streptococcus diacetylactis, Streptococcus feacalis, Streptococcus mitis and Streptococcus thermophilus (Table 1).
3.2 Biochemical composition of carob pods syrup
The carob pods syrup which has been the subject of our work has high water content 85% (Table 2), we agree that a product with high water content facilitates lactic acid bacteria proliferation and helps for a better substrate-enzyme contact [26]. Carob pods syrup will be very rich of total sugars 22 g/L. The protein fraction is considerable; therefore it can serve as a nitrogen source (0.25%). An ash content of 0.8% indicates its richness of minerals including potassium (110 mg/100 ml of MF), sodium (80) and calcium (150). Karkacier and Artik report that the fruits are ripe enough; they have 91-92% total dry matter and 62-67% total soluble solids, which consist of 34-42% sucrose, 10-12% fructose, and 7-10% glucose [7].
The content of sugars found a high energy value 17.5 kJ/g D.M. for pod of the carob [5,27,28]. Petit and Pinilla, report that the carob pods are also characterized by high sugar content 200-500 g/ kg [8]. According to the results obtained by Yousif and Alghzawi [2] and by Vaheed et al. [29], carob pod contains 45 to 56.10% total sugar and13.60 to19.00% reducing sugar. Vaheed et al. [30] show that the carob pods powder contained 9.09 moisture, 56.10 total sugars, and 19.00 reducing sugars (all as weight %). Chemical composition of carob had been studied extensively for different countries of the Mediterranean area. It had been observed that this composition is depending not only on technological Fidan and Sapundzhieva [34], carob fruit (pulps and seeds) and flour are rich in carbohydrates, proteins and also are a good source of K, Ca, Na, Fe, and Mg. According to the literature data, many factors affect the chemical composition of the fruit as well as its mineral content, for example, temperature, dryness [35], irrigation and fertilization [36] and salinity [37]. Finally, the biochemical analysis show that it can constitute a fermentation medium of good quality.
3.3 Lactic acid fermentation
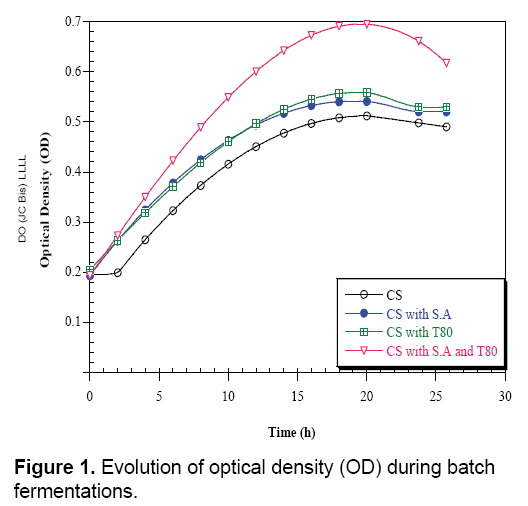
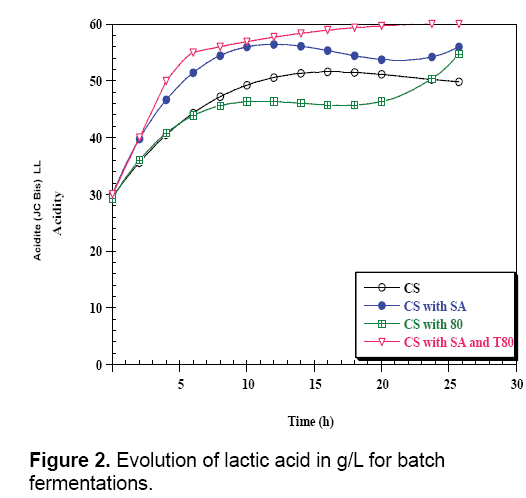
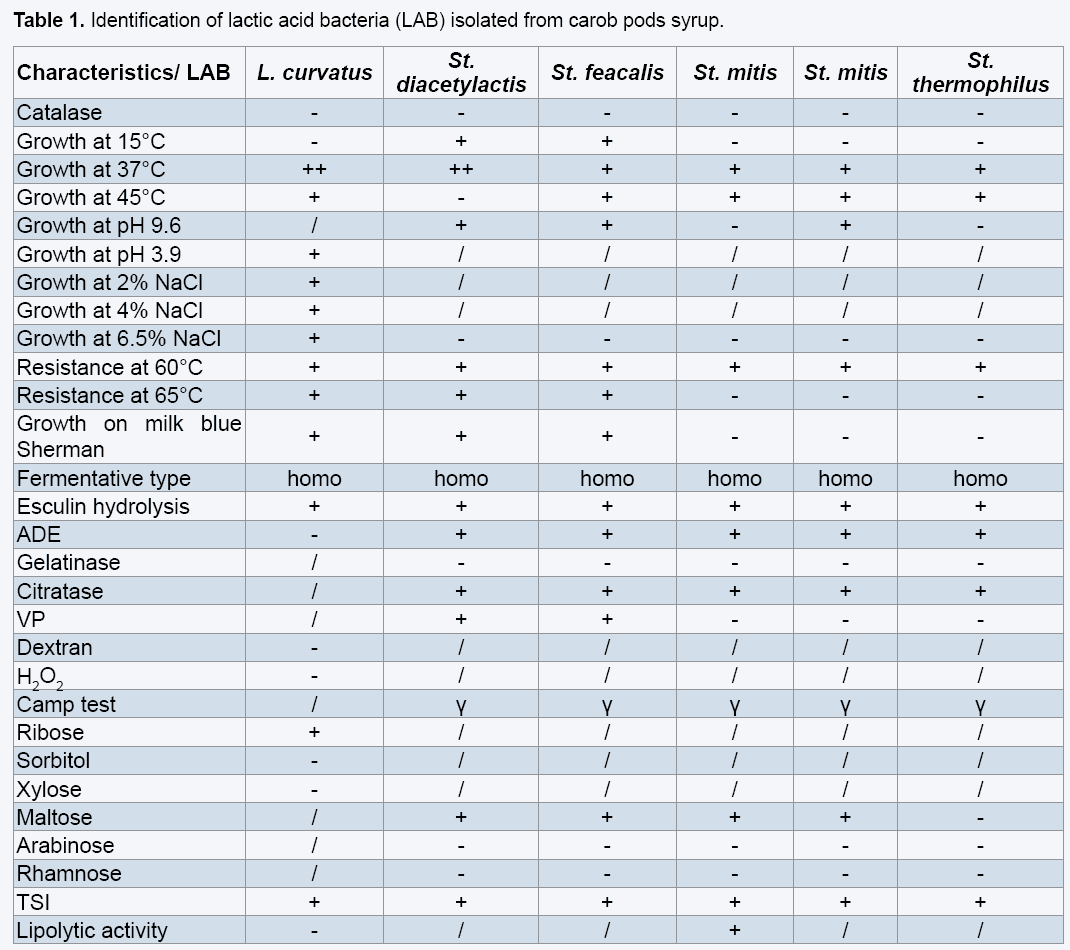
The results clearly indicated that the highest amount of biomass and lactic acid were obtained with the factors such as the extraction and analytical methodologies, but also on the genotype of the plant, the geographical origin, the climate conditions and the harvesting and storage procedures [7,31-33]. The analysis applied on the carob pods syrup shows that it is poor, with minerals such as Magnesium, Manganese and fatty-acids, so the addition of these elements (growth factors) is necessary to the syrup in order to import this quantity of lactic acid. According to carob enriched with sodium Acetate and Tween 80 (Table 3). The production curves of lactic acid in enriched and un-enriched mediums looked the same, the production in the un-enriched (carob syrups: CS) begins with an initial content of 30 g/L to achieve 50 g/L of lactic acid in 26 h of fermentation (Figure 1).
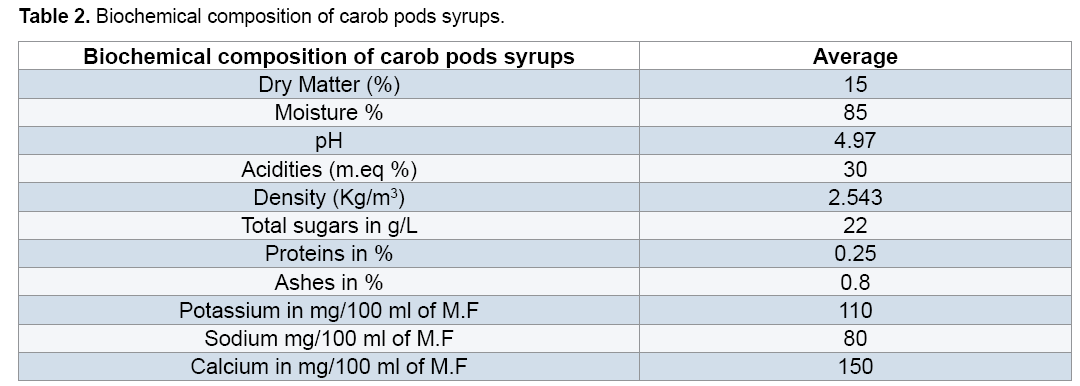
In parallel, in the medium enriched with sodium acetate (CS with SA), Tween 80 (CS with T80), and two components (CS with SA and T80), the lactic acid rate evolves gradually to achieve the end of fermentation 60 g/L. The addition of Tween 80 and sodium acetate in carob syrup increase the lactic acid production. On the other hand, the biomass evolution in the un-enriched medium (CS) starts with an initial concentration (OD=0.19) and after 26 h of fermentation it reaches a maximum value OD=0.5 (Figure 2).
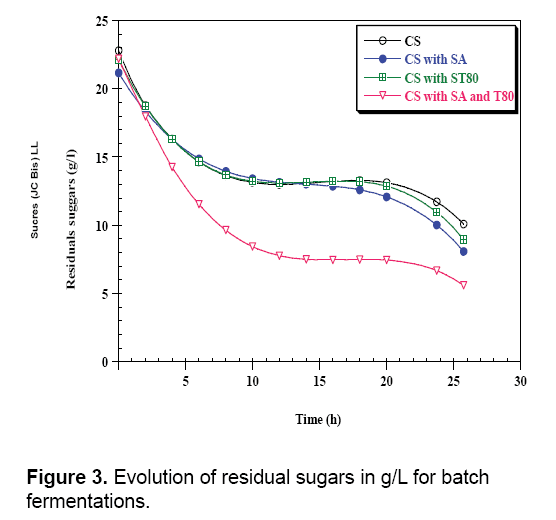
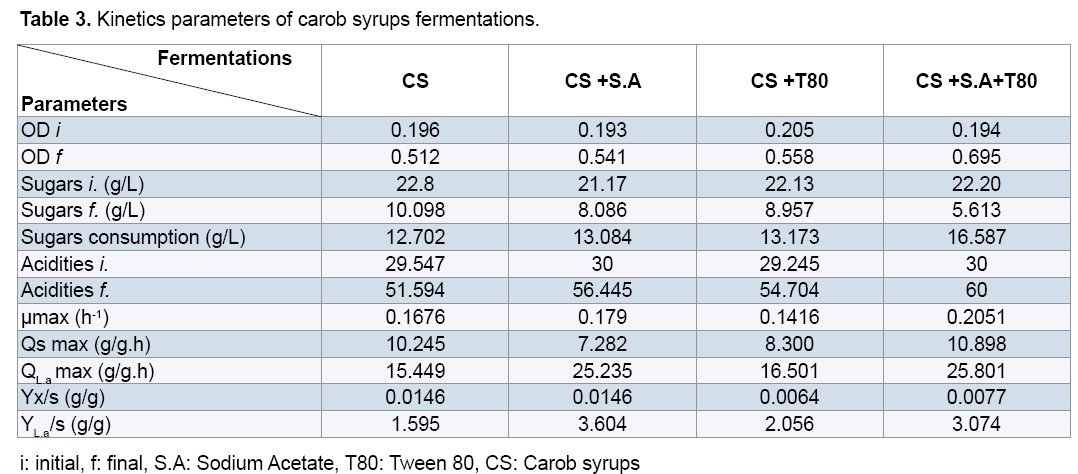
In the case of enriched (CS with SA and T80), we observe a low initial concentration of biomass and in the end of fermentation optical density reaches a maximum value 0.7. Tween 80 (commercial form of oleic acid) is essential for growth of lactic acid bacteria and acetate is a buffer and stimulating [26]. For different fermentations, there is a total absence of the lag phase indicating the perfect adaptation of Lactobacillus curvatus to the different medium used. However, decreasing of sugar rates is very faster in the medium supplemented with Sodium acetate and Tween 80. Where the Lactobacillus curvatus consumes about half quantity of initial total sugars, during 26 h of fermentation from an initial quantity of 22.2 g/L of sugars, it remains 5.6 g/L which implies an amount of 16.58 g/L (Figure 3).
Comparing the results for both fermentations indicated that the addition of growth factors in culture medium has a positive and beneficial effect on fermentation, since the growth rate and lactic acid production increase after the enrichment of carob pods syrup. The addition of Tween 80 allows better cells excretion of lactic acid by creating pores in the membrane and plays the role of surfactant which makes a good contact between bacteria and nutriments, and for more it is considered as a source of carbon and energy for electrons [26]. From these results, the enriched mediums present an interesting result. Overall, when the carob syrups fermentation medium was compared with carob syrups enriched with SA and T80, in terms of lactic acid produced, maximum specific rate of growth (μmax), maximum consumption specific rate (Qs), maximum production specific rate (QL.A), yield of lactic acid production (YL.A/s), there were significant differences (Table 3). Moreover, kinetic parameters show clear differences between CS and CS with SA and T80. Fermentation CS with SA and T80 resulted in significantly higher lactic acid productivity compared to CS. Overall, the lactic acid yield, the maximum consumption rate, and the specific growth rate were higher in CS with SA and T80 compared to CS.
4. Conclusion
The bioconversion of agricultural by- products mainly the ones rich in fermentable sugars has an economic and strategic interest. The main aim of this study was to isolation, identification and kinetics fermentation of Lactobacillus curvatus from carob syrups without and with addition of Sodium Acetate and Tween 80. Six isolates of lactic acid bacteria LAB were screened and five strains were identified as Lactobacillus curvatus, Streptococcus diacetylactis, Streptococcus feacalis, Streptococcus mitis and Streptococcus thermophilus. This identification was based on phenotypic characters and the API system. By its biochemical composition, the carob syrup is very rich in carbohydrates 22 g/L. which make it a substrate of choice for the development of high value substances. In producing countries, carob pods have traditionally been used as animal and human food and currently the main use is the seed for gum extraction, carob bean gum (CBG) or locust bean gum (LBG) [1]. The carob powder is being acclaimed as an ingredient with a marked nutritional value due to its high levels of dietary fibre (preventative role against heart disease) and phenol compounds (antioxidant activity) [38]. Regarding the high sugar content in carob pod, there have been some studies on the production of the value added product [30,39,40]. Lactic acid can be one of these value added products due to its current and future potentials. Kinetics study of growth of Lactobacillus curvatus in carob syrup showed that the addition of sodium acetate combined with Tween 80 to the culture medium increases the yield of lactic acid production.
Acknowledgement
We gratefully acknowledge Pr. I. Chevalot to have me to help to realize this work as well as all the team of the laboratory LRGP, ENSAIA, Nancy, France.
References
- Batlle I, Tous J.(1997). Carob tree Ceratoniasiliqua L. Promoting the conservation and use of underutilized and neglected crops. 17. Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute, Rome, Italy. 97.
- Yousif AK, Alghzawi HM. (2000). Processing and characterization of carob powder. Food Chemistry. 69: 283–287.
- Winer N.(1980). The potential of the carob (Ceratoniasiliqua L.). Int tree crop J. 1: 15-26.
- Girolamo R, Laura D. (2002). Evaluation and preservation of genetic resources of carob (Ceratoniasiliqua L.) in southern of Italy for pharmaceutical use. Breeding Res Aromatic Med. Plant. 9: 367-372.
- Biner B, Gubbuk H, Karhan M, Aksu M, Pekmezci M. (2007). Sugar profiles of the pods of cultivated and wild types of carob bean (Ceratoniasiliqua L.) in Turkey. Food Chem.100: 1453-1455.
- Shawakfeh K, Ereifej KI. (2005). Pod Characteristics of two Ceratoniasiliqua L. Varieties from Jordan. Ital. J. Food Sci.17: 187-194.
- Karkacier M, Artik N. (1995). Physical properties, chemical composition and extraction conditions of carob (CeratoniasiliquaL.). Gida.20:131-136.
- Petit MD, Pinilla JM. (19995). Production and purification of a sugar syrup from carob pods, LWT. Food Sci. Technol.28: 145-152.
- Thomson P. (1971). The carob in California. California Rare Fruit Growers Yearbook.3: 61-102.
- Owen RW, Haubner R, Hull WE, et al. (2003). Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem.Toxicol.41: 1727-1738.
- Harrigan W, McCance M. (1990). Laboratory Methods in Food and Dairy Microbiology, 8th edn. Academic Press, London, UK.
- Badis A, Guetarni D, MoussaBoudjema B, Henni DE, Kihal M. (2004). Identification and technological properties of lactic acid bacteria isolated from raw goat milk of four Algerian races. Food Microbiol.21: 579-588.
- Papamanoli E, Tzanetakis N, Litopoulou-Tzanetaki E, Kotzekidou P. (2003). Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci.65: 859-867.
- Buchanan RE, Gibbons NE, Cowan ST, et al.(1974). Bergey’s Manual of Determinative Bacteriology. Eds: Williams and Wilkins Co: Baltimore.
- Klein G. (2001). International Committee of Systematic Bacteriology. Subcommittee on the Taxonomy of Bifidobacterium, Lactobacillus and Related Organisms. Minutes of the Meeting. IntJ SystEvolMicrobiol.51: 259-261.
- Dykes GA, Britz TJ, Von Holy A. (1994). Numerical taxonomy and identification of lactic acid bacteria from spoiled, vacuum packaged Vienna sausages. J ApplBacteriol.76: 246-252.
- Stiles ME, Holzapfel WH. (1997). Lactic acid bacteria of food and their current taxonomy. Int J Food Microbiol.36: 1-27.
- Samelis J, Maurogenakis F, Metaxopoulos J. (1994). Characterization of lactic acid bacteria isolated from naturally fermented Greek dry Salami. Int J Food Microbiol.23: 179-196.
- Kempler GM, Mc Kay LL. (1980). Improved medium for detection ofcitrate-f ermentingStreptococcuslactis subsp. diacetylactis. J Appl Environ Microbiol.39: 927-956.
- Mayeux JV, Sandine WWE, Elliker PR. (1962). A selective medium for detecting Leuconostocorganisms in mixed isolate starter cultures. J Dairy Sci.45: 56-65.
- Clark WM, Lubs HA. (1915). The differentiation of bacteria of the colon-aerogenes family by the use of indicators. J Infect Dis.17: 160-173.
- Turhan I, Demirci A, Karhan M. (2008). Ethanol production from carob extract by Saccharomyces cerevisiae. ASABE Paper No. NABEC 08-009. St. Joseph, MI: American Society of Agricultural Engineers.
- AOAC. (2007). Official methods of Analysis of AOAC international. Gaithersburg. Maryland: AOAC.2: 955.04-945.46.
- Dubois MK. (1956). Colorimetric Method for Determination of Sugar and Related Substances. Anal and Chem Jour.28: 350.
- Muhamed M. (1983). Physical and chemical characterization of the major date varieties grown in Saudi-Arabia. Date Pal. Jou. 2: 183-196.
- Bouhadi D, Abbouni B, Hariri A, Ibri k,OuisNawel. (2012). Study of the Behaviour of Lactobacillus delbrueckii subsp. bulgaricus in Date Syrup in Batch Fermentation with Controlled pH.J Biotechnol Biomaterial.2: 1-5.
- Avallone R, Plessi M, Baraldi M, Monzani A. (1997). Determination of Chemical Composition of Carob (Ceratoniasiliqua): Protein, Fat, Carbohydrates, and Tannins.Journal of food composition and analysis.10: 166-172.
- Dakia P, Wathelet B, Paquot M.(2007). Isolation and chemical evaluation of carob (CeratoniasiliquaL.) seed germ.Food Chemistry.102: 1368-1374.
- Vaheed H, Shojaosadati SA, Galip H. (2011). Evaluation and optimization of ethanol production from carob pod extract by Zymomonasmobilisusing response surface methodology. J IndMicrobiol Biotech.38:101-111.
- Vaheeda H, Mousavib SM, Shojaosadati SA,Galipa H. (2014). Evaluation of Ethanol Production from Tannin-Reduced Carob Pod Extracts by Zymamonasmobilis.JREE.1: 8-19.
- Naghmouchi S, Khouja ML, Romero A, Tous J, Boussaid M. (2009). Tunisian carob (Ceratoniasiliqua L.) populations: Morphological variability of pods and kernel. Sci Hort.121: 125-130.
- Sidina MM, Elhansali M, Wahid N, et al. (2009). Fruit and seed diversity of domesticated carob (Ceratoniasiliqua L.) in Morocco. Sci Hort.123: 110-116.
- Haddarah A, Ismail A, Bassal A, Hamieh T, Ghoul M.(2013). Morphological and chemical variability of Lebanese carob varieties. European Scientific Journal.9: 353-369.
- Fidan H, Sapundzhieva T. (2015). Mineral composition of pods, seeds and flour of grafted carob (Ceratoniasiliqua L.). FRUITS Scientific Bulletin. Series F Biotechnologies.19: 136-139.
- Nunes MA, Ramalho C, Domingos J, Silva Rijo Pd. (1992). Seasonal changes in some photosynthetic properties of Ceratoniasiliqua (carob tree) leaves under natural conditions. Physiol. Plant.86: 381-387.
- Correia P, Martins-Loucao M. (1997). Leaf nutrient variation in mature carob (Ceratoniasiliqua) trees in response to irrigation and fertilization. Tree Physiol.17: 813-819.
- El-Dengawy ERF, Hussein AA, Alamri SA. (2011). Improving growth and salinity tolerance of carob seedlings (Ceratoniasiliqua L.) by Azospirillum inoculation.AmEurasian J Agric Environ. Sci.11: 371-384.
- Youssef MKE, El-Manfaloty MM, Ali HM. (2013). Assessment of Proximate Chemical Composition, Nutritional Status, Fatty Acid Composition and Phenolic Compounds of Carob (CeratoniaSiliqua L.). Food and Public Health.3: 304-308.
- Roukas T. (1993). Ethanol-production from carob pods by Saccharomyces cerevisae. Food Biotechnol.7:159-176.
- Turhan I, Bialka K, Demirchi A, Karhan M. (2010). Ethanol productionfrom carob extract by using Saccharomycescerevisiae. Bioresour Technol.101:5290-5296.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences