Uncarboxylated Osteocalcin Increases Serum Nitric Oxide Levels and Ameliorates Hypercholesterolemia in Mice Fed an Atherogenic Diet
Akihiko Kondo, Tomoyo Kawakubo-Yasukochi, Akiko Mizokami, Sakura Chishaki, Hiroshi Takeuchi, Masato Hirata
1Laboratory of Molecular and Cellular Biochemistry, Faculty of Dental Science, Kyushu University, Fukuoka, Japan
2Department of Immunological and Molecular Pharmacology, Faculty of Pharmaceutical Science, Fukuoka University, Fukuoka, Japan
3 OBT Research Center, Faculty of Dental Science, Kyushu University, Fukuoka, Japan
4 Division of Applied Pharmacology, Kyushu Dental University, Kitakyushu, Japan.
- *Corresponding Author:
- Tel: +81-92-871-6631
Fax: +81-92-863-0389
Email: tomoyoyasu@fukuoka-u.ac.jp; - Tel: +81-92-642-6317
Fax: +81-92-642-6322
E-mail: hirata1@dent.kyushu-u.ac.jp
Received date: November 29, 2016; Accepted date: December 19, 2016; Published date: December 26, 2016
Citation: Kondo A, Kawakubo-Yasukochi TK, Mizokami A, et al. Uncarboxylated Osteocalcin Increases Serum Nitric Oxide Levels and Ameliorates Hypercholesterolemia in Mice Fed an Atherogenic Diet. Electronic J Biol, 13:1
Abstract
Background: Osteocalcin (OC), an osteoblastderived bone matrix protein, may be involved in atherosclerosis pathophysiology. The physiological action of OC and the effect of OC administration under atherogenic conditions in vivo have not yet been examined.
Methods and findings: Here we demonstrate that acute or Chronic Uncarboxylated OC (GluOC) injection in wild-type C57BL/6 female mice fed a normal diet or a diet supplemented with cholesterol and cholic acid resulted in increased serum Nitric Oxide (NO) levels. Furthermore, our study shows that GluOC treatment led to elevated expression levels of liver X receptor α (LXRα), a key transcription factor in cholesterol metabolism, in white adipose tissue and liver and ameliorated hypercholesteromia in those mice.
Conclusion: We thus propose that GluOC exhibited potential atheroprotective effects through serum NO production and hypercholesterolemia improvement in wild-type mice.
Keywords
Uncarboxylated osteocalcin; Hypercholesterolemia; Liver X receptor α; Nitric oxide.
Abbreviations
OC: Osteocalcin; GluOC: Uncarboxylated Osteocalcin; GlaOC: γ-Carboxylated Osteocalcin; HAECs: Human Aortic Endothelial Cells; NO: Nitric oxide; eNOS: Endothelial Nitric Oxide Synthase; p-eNOS; Phosphorylated Endothelial Nitric Oxide Synthase; LXRα: Liver X Receptor α; SREBP1: Sterol- Regulatory-Element-Binding Protein 1; SREBP2: Sterol-Regulatory-Element-Binding Protein 2; ABCA1: ATP-Binding Cassette Protein A1; ABCG5: ATPBinding Cassette sub-family G member 5; ABCG8: ATP-Binding Cassette sub-family G member 8; T-CHO: Total Cholesterol; LDL-C: Low-Density Lipoprool; TG: Triglyceride
1. Introduction
Recent studies have indicated broad roles for Osteocalcin (OC), especially uncarboxylated OC (GluOC), including the regulation of whole body metabolism, reproduction and cognition [1,2]. In addition, there is a critical relevance between OC and atherosclerosis, such as the existence of OC in atherosclerotic plaques and a negative correlation between serum OC levels and atherosclerosis development [3-5]. There is a study to examine the therapeutic effect of OC administration on high fatdiet- induced impairment using genetically modified animals [6]; however, the physiological role of OC in wild-type living animals has not yet been elucidated.
There is also evidence that Nitric Oxide (NO) production is stimulated by GluOC in vascular endothelial cells via endothelial NO synthase (eNOS) phosphorylation in vitro [6,7]. NO is a multifunctional signaling molecule critically involved in the maintenance of metabolic and cardiovascular homeostasis. Previous studies have demonstrated that endothelium-derived NO suppresses key processes in vascular lesion formation and potently opposes atherogenic progression [8-12]. Therefore, in the present study, we used wild-type female C57BL/6 mice fed a normal diet or a diet supplemented with cholesterol and cholic acid to measure serum NO values following GluOC or γ-carboxylated osteocalcin (GlaOC) treatment.
Furthermore, we examined a potential antiatherogenic role of GluOC in vivo. Recent studies determined that serum OC levels were negatively correlated with hypercholesterolemia, a primary risk factor for atherosclerosis [4,13]. To address this, we measured serum lipid profiles in the mice and further explored the effects of GluOC application on molecules involved in cholesterol metabolism.
2. Methods
2.1 Animals
All experiments were approved by the animal ethics committee of Kyushu University (permission no. A27-138). Wild-type C57BL/6 mice were maintained as previously described [14]. CRF- 1 and F2HFD1 (Oriental Yeast, Tokyo, Japan) were used as the normal and atherogenic diets, respectively. F2HFD1 consisted of 72% CRF-1 and 28% other components, including 7.5% cacao butter, 0.5% cholic acid, 1.25% cellulose, 1.0% mineral composition, 1.625% glucose, 0.125% choline chloride, 1.25% cholesterol, 7.5% casein, 1.0% vitamin composition, 1.625% sucrose, 1.625% dextrin and 3.0% lard.
2.2 Materials
Recombinant GluOC was prepared as previously described [1,15]. GlaOC was purchased from AnaSpec, Inc. (Fremont, CA). Human aortic endothelial cells (HAECs) and MV2 medium kit were purchased from Promocell (Heidelberg, Germany). We obtained eNOS and phosphorylated eNOS (p-eNOS) (diluted 1/2000, BD Biosciences, Franklin Lakes, NJ), liver X receptor α (LXRα) (diluted 1/1000, R&D Systems, Inc., Minneapolis, MN), sterol-regulatory-element-binding protein 2 (SREBP2) (diluted 1/3000, MBL Co., Ltd., Nagoya, Japan), β-actin (diluted 1/3000, SIGMA-Aldrich, St. Louis, MO) and GAPDH (diluted 1/40000, Biosource International, Camarillo, CA) antibodies.
2.3 NO measurement
Following deproteinization with an Ultracel-10 membrane (Merck Millipore, Billerica, MA), serum NO was measured with an NO2/NO3 Assay kit-FX (Dojindo, Kumamoto, Japan).
2.4 Immunoblot analysis
Cell lysates were separated by SuperSepTM Ace, 5–20% (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and transferred to polyvinylidene difluoride membranes (Merck-Millipore). After blocking with Blocking One (Nacalai Tesque Inc., Kyoto, Japan), the membranes were incubated with primary antibody at 4°C overnight. Immune complexes were detected with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Inc., Danvers, MA) and an enhanced chemiluminescence substrate kit (GE Healthcare, Pittsburgh, PA).
2.5 Quantitative RT-PCR analysis
Quantitative RT-PCR was performed as previously described, with the use of the LightCycler® 480 system (Roche, Basel, Switzerland) [14]. Amplicon sizes were as follows: β-actin, 104 bp; ATP-binding cassette protein A1 (Abca1), 69 bp; ATP-binding cassette sub-family G member 5 (Abcg5), 85 bp; ATP-binding cassette sub-family G member 8 (Abcg8), 94 bp; and Sterol-regulatory-element-binding protein 1 (Srebp1), 111 bp. Primers used to amplify the transcripts were as follows: β-actin, (forward) ctaaggccaaccgtgaaaag, (reverse) accagaggcatacagggaca; Abca1, (forward) gcagatcaagcatcccaact, (reverse) ccagagaatgtttcattgtcca; Abcg5, (forward) tcctgcatgtgtcctacagc, (reverse) atttgcctgtcccacttctg; Abcg8, (forward) aaccctgcggacttctacg, (reverse) ctgcaagagactgtgccttct; and Srebp1, (forward) tcaagcaggagaacctgacc, (reverse) tcatgccctccatagacaca. Light Cycler Universal Probe Master (Roche) specific for each sequence was applied to number 64 (for β-actin), number 1 (for Abca1), number 31 (for Abcg5), number 10 (for Abcg8), and number 25 (for Srebp1). β-actin was used as an endogenous control.
2.6 Statistical analysis
Student’s t-tests were performed for statistical analysis using StatPlus software. P values<0.05 were considered to be statistically significant. Results are expressed as the mean ± standard deviation (SD).
3. Results
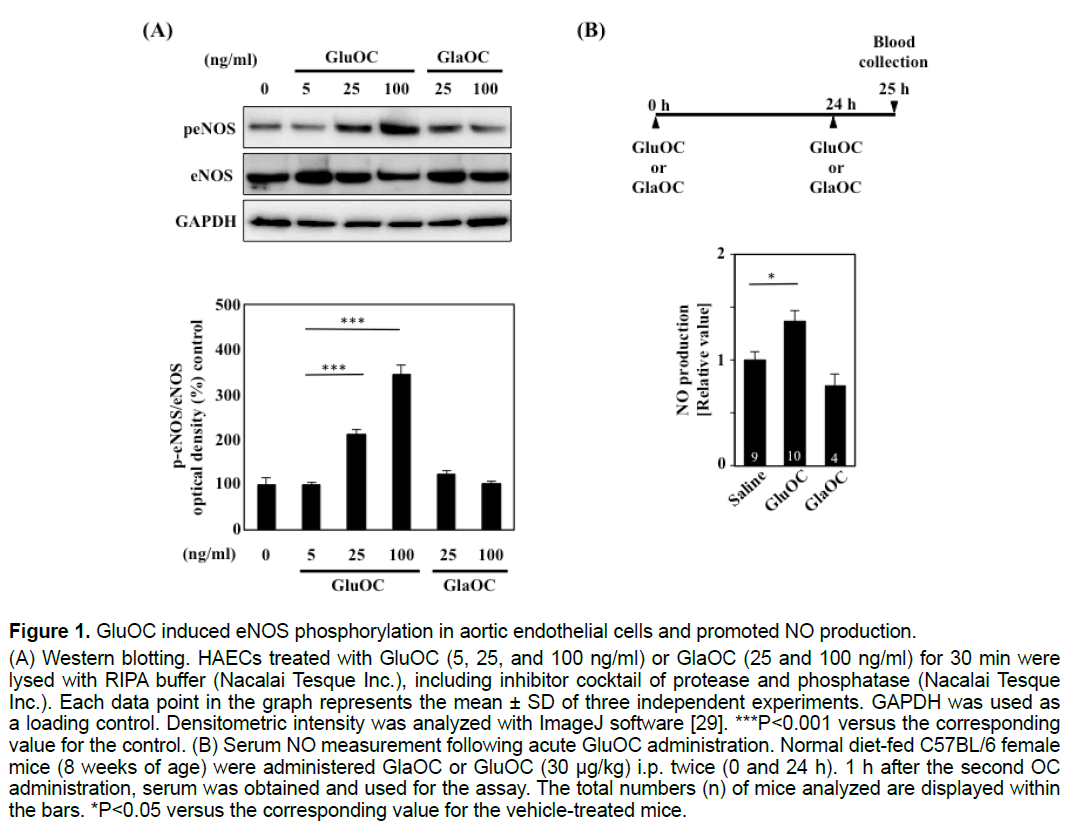
3.1 GluOC, but not GlaOC, induced eNOS phosphorylation in aortic endothelial cells and promoted NO production in vivo
We first examined whether GluOC and GlaOC promoted eNOS phosphorylation in HAECs. eNOS phosphorylation was significantly increased in response to 25 and 100 ng/ml GluOC but not to the same doses of GlaOC (Figure 1A). Furthermore, intraperitoneal administration of 30 μg/kg GluOC, but not GlaOC, resulted in a significant increase in serum NO concentrations in 8-week-old female C57BL/6 mice fed a normal chow diet (Figure 1B).
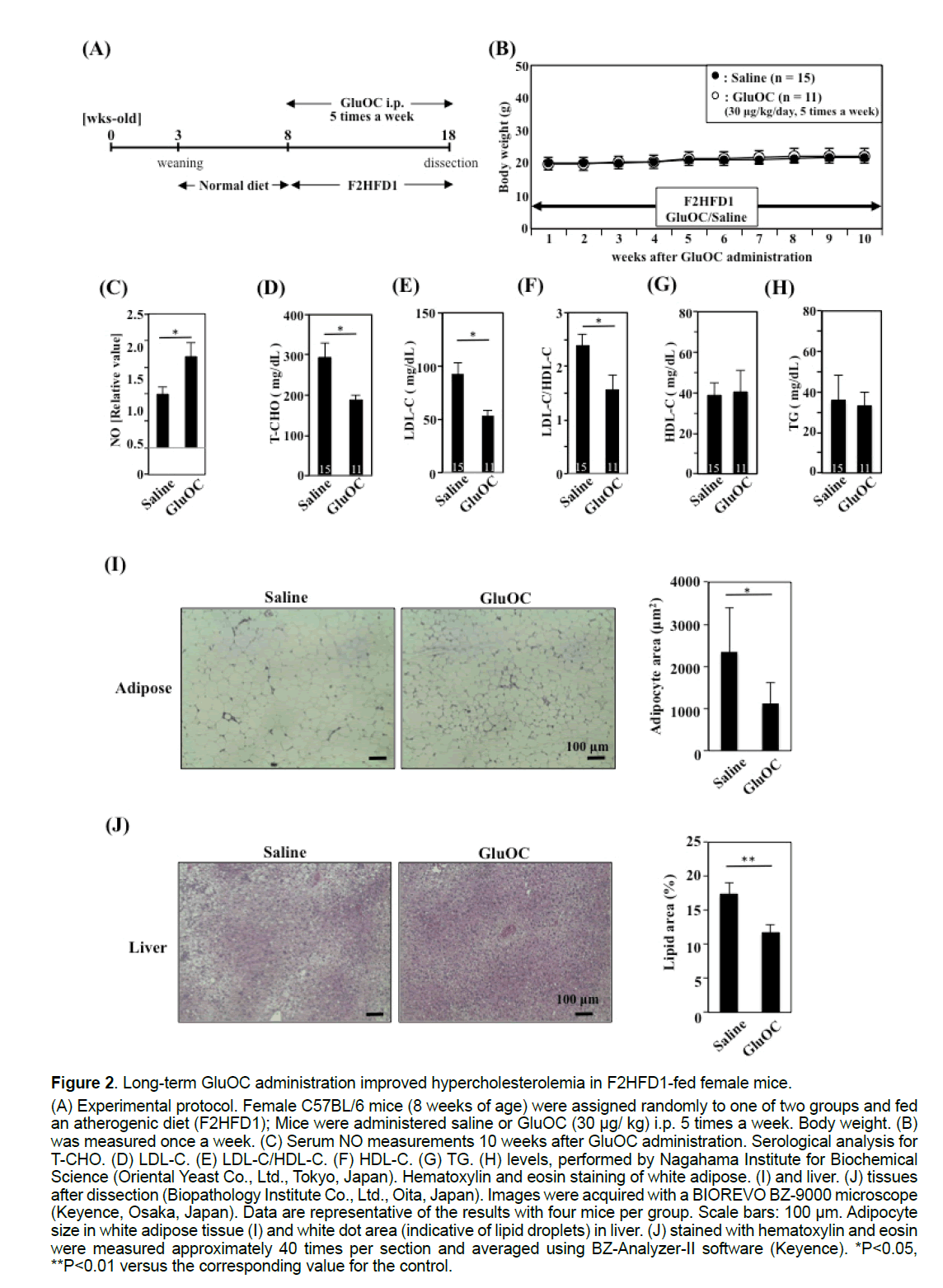
3.2 Long-term GluOC administration improved hypercholesterolemia in F2HFD1-fed female mice
GluOC (30 μg/kg) was administered five times per week for 10 weeks to female F2HFD1-fed C57BL/6 mice to investigate the atheroprotective effect of GluOC (Figure 2A). No difference in body weight gain was observed between saline- and GluOC-injected mice during the experimental period (Figure 2B). Furthermore, aortic atherosclerotic plaques were not detected in either group by histological analysis (data not shown). However, serum NO concentrations were increased approximately 1.7-fold in GluOCtreated mice, compared with those of the control mice, after 10 weeks (Figure 2C). In addition, total serum cholesterol (T-CHO) levels were elevated following F2HFD1 feeding, reaching levels as high as 300 mg/dL (Figure 2D), compared with those in C57BL/6 mice fed a normal diet (CRF-1; 79.4 ± 5.38 mg/dL; Charles River Laboratories Japan, Inc., Yokohama, Japan). GluOC administration reduced T-CHO levels by approximately 40% (Figure 2D) and improved low-density lipoprotein cholesterol (LDL-C) levels (Figure 2E) and the LDL-C to high-density lipoprotein cholesterol (HDL-C) ratio (Figure 2F), which are thought to be predictors of atherosclerosis risk [16,17]. However, no significant differences were detected in serum HDL-C (Figure 2G) or triglyceride (TG) (Figure 2H) levels. Additionally, we performed a histological analysis of white adipose tissue (Figure 2I) and liver (Figure 2J) from F2HFD1-fed mice. White adipose tissue adipocyte size (Figure 2I) and liver white dot area (lipid droplet area) (Figure 2J) were significantly decreased by GluOC administration.
Figure 1:GluOC induced eNOS phosphorylation in aortic endothelial cells and promoted NO production.
(A) Western blotting. HAECs treated with GluOC (5, 25, and 100 ng/ml) or GlaOC (25 and 100 ng/ml) for 30 min were
lysed with RIPA buffer (Nacalai Tesque Inc.), including inhibitor cocktail of protease and phosphatase (Nacalai Tesque
Inc.). Each data point in the graph represents the mean ± SD of three independent experiments. GAPDH was used as
a loading control. Densitometric intensity was analyzed with ImageJ software [29]. ***P<0.001 versus the corresponding
value for the control. (B) Serum NO measurement following acute GluOC administration. Normal diet-fed C57BL/6 female
mice (8 weeks of age) were administered GlaOC or GluOC (30 μg/kg) i.p. twice (0 and 24 h). 1 h after the second OC
administration, serum was obtained and used for the assay. The total numbers (n) of mice analyzed are displayed within
the bars. *P<0.05 versus the corresponding value for the vehicle-treated mice.
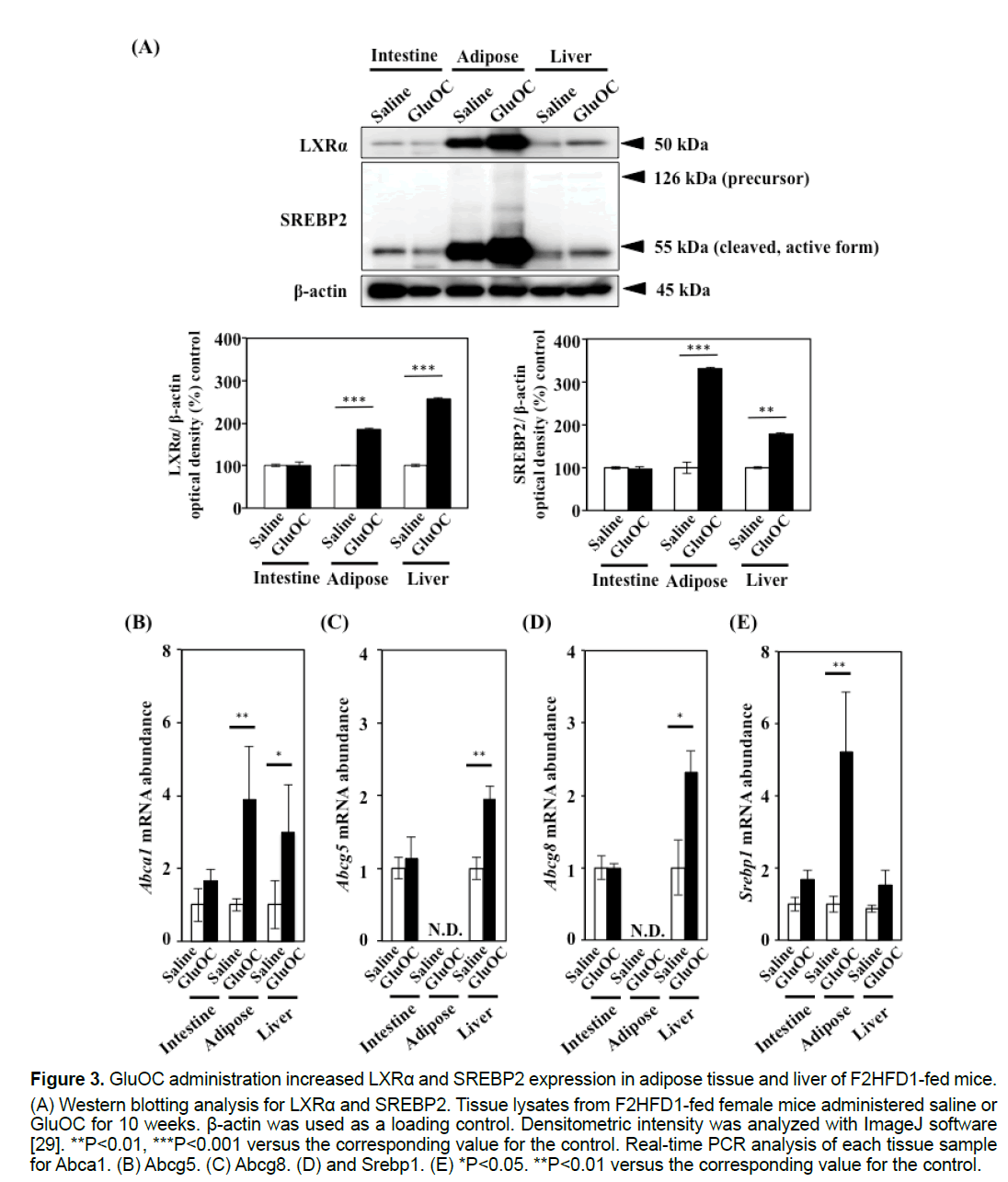
3.3 GluOC administration increased LXRα and SREBP2 expression in the white adipose tissue and liver of F2HFD1-fed female mice
Consistent with previous data, our study revealed that GluOC treatment improved hypercholesterolemia in F2HFD1-fed mice (Figures 2D–2F) [4,13,18]. To understand how GluOC regulates serum cholesterol levels, we investigated the expression levels of cholesterol metabolism-related transcription factors, LXRα and SREBP2. The active forms of both transcription factors were up-regulated by GluOC administration in white adipose tissue and liver, but not in the intestine (Figure 3A). LXRα controls cholesterol efflux by regulating expression of the genes encoding the ATP-binding cassette (ABC) transporters, such as Abca1, Abcg5 and Abcg8, and is also a regulator of Srebp1 [18,19]. These mRNA levels were up-regulated or tended to be upregulated, in the adipose tissue and liver of GluOCtreated mice compared with those of control mice (Figures 3B–3E).
4. Discussion
In this study, we reported that GluOC administration increased serum NO concentration and improved dietary hypercholesterolemia, which is an important risk factor for atherosclerosis in vivo [4,13]. There is evidence that endothelial cell NO production, which exerts a number of atheroprotective effects, is potentially increased by GluOC treatment via eNOS phosphorylation [6-8,12]. However, the actual NO values in cell culture supernatant or serum after GluOC treatments have not been verified in those studies. Additionally, our study revealed that GluOC, but not GlaOC, treatment promoted eNOS phosphorylation in HAECs. Accordingly, we investigated GluOC-induced NO production in wildtype mice fed an atherogenic diet to evaluate the atheroprotective effect of GluOC. Both acute and chronic GluOC treatment in vivo increased serum NO levels. Given that adiponectin, which is up-regulated by GluOC in adipocytes, also activates eNOS phosphorylation, we propose that GluOC treatment would confer cardiovascular benefits by enhancing NO bioavailability [20,21].
Figure 2:Long-term GluOC administration improved hypercholesterolemia in F2HFD1-fed female mice.
(A) Experimental protocol. Female C57BL/6 mice (8 weeks of age) were assigned randomly to one of two groups and fed
an atherogenic diet (F2HFD1); Mice were administered saline or GluOC (30 μg/ kg) i.p. 5 times a week. Body weight. (B)
was measured once a week. (C) Serum NO measurements 10 weeks after GluOC administration. Serological analysis for
T-CHO. (D) LDL-C. (E) LDL-C/HDL-C. (F) HDL-C. (G) TG. (H) levels, performed by Nagahama Institute for Biochemical
Science (Oriental Yeast Co., Ltd., Tokyo, Japan). Hematoxylin and eosin staining of white adipose. (I) and liver. (J) tissues
after dissection (Biopathology Institute Co., Ltd., Oita, Japan). Images were acquired with a BIOREVO BZ-9000 microscope
(Keyence, Osaka, Japan). Data are representative of the results with four mice per group. Scale bars: 100 μm. Adipocyte
size in white adipose tissue (I) and white dot area (indicative of lipid droplets) in liver. (J) stained with hematoxylin and eosin
were measured approximately 40 times per section and averaged using BZ-Analyzer-II software (Keyence). *P<0.05,
**P<0.01 versus the corresponding value for the control.
Figure 3:GluOC administration increased LXRα and SREBP2 expression in adipose tissue and liver of F2HFD1-fed mice.
(A) Western blotting analysis for LXRα and SREBP2. Tissue lysates from F2HFD1-fed female mice administered saline or
GluOC for 10 weeks. β-actin was used as a loading control. Densitometric intensity was analyzed with ImageJ software
[29]. **P<0.01, ***P<0.001 versus the corresponding value for the control. Real-time PCR analysis of each tissue sample
for Abca1. (B) Abcg5. (C) Abcg8. (D) and Srebp1. (E) *P<0.05. **P<0.01 versus the corresponding value for the control.
The relationship between bone metabolism and serum lipid profiles under pathophysiological conditions has been controversial [22,23]. We determined that GluOC treatment up-regulated two cholesterolregulating transcription factors, LXRα and SREBP-2, in white adipose tissue and liver. Furthermore, the mRNA expression levels of the direct LXRα target genes (Abca1, Abcg5, and Abcg8), which are related to sterol excretion and reducing intestinal cholesterol absorption, were similarly increased.
A previous study demonstrated that an increase in ABC transporters led to a compensatory increase in cholesterol synthesis [24]. The present study revealed that SREBP2, which up-regulates genes involved in cholesterol biosynthesis and uptake, was also increased in white adipose tissue and liver of GluOCtreated mice. In addition, SREBP1, which activates fatty acid synthesis-related gene transcription and leads to fatty liver, is also an LXRα target gene [25,26]. Indeed, Srebp1 mRNA expression was significantly increased in white adipose tissue from GluOC-treated mice compared with that of the control. However, serum TG levels, adipocyte size in white adipose tissue, and lipid droplet area in the liver were ameliorated by GluOC administration. Based on these results, we concluded that GluOC treatment improved dietary hypercholesterolemia, likely by increasing LXRα expression, resulting in the promotion of lipid excretion and absorption inhibition rather than synthesis.
In this study, we used wild-type mice without any genetic modification to describe a physiological role for GluOC in atherogenic development. Given that wild-type rodents are resistant to atherosclerosis development, future studies using genetically modified mice, such as LDL receptor-deficient and apolipoprotein E-deficient mice, are warranted to investigate the GluOC therapeutic process [6,27,28].
5. Acknowledgement and Funding
The authors thank Dr. Miho Matsuda (Kyushu University) for the help with animal experiments. This work was supported by the Japan Society for the Promotion of Science (KAKENHI grants 24229009 to M.H., 26861554 and 16K11469 to T.K-Y. and 26861553 and 16K20421 to A.M.), the Central Research Institute of Fukuoka University (No. 157103 to T.K-Y.) and the Mishima Kaiun Foundation (to T.K-Y.).
References
- Mizokami A, Yasutake Y, Higashi S, et al. (2014). Oral administration of osteocalcin improves glucose utilization by stimulating glucagon-like peptide-1 secretion. Bone. 69: 68-79.
- Zoch ML, Clemens TL, Riddle RC. (2016). New insights into the biology of osteocalcin. Bone. 82: 42-49.
- Fleet JC, Hock JM. (1994). Identification of osteocalcin mRNA in nonosteoid tissue of rats and humans by reverse transcription-polymerase chain reaction. J Bone Miner Res. 9: 1565-1573.
- Magni P, Macchi C, Sirtori CR, et al. (2016). Osteocalcin as a potential risk biomarker for cardiovascular and metabolic diseases. Clin Chem Lab Med. 54: 1579-1587.
- Ma H, Lin H, Hu Y, et al. (2014). Serum levels of osteocalcin in relation to glucose metabolism and carotid atherosclerosis in Chinese middle-aged and elderly male adults: The Shanghai Changfeng Study. Eur J Int Med. 25: 259-264.
- Dou J, Li H, Ma X, et al. (2014). Osteocalcin attenuates high fat diet-induced impairment of endothelium-dependent relaxation through Akt/eNOS-dependent pathway. Cardiovasc Diabetol. 13: 74.
- Jung CH, Lee WJ, Hwang JY, et al. (2013). The preventive effect of uncarboxylated osteocalcin against free fatty acid-induced endothelial apoptosis through the activation of phosphatidylinositol 3-kinase/Akt signaling pathway. Metabolism. 62: 1250-1257.
- Kuhlencordt PJ, Gyurko R, Han F, et al. (2001). Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 104: 448-454.
- Naruse K, Shimizu K, Muramatsu M, et al. (1994). Lond-term inhibition of NO synthesis promotes atherosclerosis in the hypercholesterolemic rabbit thoracic aorta. PGH2 does not contribute to impaired endothelium-dependent relaxation. Arterioscler Thromb. 14: 746-752.
- Cooke JP, Singer AH, Tsao P, et al. (1992). Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 90: 1168-1172.
- Kojda G, Noack E. (1995). Effects of pentaerythrity-tetranitrate and isosorbide-5-mononitrate in experimental atherosclerosis. Agents Actions Suppl. 45: 201-206.
- Hogg N, Kalyanaraman B, Joseph J, et al. (1993). Inhibition of low-density lipoprotein oxidation by nitric oxide. FEBS. 334: 170-174.
- Filip R, Possemiers S, Heyerick A, et al. (2015). Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J Nutr Health Aging. 19: 77-86.
- Yasukochi KT, Kondo A, Mizokami A, et al. (2016). Maternal oral administration of osteocalcin protects offspring from metabolic impairment in adulthood. Obesity. 24: 895-907.
- Mizokami A, Yasutake Y, Gao J, et al. (2013). Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS One. 8: e57375.
- Fernandez ML, Webb D. (2008). The LDL to HDL cholesterol ratio as a valuable tool to evaluate coronary heart disease risk. J Am Coll Nutr. 27: 1-5.
- Nicolosi RJ, Rogers EJ, Kritchevsky D, et al. (1997). Dietary conjugated linoleic acid reduces plasma lipoproteins and early aortic atherosclerosis in hypercholesterolemic hamsters. Artery. 22: 266-277.
- Chen L, Li Q,Yang Z, et al. (2013). Osteocalcin, glucose metabolism, lipid profile and chronic low-grade inflammation in middle-aged and elderly Chinese.Diabet Med. 30: 309-317.
- Zhang Y, Breevoort SR, Angdisen J, et al. (2012). Liver LXRa expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J Clin Invest. 122: 1688-1699.
- Otani T, Mizokami A, Hayashi Y, et al. (2015). Signaling pathway for adiponectin expression in adipocytes by osteocalcin. Cell Signal. 27: 532-544.
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. (2003). Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 278: 45021-45026.
- Niemeier A, Schinke T, Heeren J, Amling M. (2012). The role of apolipoprotein E in bone metabolism. Bone. 50: 518-524.
- During A, Penel G, Hardouin P. (2015). Understanding the local actions of lipids in bone physiology. Prog Lipid Res. 59:126-146.
- Yu L, Li-Hawkins J, Hammer RE, et al. (2002). Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 110: 671-680.
- Schultz JR, Tu H, Luk A, et al. (2000). Role of LXRs in control of lipogenesis. Genes Dev. 14: 2831-2838.
- Repa JJ, Liang G, Ou J, et al. (2000). Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 14: 2819-2830.
- Hsueh W, Abel ED, Breslow JL, et al. (2007). Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 100: 1415-1427.
- Jeon US, Choi JP, Kim YS, et al. (2015). The enhanced expression of IL-17-secreting T cells during the early progression of atherosclerosis in ApoE-deficient mice fed on a western-type diet. Exp Mol Med. 47: e163.
- Schneider CA, Rasband WS, Eliceiri KW. (2012). NIH image to ImageJ: 25 years of image analysis. Nature Methods. 9: 671-675.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences