Type II Cyclic Guanosine Monophosphate-Dependent Protein Kinase Inhibits Vegf-A/Vegfr-2 Pathway Activation In Gastric Cancer Cells
Ying Wang, Weihui Zhang, Yan Wu, Min Wu, Hai Qian, Yongchang Chen
Ying Wang, Weihui Zhang, Yan Wu, Min Wu, Hai Qian, Yongchang Chen*
School of Medicine, Jiangsu University, Jiangsu Province, PR China
Received: date June 13, 2016; Accepted date : August 24, 2016; Published date: August 31, 2016
Citation: Wang Y, Zhang W, Wu Y, et al. Type II Cyclic Guanosine Monophosphate-Dependent Protein Kinase Inhibits Vegf-A/ Vegfr-2 Pathway Activation In Gastric Cancer Cells. Electronic J Biol, 12:4
Abstract
Our previous study found that Type II cyclic guanosine monophosphate-dependent protein kinase (PKG II) inhibited VEGF-induced tyrosine phosphorylation/ activation of VEGFR-2. But how does PKG II inhibit VEGFR-2 is not clear yet. The aim of this paper was to investigate the molecular mechanism of PKG II inhibition on VEGFR-2. Human gastric cancer cell line HGC-27 and Human Umbilical Vein Endothelial Cells (HUVECs) were infected with adenoviral construct encoding cDNA of PKG II (Ad-PKG II) to increase the expression of PKG II and treated with 8-pCPT-cGMP to activate the kinase. Trans-well migration assay results showed that PKG II inhibited VEGF-induced migration of gastric cancer cells. Tube formation assay results showed that PKG II inhibited VEGF-induced tube formation of HUVEC cells. Co- Immunoprecipitation results indicated that PKG II combined with VEGFR-2. Immunoprecipitation and Western blotting results showed that PKG II caused Serine/Threonine phosphorylation of VEGFR-2. Mutagenesis and Western blotting results showed that when Threonine 439 and Serine1231 of VEGFR-2 were mutated to Alanine which could not be phosphorylated, the inhibition of PKG II on VEGFR-2 disappeared. The results suggest that PKG II inhibits VEGF-induced activation of VEGFR-2, migration and tube formation through phosphorylating Threonine439 and Serine1231 of VEGFR-2.
Keywords
Type II cGMP-dependent protein kinase(PKG II); VEGF; VEGFR-2; Gastric cancer cells; Phosphorylation; Inhibition.
1. Introduction
Gastric cancer is one of the most common cancers in worldwide and especially in Asia [1]. Metastasis and angiogenesis are critical processes in gastric cancer development. The vascular endothelial growth factor (VEGF) family plays important role in angiogenesis in tumors [2]. VEGF expression is affected by several factors such as hypoxia, acidosis, and mechanical stress [3]. Overexpression of VEGF occurs in most tumors including colon, pancreatic and gastric cancer, and is related to tumor progression and poor prognosis [4,5]. VEGF-A, a member of VEGF family, is usually noted as VEGF because it is a key regulator of angiogenesis and differentiation of progenitor endothelial cell [6]. Ozdemir et al. reported that the presence of VEGF expression was significantly associated with angiogenesis and the prognosis in gastric cancer patients [7]. VEGFR-1 and VEGFR-2 are major VEGF receptors activated by VEGF-A. A growing body of evidence demonstrates that VEGFR-2 is predominantly responsible for responses of vascular endothelial cells to VEGF under both physiological and pathological conditions [6]. Once VEGF binding to VEGFR-2 on endothelial cells, VEGFR-2 is activated and triggered with a cascade of proteins inducing endothelial cell proliferation, migration, apoptosis inhibition and maturation of vascular structures [8,9]. Data from Aprile et al. showed that a recombinant monoclonal antibody that binds to VEGFR-2 was effective for patients with gastric carcinomas [10]
Type II cyclic Guanosine Monophosphate (cGMP)- dependent Protein Kinase (PKG II) is a serine/ threonine protein kinase. For many years, PKG II has been known as a biological mediator of cGMPinduced Cl-/water secretion of small intestine, normal skeletal growth and renin-release of the kidneys [11- 13]. Recently, the new functions of PKG II in cancers were discovered. Cook et al found that activation of PKG II induced apoptosis of human cultured prostatic stromal cells [14]. Fallahian et al. reported that both in estrogen receptor-positive and -negative breast cancer cell lines, cGMP caused apoptosis by activating PKG II, and they also evaluated that the mRNA expression of PKG II in breast cancer tumor was lower than that in normal breast tissue [15,16]. Swartling et al found that PKG II had an anti-proliferation role in human glioma cells [17]. In our laboratory, research has been focused on PKG II for years and the results demonstrated that PKG II inhibited EGF-EGFR related pathways including MAPK/JNK, MAPK/ERK, and PI3K/AKT mediated signal transduction pathways, resulting from the impediment of EGFR activation in gastric cancer cell lines [18-21]. In addition, we also found that PKG II effectively inhibited the phosphorylation/activation of other Receptor Tyrosine Kinases (RTKs), including Vascular Endothelial Growth Factor Receptor (VEGFR), Platelet-Derived Growth Factor Receptor (PDGFR) and insulin-like growth factor-1 receptor (IGF-1R) [22]. However, the mechanism of PKG II inhibiting RTKs is still unknown. The purpose of this study was to evaluate the function of PKG II on VEGFR-2 activation induced by VEGF-A and the related mechanism in gastric cancer cell lines.
2. Materials and Methods
2.1 Cell lines and reagents
The human gastric cancer cell line HGC-27 and simian virus 40-transformed African Green monkey kidney cell line COS-7 were provided by the Institute of Cell Biology (Shanghai, China). Human Umbilical Vein Endothelial Cells (HUVECs) were from the American Type Culture Collection (Manassas, VA, USA). Adenoviral vectors encoding cDNA of β-galactosidase (Ad-LacZ) and PKG II (Ad-PKG II) were provided by Dr. Gerry Boss and Dr. Renate Pilz of the University of California (San Diego, CA, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). Endothelial Cell Growth Medium-2 (EGM-2) was purchased from Lonza Inc. (Allendale, NJ, USA). VEGF-A was purchased from PeproTech (Rocky Hill, NJ, USA). 8-pCPT-cGMP was purchased from BioLog (Bremen, GER). The polyclonal rabbit anti-human p-Serine/Threonine antibody was from Abcam (Cambridge, MA, USA) and the application dilution was 1:1,000. The polyclonal rabbit anti-human PKG II and the monocolonal mouse anti-human VEGFR-2 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) and the application dilutions were 1:200. The polyclonal rabbit anti-human p-VEGFR-2 (Y1175) antibody was purchased from Bioworld Technology (St. Louis Park, MN, USA) and the application dilution was 1:200. QuikChange Site-Directed Mutagenesis Kit was purchased from Agilent Technologies (Santa Clara, CA, USA).
2.2 Preparation of adenoviral vectors
293A cells were transfected with adenoviral vector encoding cDNA of LacZ and PKG II respectively and cultured for up to ten days until cytopathic effect (CPE) was seen. The cells and the culture medium were harvested and underwent three freezing-thawing cycles. The supernatants containing adenoviruses (Ad-LacZ and Ad-PKG II) were used to infect new 293A cells to amplify adenoviruses. The amplified adenoviral preparations were titrated and the pfu/ml was determined, and kept in -80ºC until use.
2.3 Construction of mutant plasmids
The plasmid pCMV3-Flag-VEGFR-2 encoding full length cDNA of VEGFR-2 with N terminal Flag tag was purchased from Sino Biological Inc. (Beijing, CHN). Mutants of VEGFR-2 were generated by using the QuikChange Site-Directed Mutagenesis Kit. For mutant VEGFR-2 (T393A) whose Threonine 393 was mutated to Alanine, the forward primer was 5’-ATGACAGTGTAATTTCCTGCGTCTCTTTCACTCACTTCC- 3’ and the reverse primer was 5’-GGAAGTGAGTGAAAGAGACGCAGGAAATTACACTGTCAT- 3’. For mutant VEGFR-2 (T439A) whose Threonine 439 was mutated to Alanine, the forward primer was 5’-CAGCGTTTGAGTGGCGCCGTACTGGTAGG- 3’ and the reverse primer was 5’-CCTACCAGTACGGCGCCACTCAAACGCTG- 3’. For mutant VEGFR-2 (S1231A) whose Serine 1231 was mutated to Alanine, the forward primer was 5’-GCAGAACAGTAAGCGAAAGGCCCGGCCTGTGAGT- 3’ and the reverse primer was 5’-ACTCACAGGCCGGGCCTTTCGCTTACTGTTCTGC- 3’. The mutant plasmids were sequenced and the mutations were confirmed.
2.4 Cell culture, transfection and infection
HGC-27 and COS-7 cells were cultured in DMEM supplemented with 10% FBS. HUVECs were cultured in EGM-2 containing 10% FBS. The cells were maintained at 37ºC in a humidified atmosphere of 95% air and 5% CO2. The culture medium was replaced every other day until the cells reached confluence. Passage 3 and 4 cells of HUVECs were used for experiments. For transfection with plasmids, cells were sub-cultured the previous day, and transfection was conducted according to the manufacturer's instructions. For infection with Ad- LacZ and Ad-PKG II, cells were freshly seeded with 70-80% confluence at 1 day prior to the infection.
2.5 Transwell migration assay
The migration activity of HGC-27 cells was detected by using a transwell system (BD BioCoat Control, 8.0 μm PET membrane, 24-well cell culture inserts; BD Biosciences, San Jose, CA, USA). HGC-27 cells were infected with Ad-LacZ or Ad-PKG II for 24 h and serum starved overnight. Following trypsinization, 5×104 cells were seeded into the upper chamber in serumfree culture medium. Then cells in the upper chamber were treated with VEGF-A (10 ng/ml), or treated with 8-pCPT-cGMP (250 μM) for 1 h and then with VEGF-A (10 ng/ml). Migration of the cells to the bottom of the membrane was induced by incubation with medium containing 10% FBS in the lower chamber for 12 h at 37ºC in a tissue culture incubator. The cells remaining in the upper chamber were removed with cotton swabs. Cells that had migrated to the lower side of the membrane were fixed in 4% paraformaldehyde solution for 30 min, stained by Giemsa solution for 10 min and rinsed with water. Stained cells were subsequently examined by light microscopy. Migrated cells were counted in five randomly selected fields per insert, and mean values were calculated. All experiments were performed in triplicate for each migration condition.
2.6 Tube formation assay
Tube formation assay was performed by following the published protocol by Arnaoutva et al [23]. Briefly, 96-well plates coated with BD Growth Factor Reduced Basement Membrane Matrix (50 μl per well, BD Biosciences, Franklin Lakes, NJ, USA) were incubated at 37ºC for 1 h to promote solidification. HUVECs were infected with Ad-LacZ or Ad-PKG II for 24 h and serum starved overnight. Then the cells were suspended in EGM-2 medium containing 10% FBS, and were treated with or without 8-pCPT-cGMP (250 μM) for 1 h. The treated cells were seeded at a density of 1 × 104 cells per well into previously coated 96-well plates and incubated with EGM-2 medium containing 10% FBS with or without VEGF-A (10 ng/ ml). After overnight incubation at 37°C in a 5% CO2 chamber, morphological changes were observed under an Axio observer A1 inverted microscope (Carl Zeiss, Jena, GER). Images were taken by using a phase-contrast microscope at a magnification of 100x. The capillary tubes were quantified by counting the average numbers of completed tubule structures in three randomly selected fields.
2.7 Western blotting
Protein samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDSPAGE) and the 8-12% gel was chosen according to the molecular size of target protein. The electrophoresis and membrane transfer was performed following the Bio-Rad manufacturer’s protocol (Hercules, CA, USA). The primary antibodies were incubated over night at 4ºC in Tris buffered saline containing 1% Tween-20 (TBS-T), and the corresponding secondary antibodies were incubated for 1 h at room temperature (RT) in TBS-T, with three washes after each incubation. ECL reagents were used to show the positive bands on the membrane. To perform densitometry analysis, digital images of the positive bands were obtained with Chemidoc XRS and analyzed by using the image analysis program Quantity One (Bio-Rad, Hercules, CA, USA).
2.8 Co-Immunoprecipitation (Co-IP)
Two days after transfection and/or infection, the cells growing on 10 cm culture plate were washed two times with cold phosphate buffer saline (PBS) and lysed by adding RIPA buffer (50 mM Tris-HCl pH 7.4, 1% Triton X-100, 1 mM EDTA, 1 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 10 mM NaF, 1 mM Na3VO4). The supernatant was obtained by centrifugation (12,000 rpm, 10 min). Then half of the supernatant was incubated with antibodies against VEGFR2 or PKG II and the remainder with matched immunoglobulin as a negative control for 12 h rocking at 4ºC. Fresh protein A/G conjugated to agarose was then added, followed by 2 to 3 h rocking at 4ºC. Immuno-precipitates were centrifuged at 2,000 rpm for 2 min at 4ºC. The supernatant was then discarded, and the pellet was washed four times with binding buffer and then re-suspended with the same volume of 2x SDS sample buffer. The precipitates were probed by Western blotting with antibodies against PKG II and VEGFR-2 respectively.
2.9 Combination of immunoprecipitation and western blotting
The cells were treated same as in Co-IP. The supernatant was incubated with antibody against VEGFR2 or Flag respectively at 4ºC overnight and then with protein A/G conjugated to agarose for 2 h at 4ºC. The precipitates were obtained and treated according to the procedures same as in Co-IP. Then the precipitates were probed by Western blotting with antibodies against pan p-Serine/Threonine or p-VEGFR2 (Y1175).
2.10 Statistical analysis
The data were expressed as means ± standard deviation (SD). Statistical significance was performed using a two-tailed ANOVA with SPSS statistical software. A P-value of less than 0.05 was considered significant.
3. Results
3.1 PKG II inhibits VEGF-induced cell migration and tube formation
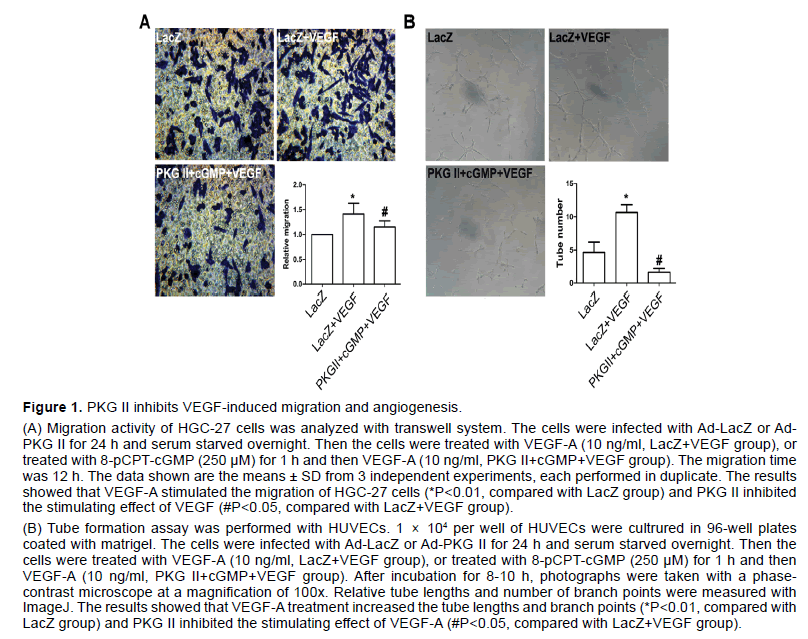
VEGF has been shown to stimulate endothelial cell mitogenesis and cell migration in vitro. To evaluate the effect of PKG II on VEGF-induced migration in gastric cancer, HGC-27 cells were treated as described in “Transwell migration assay”. The results showed that the migration activity of HGC-27 cell line increased obviously after VEGF-A treatment (P<0.05, compared to control group, Figure 1A), whereas activated PKG II by 8-pCPT-cGMP markedly inhibited migration induced by VEGF-A in HGC-27 cells (P<0.05, compared to LacZ+VEGF group, Figure 1A).
VEGF is more potent in pathological angiogenesis relative to tumor growth [24]. To define the functional significance of PKG II in VEGF-induced angiogenesis, the tube formation assay was performed with HUVECs which was most widely used in vitro to model the reorganization stage of angiogenesis. The cells were treated as described in “Tube formation assay”. The results indicated that VEGF-A obviously promoted tube network formation, being indicated by the increase of branching points (P<0.05, compared to control group, Figure 1B). However, the number of branching point per field decreased markedly in cells infected with Ad- PKG II and treated with 250 μM 8-pCPT-cGMP for 1 h prior to treatment with VEGF-A (P<0.05, compared to LacZ+VEGF group, Figure 1B).
The above results suggested that PKG II inhibited both VEGF-induced cell migration in HGC-27 cells and VEGF-stimulated tube formation in HUVECs.
3.2 PKG II phosphorylates VEGFR-2 by combination with VEGFR-2
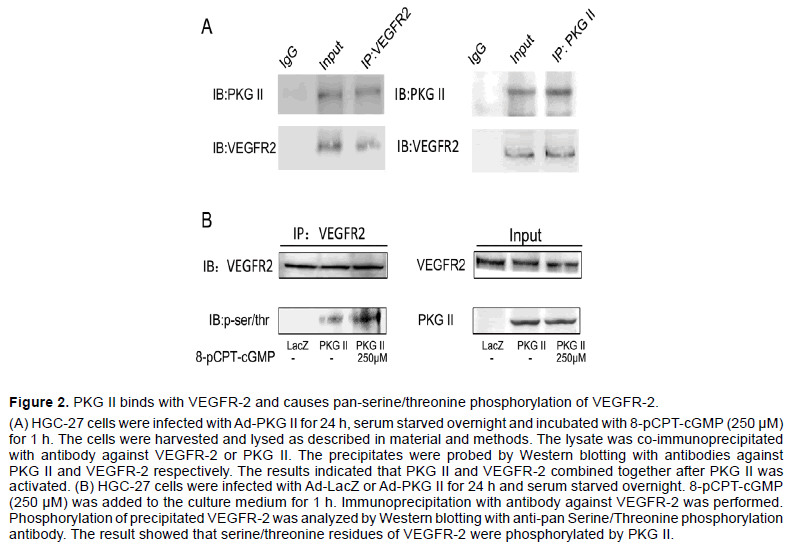
We previously reported that PKG II prevented Tyrosine 951 (Tyr951) phosphorylation of VEGFR-2 and activation of downstream ERK and PI3K pathways induced by VEGF-C [22]. We also reported before that PKG II inhibited EGF-induced phosphorylation of EGFR and HER2 by combining with EGFR and HER2 directly [18,25]. To explore the possibility of interaction between PKG II and VEGFR-2, Co-IP assay was used to detect the relationship between PKG II and VEGFR-2. In HGC-27 cells infected with Ad-PKG II and then stimulated with 8-pCPT-cGMP, binding between PKG II and VEGFR-2 was detected (Figure 2A).
Figure 1:PKG II inhibits VEGF-induced migration and angiogenesis
(A) Migration activity of HGC-27 cells was analyzed with transwell system. The cells were infected with Ad-LacZ or Ad- PKG II for 24 h and serum starved overnight. Then the cells were treated with VEGF-A (10 ng/ml, LacZ+VEGF group), or treated with 8-pCPT-cGMP (250 μM) for 1 h and then VEGF-A (10 ng/ml, PKG II+cGMP+VEGF group). The migration time was 12 h. The data shown are the means ± SD from 3 independent experiments, each performed in duplicate. The results showed that VEGF-A stimulated the migration of HGC-27 cells (*P<0.01, compared with LacZ group) and PKG II inhibited the stimulating effect of VEGF (#P<0.05, compared with LacZ+VEGF group).
(B) Tube formation assay was performed with HUVECs. 1 × 104 per well of HUVECs were cultrured in 96-well plates coated with matrigel. The cells were infected with Ad-LacZ or Ad-PKG II for 24 h and serum starved overnight. Then the cells were treated with VEGF-A (10 ng/ml, LacZ+VEGF group), or treated with 8-pCPT-cGMP (250 μM) for 1 h and then VEGF-A (10 ng/ml, PKG II+cGMP+VEGF group). After incubation for 8-10 h, photographs were taken with a phasecontrast microscope at a magnification of 100x. Relative tube lengths and number of branch points were measured with ImageJ. The results showed that VEGF-A treatment increased the tube lengths and branch points (*P<0.01, compared with LacZ group) and PKG II inhibited the stimulating effect of VEGF-A (#P<0.05, compared with LacZ+VEGF group).
As a serine/threonine protein kinase, PKG II often phosphorylates its substrates when binds with them. To examine whether PKG II causes serine/threonine phosphorylation of VEGFR-2, HGC-27 cells were infected with Ad-PKG II or Ad-LacZ, and then treated with or without 8-pCPT-cGMP. The VEGFR-2 in the lysates of the cells was separated/enriched by immunoprecipitation and then subjected to Western blotting with antibody against pan-phosphorylation of serine/threonine. The results showed that in cells infected with Ad-PKG II and treated with 8-pCPTcGMP, there was an obvious increase of serine/ threonine phosphorylation of VEGFR-2 (Figure 2B). These results indicated that PKG II resulted in serine/ threonine phosphorylation of VEGFR-2 through direct combination.
PKG II inhibits VEGF-induced activation of VEGFR-2 by phosphorylating VEGFR-2 at Thr439 and Ser1231.
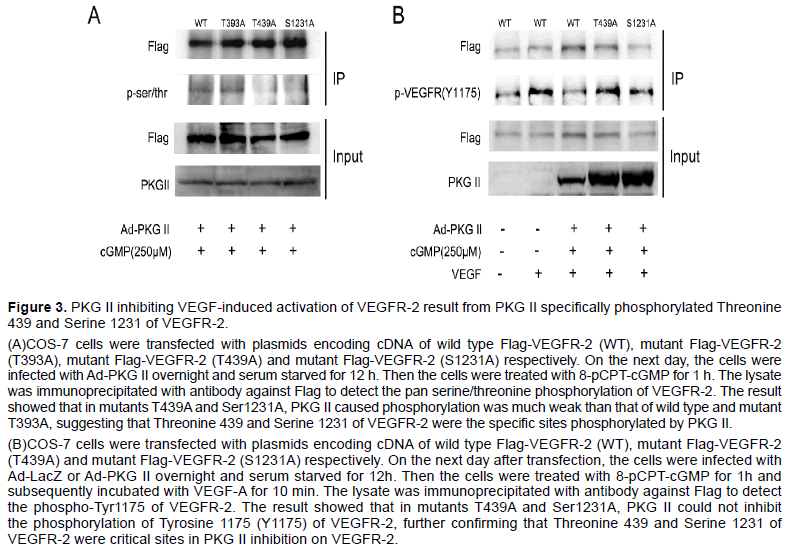
Group-based Prediction System 2.0 (GPS2.0) software could predict kinase-specific phosphorylation sites for 408 human Protein Kinases in hierarchy including PKG II [26]. We used GPS2.0 software to predict PKG II specific serine/threonine phosphorylation sites on VEGFR-2 and the results showed that Threonine 393 (T393), Threonine 439 (T393) and Serine 1231 (S1231) were potential phosphorylating sites. To determine whether these sites were real or not, plasmids encoding cDNA of mutant VEGFR-2 in which threonine and serine were mutated to alanine were constructed (T393A, T439A and S1231A, tagged with Flag). COS-7 cells were transfected respectively with the plasmids of T393A, T439A and S1231A, and the expression products of VEGFR-2 mutants were isolated by immunoprecipitation with antibody against Flag. Western blotting with antibody against pan serine/threonine phosphorylation was applied to detect the phosphorylation of the mutant VEGFR-2. The results showed that when PKG II activity was increased, the serine/threonine phosphorylation was down-regulated in VEGFR-2 mutants of T439A and S1231A but not in VEGFR-2 mutant of T393A (Figure 3A). Furthermore, PKG II could not inhibit tyrosine phosphorylation of VEGFR-2 mutants of T439A and S1231A (Figure 3B). These results confirmed that Threonine 439 and Serine 1231 were the PKG II specific phosphorylation sites on VEGFR-2 and the phosphorylation of both sites were crucial for the inhibition of PKG II on VEGFR-2 activation.
Figure 2:PKG II binds with VEGFR-2 and causes pan-serine/threonine phosphorylation of VEGFR-2.
(A) HGC-27 cells were infected with Ad-PKG II for 24 h, serum starved overnight and incubated with 8-pCPT-cGMP (250 µM) for 1 h. The cells were harvested and lysed as described in material and methods. The lysate was co-immunoprecipitated with antibody against VEGFR-2 or PKG II. The precipitates were probed by Western blotting with antibodies against PKG II and VEGFR-2 respectively. The results indicated that PKG II and VEGFR-2 combined together after PKG II was activated. (B) HGC-27 cells were infected with Ad-LacZ or Ad-PKG II for 24 h and serum starved overnight. 8-pCPT-cGMP (250 µM) was added to the culture medium for 1 h. Immunoprecipitation with antibody against VEGFR-2 was performed. Phosphorylation of precipitated VEGFR-2 was analyzed by Western blotting with anti-pan Serine/Threonine phosphorylation antibody. The result showed that serine/threonine residues of VEGFR-2 were phosphorylated by PKG II.
Figure 3:PKG II inhibiting VEGF-induced activation of VEGFR-2 result from PKG II specifically phosphorylated Threonine 439 and Serine 1231 of VEGFR-2.
(A)COS-7 cells were transfected with plasmids encoding cDNA of wild type Flag-VEGFR-2 (WT), mutant Flag-VEGFR-2 (T393A), mutant Flag-VEGFR-2 (T439A) and mutant Flag-VEGFR-2 (S1231A) respectively. On the next day, the cells were infected with Ad-PKG II overnight and serum starved for 12 h. Then the cells were treated with 8-pCPT-cGMP for 1 h. The lysate was immunoprecipitated with antibody against Flag to detect the pan serine/threonine phosphorylation of VEGFR-2. The result showed that in mutants T439A and Ser1231A, PKG II caused phosphorylation was much weak than that of wild type and mutant T393A, suggesting that Threonine 439 and Serine 1231 of VEGFR-2 were the specific sites phosphorylated by PKG II.
(B)COS-7 cells were transfected with plasmids encoding cDNA of wild type Flag-VEGFR-2 (WT), mutant Flag-VEGFR-2 (T439A) and mutant Flag-VEGFR-2 (S1231A) respectively. On the next day after transfection, the cells were infected with Ad-LacZ or Ad-PKG II overnight and serum starved for 12h. Then the cells were treated with 8-pCPT-cGMP for 1h and subsequently incubated with VEGF-A for 10 min. The lysate was immunoprecipitated with antibody against Flag to detect the phospho-Tyr1175 of VEGFR-2. The result showed that in mutants T439A and Ser1231A, PKG II could not inhibit the phosphorylation of Tyrosine 1175 (Y1175) of VEGFR-2, further confirming that Threonine 439 and Serine 1231 of VEGFR-2 were critical sites in PKG II inhibition on VEGFR-2.
4. Discussion
The VEGF mediated signaling pathway is the main signaling pathway governing angiogenesis in physiological condition [27]. VEGF also has vasculogenic roles in tumor development, and VEGFR-2 is predominantly responsible for tumor angiogenesis. Some researchers found that VEGF released from tumor cells activates VEGFR-2, which is crucial to drive tumor angiogenesis [7,28]. As a classical protein kinase, the new functions of PKG II in cancers are continually established. More and more data suggested that this kinase was a potential anti-tumor factor. The results of this study confirmed that PKG II inhibited VEGF-stimulated migration of gastric cancer cells and tube formation of the endothelial cells. This not only gives hint to the strategy of VEGFR-2 targeted anti-cancer therapy but also provides evidence to confirm PKG II as an anti-cancer factor.
VEGFR-2 is the major receptor in the VEGF signaling pathway that regulates cell migration, proliferation, and angiogenesis. Once VEGFR-2 binding with its ligand VEGF, the trans/auto phosphorylation of intracellular tyrosine residues could be activated. In the previous research, we found that the activation of RTKs family members, including EGFR, HER2, PDGFR, and IGF-1R, were inhibited by PKG II [18,22,25]. Among them, we confirmed that inhibition of PKG II on EGF-induced EGFR/HER2 activation resulted from phosphorylating serine/threonine of EGFR/HER2. In previous study we also found that PKG II inhibited VEGFR-2 tyrosine phosphorylation induced by VEGF-C and in turn the VEGFR-2- mediated signaling pathway [22]. Therefore we wondered whether the mechanism of inhibition of PKG II on VEGFR-2 was similar to that of EGFR/ HER2. In the present study, our results revealed that PKG II combined with VEGFR-2 directly and caused serine/threonine phosphorylation of VEGFR-2. This result was consistent with our previous findings: PKG II impeded tyrosine kinase activity of RTKs by increasing their serine/threonine phosphorylation.
To further explore the possible PKG II specific phosphorylation site of VEGFR-2, GPS2.0 software was used to predict the sites first. Then, the recommended PKG II specific serine/threonine sites of VEGFR-2 were mutated into alanine which could not be phosphorylated by PKG II and the phosphorylating action of PKG II on the mutants was detected. The results showed that phosphorylation of both T439A and S1231A mutants of VEGFR-2 decreased compared to wild type after PKG II was activated. Auto-phosphorylation is the representative process in activation of RTKs. Tyr1175 is one of the major auto-phosphorylation sites of VEGFR-2. By detecting Tyr1175 phosphorylation of VEGFR-2, we further confirmed that PKG II inhibited activation of VEGFR-2 through phosphorylating Thr439 and Ser1231.
5. Conclusion
In conclusion, our present study elucidates that PKG II inhibits VEGF-induced angiogenesis and migration. Preliminary investigations into mechanism demonstrate that PKG II directly inhibits VEGFR-2 activation through binding with and phosphorylating Thr439 and Ser1231 of VEGFR-2. These results support that PKG II is a potential tumor inhibitor and will provide novel thought on strategy of cancer therapy.
6. Acknowledgement
This study was supported by grants from the Science and Technology Cooperation Project of Jiangsu University (No. 2014079); the National Natural Science Foundation of China (No. 81272755, 81201959); China Postdoctoral Science Foundation (No. 2014M561599); Postdoctoral Research Funding Plan in Jiangsu Province (No. 1401144C); Graduate Student Research and Innovation Program of Jiangsu Province (No. CXZZ13_0701). We thank Dr. Gerry Boss and Dr. Renate Pilz in University of California, San Diego, U.S.A. for the kind gifts of Adenoviral constructs.
References
- Ferlay J, Shin HR, Bray F, et al. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer.127:2893–2917.
- Dey N, De P, Brian LJ. (2015). Evading anti-angiogenic therapy: Resistance to anti-angiogenic therapy in solid tumors. Am J Transl Res. 7:1675-1698.
- Gasparini G, Longo R, Toi M, Ferrara N. (2005).Angiogenic inhibitors: A new therapeutic strategy in oncology. Nat ClinPractOncol.2:562–577.
- Mihalache A, Rogoveanu I. (2014). Angiogenesis factors involved in the pathogenesis of colorectal cancer. Curr Health Sci J.40:5-11.
- Costache MI, Ioana M, Iordache S, et al. (2015). VEGF expression in pancreatic cancer and other malignancies: A review of the literature. Rom J Intern Med.53:199-208.
- Takahashi S. (2011). Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull.34:1785-1788.
- Ozdemir F, Akdogan R, Aydin F, et al. (2006). The effects of VEGF and VEGFR-2 on survival in patients with gastric cancer. J ExpClin Cancer Res. 25:83-88.
- Takahashi T, Shibuya M. (1997). The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma N pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 14: 2079–2089.
- Quinn TP, Peters KG, De Vries C, et al. (1993). Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. ProcNatlAcadSci USA.90:7533–7537.
- Aprile G, Ferrari L, Cremolini C, et al. (2016). Ramucirumab for the treatment of gastric cancers, colorectal adenocarcinomas and other gastrointestinal malignancies. Expert Rev ClinPharmacol.9:877-885.
- Forte LR, London RM, Krause WJ, et al. (2000). Mechanisms of guanylin action via cyclic GMP in the kidney. Annu Rev Physiol.62: 673–695.
- Rangaswami H, Marathe N, Zhuang S, et al. (2009). Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J Biol Chem.284: 14796–14808.
- Gambaryan S, Wagner C, Smolenski A, et al. (1998). Endogenous or overexpressed cGMP-dependent protein kinases inhibit cAMP dependent renin release from rat isolated perfused kidney, micro-dissected glomeruli, and isolated juxtaglomerular cells. ProcNatlAcadSci USA.95: 9003–9008.
- Cook AL, Haynes JM. (2004). Protein kinase G II-mediated proliferative effects in human cultured prostatic stromal cells. Cell Signal.16: 253–261.
- Fallahian F, Karami-Tehrani F, Salami S. (2011). Cyclic GMP induced apoptosis via protein kinase G in oestrogen receptor-positive and -negative breast cancer cell lines. FEBS J.278: 3360–3369.
- KaramiTehrani F, Fallahian F, Atri M. (2012). Expression of cGMP-dependent protein kinase, PKGIa, PKG Iß and PKG II in malignant and benign breast tumors. Tumor Bio.33: 1927-1932.
- Swartling FJ, Ferletta M, Kastemar M, et al. (2009). Cyclic GMP-dependent protein kinase II inhibits cell proliferation, Sox9 expression and Akt phosphorylation in human glioma cell lines. Oncogene. 28:3121-3131.
- Jiang L, Lan T, Chen Y, et al. (2013). PKG II inhibits EGF/EGFR induced migration of gastric cancer cells. PLoS One.8: e61674.
- Wu Y, Chen Y, Qu R, et al. (2012). Type II cGMP dependent protein kinase inhibits EGF triggered signal transduction of the MAPK/ERK mediated pathway in gastric cancer cells. Oncol Rep.27: 553 558.
- Lan T, Chen Y, Sang J, et al. (2012). Type II cGMP dependent protein kinase inhibits EGF induced MAPK/JNK signal transduction in breast cancer cells. Oncol Rep.27: 2039-2044.
- Wu M, Chen Y, Jiang L, et al. (2013). Type II cGMP-dependent protein kinase inhibits epidermal growth factor-induced phosphatidylinostol3-kinase/Akt signal transduction in gastric cancer cells. OncolLett.6:1723-1728.
- Jiang L, Chen Y, Sang J, et al. (2013). Type II cGMP-dependent protein kinase inhibits activation of key members of the RTK family in gastric cancer cells. Biomed Rep.1: 399-404.
- Arnaoutova I, George J, Kleinman HK, Benton G. (2009). The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis.12:267–274.
- Ferrara N, Davis-Smyth T. (1997). The biology of vascular endothelial growth factor. Endocr Rev.18:2-25.
- Zhu ML, Yao XY, Wu M, et al. (2016). Type II cGMP-dependent protein kinase directly inhibits HER2 activation of gastric cancer cells. Mol Med Rep.13: 1909-1913.
- Xue Y, Ren J, Gao XJ, et al. (2008). GPS 2.0, a Tool to Predict Kinase-specific Phosphorylation Sites in Hierarchy. Mol Cell Proteomics.7: 1598-1608.
- Ferrara N, Gerber HP, LeCouter J. (2003). The biology of VEGF and its receptors. Nat Med.9: 669-676.
- Olsson AK, Dimberg A, Kreuger J, Claesson Welsh L. (2006). VEGF receptor signaling-in control of vascular function. Nat Rev Mol Cell Biol.7:359–371.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences