The Comparison of Histopathological Results of the Repair of Dura Defects on Rats Using Different Collagen Based Dura Grafts
Mehmet Atif Erol Aksekili, Okan Ateş, Kaan Yüksel, Mahmut Uğurlu, Aydan Kiliçarslan, Nihat Tosun
1Department of Orthopedic Surgery, Atatürk Training and Research Hospital, University of Yıldıırım Beyazıt, Turkey
2Orthopedic Department, Haymana Public Hospital, Turkey
3Institute of Pathology, University of Yıldıırım Beyazıt, Turkey
Received date: April 18, 2017; Accepted date: May 22, 2017; Published date: May 29, 2017
Citation: Aksekili MAE, Ateş O, Yüksel K, et al. The Comparison of Histopathological Results of the Repair of Dura Defects on Rats Using Different Collagen Based Dura Grafts. Electronic J Biol, 13:2
Abstract
Introduction: In cases of celebral cortex damage, such as head traumas or intracranial operations, there is a need to use biocompatible materials (semi-synthetic dural graft) which will allow natural dura formation, prevent the leakage of Cerebral Spinal Fluid (CSF) and which will be completely removed from the body in 3-6 months. Therefore, the spongeous structure of collagen, which is a natural protein of the body, is given membrane form and implanted to the defect area. The aim of this study was to determine the best dura graft by creating a dura mater defect on rats. Methods: A total of 31 female Wistar rats were randomly divided into 5 groups as Group 1 (n=8) dural defect with bovine collagen-origin dura, Group 2 (n=8) dural defect with collagen-based synthetic dura produced from bovine type 1 collagen, Group 3 (n=7) reconstruction with autograft taken from suboccipital fascia, Group 4 (n=5) control group-dural defect with no further procedure and Group 5 (n=3) sham group. At 90 days after the surgical procedures, all the rats were sacrificed under deep anaesthesia. Results: Parenchymal tissue was examined histologically in respect of fibrosis, capillary formation, cellular reaction, capsule formation and foreign body reaction. In leptomeningeal tissues, foreign body reaction, capsule development, integration of artificial brain membrane, inflammation of artificial brain membrane and histological adhesion were examined and the results were compared. While no significant difference was seen between the groups in respect of fibroblastic activity and inflammation, capillary formation was significantly positive in Group 3 autograft and artificial brain membrane integration was significantly positive in Groups 1 and 2, to a greater degree in Group 2, in semi-synthetic collagen based artificial brain membrane. Conclusion: Thus it was concluded that synthetic grafts are adequate and usable in terms of duraplasty.
Keywords
Collagen based artificial brain membrane; Histological adhesion; Artificial brain membrane integration; Duraplasty.
1. Introduction
The dura mater is the most important internal barrier in front of the spinal cord. Therefore, the dural closure is one of the most significant factors determining mortality and morbidity in neurosurgery. Dural closure must be applied with watertight suturing of autografts such as pericranium, temporal fascia or artificial dura grafts. Otherwise, complications such as infection, brain herniation, CSF (cerebrospinal fluid) fistula, meningocerebral adhesion and brain scars are inevitable [1-10]. In cases such as head traumas, or brain surgery where the cerebral cortex is damaged, biocompatible materials must be used for natural dura formation, which prevent CSF leakage and are completely re-absorbed (biodegradable) into the system within the expected time period (3-6 months) Autografts used for this purpose are excellent in terms of biocompatibility, toxicity and immunogenicity, although there are the disadvantages of an enlarged surgical incision and prolonged operating time [11-16]. Cadaveric grafts are also used for this purpose, but are not a common preference due to the risk of Creutzfeldt-Jakob Disease. Biological animal materials (sheep and bovine pericardium, pig peritoneum) are quite good biocompatible materials, although there is the risk of immunoreaction and contagious animal infections [17]. Artificial biological materials are another option. Synthetic materials such as PGA (Polyglycolic Acid), PLGA (poly lactic-co-glycolic acid) have been used for many years [18]. Mechanically, these are nontoxic, strong materials which provide long-term stability. Other natural materials such as gelatine, and silk fibroin have also been used for the same purpose [19-27]. A sponge membrane form is given to collagen, which is a natural system protein, and it is implanted in the defect area. This material is expected to both enable the natural dura formation and prevent CSF leakage, and then by spontaneous re-absorption, it is replaced by the natural dura. The aim of this study was to evaluate the formation of dura mater and the impermeability of the repair with these materials applied to a dural defect in an experimental rat model.
2. Materials and Methods
Approval for the study was granted by the Local Ethics Committee, Ankara, Turkey (protocol no 120, dated 03/10/2014). All the procedures were applied in accordance with the policies of the Local Ethics Commitee policies of the Experimental Research Animals Laboratory. The rats used in the study were obtained from those bred in the Experimental Research Animals Laboratory, Ankara, Turkey. The study included a total of 31 female Wistar rats, aged 8-10 weeks and each weighing 300 g ± 10%. The animals were kept in plastic-based cages bottoms with free access to food and water. The cages were lined with sawdust and cleaned 4 times per week. The rats were kept at a room temperature of 23°C with 60% humidity and a 12 h light/dark cycle and full air exchange every 12 h. Standard pellets were given as food and purified tap water was provided in a macrolon autoclavable bottle. All the processes for the animals were conducted in accordance with hygiene rules.
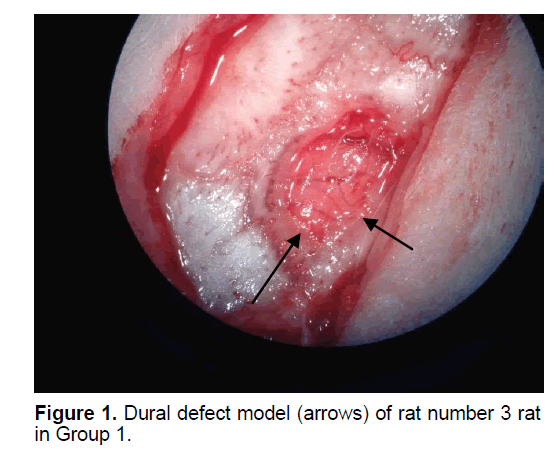
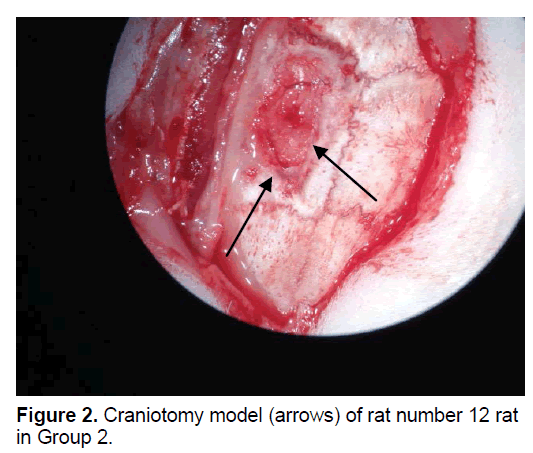
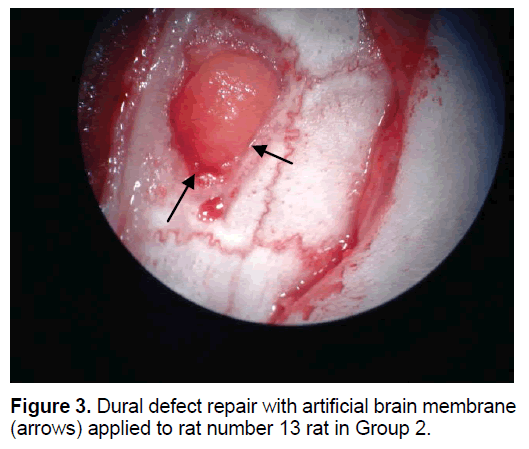
The rats were randomly assigned to 1 of 5 groups. In Group 1 (n=8), the dural defect (Figure 1) was reconstructed with artificial brain membrane of bovine origin. (Codman Duraform®). In Group 2 (n=8), the dural defect (Figure 2) was reconstructed with artificial brain membrane of bovine type 1 collagen origin (Decoll®, Desu Medical, Ankara, Turkey) (Figure 3). In Group 3 (n=7), the dural defect was reconstructed with autograft from the suboccipital fascia. Group 4 (n=5) was determined as the control group and no treatment was made to the dura defects formed in this group. Group 5 (n=3) was defined as the sham group and no dura defect was made. The purpose of Group 4 was to compare the effect of the materials used in dural reconstruction with saline and to isolate the effects of external factors. The rats were anesthetized using an intramuscular injection of 100 mg/kg ketamin (alfamine®, Egevet, 100 mg/ml) and 8 mg/kg xylazine (alfazyne, egevet, 2%) with a 30 G injector. Prophylactic antibiotic of 0.25 cc amoxicillin was injected both preoperatively and for 3 days before the surgery. Correspondingly, 0.25 ml meloxciam (moxcam) was injected subcutaneously as Non-Steroid Anti-İnflammatory Drug (NSAID) for pain relief. The rats were prepared in the prone position of sterotactic frame, then the scalp and suboccipital area were shaved. Following disinfection with Polyvinyl pyrolidone Iodine complex (Batticon%10) and draping, an incision of approximately 3 cm was made from the midline of the cranium. Osteoclastic craniectomy of 1.5 × 1 cm was applied from the right parietal area using a high speed burr. A section of dura 4 × 4 mm in size was removed under microscopic observation (KL 1500 lCD-Olympus®). Implants were cut to the appropriate sizes for Groups 1 and 2, fascia was taken from the suboccipital area with physiological saline in Group 3 and the defect was closed to extend 3 mm beyond the edges of the defect. The scalp was sutured using Polypropylene 3.0 Suture (Ethicon, USA). Oxytetracycline hydrochloride aerosol (Neo- Caf Spray®Intervet, Lazio, Italy) was applied to the suture line. For analgesic purposes, Meloxicam 0.05 ml/day (maxicam®, sanovel, 5 mg/ml) was applied subcutaneously for 3 days following the surgery with a 25 G injector. Prophylactic antibiotics, amoxicillin (amoxycure®, la-provet, 150 mg) were applied by intramuscular injection at 50 mg/kg dose with a 21 G injector for 3 days. The rats were checked daily by a vet experienced with laboratory animals and the cages were changed three times a week.
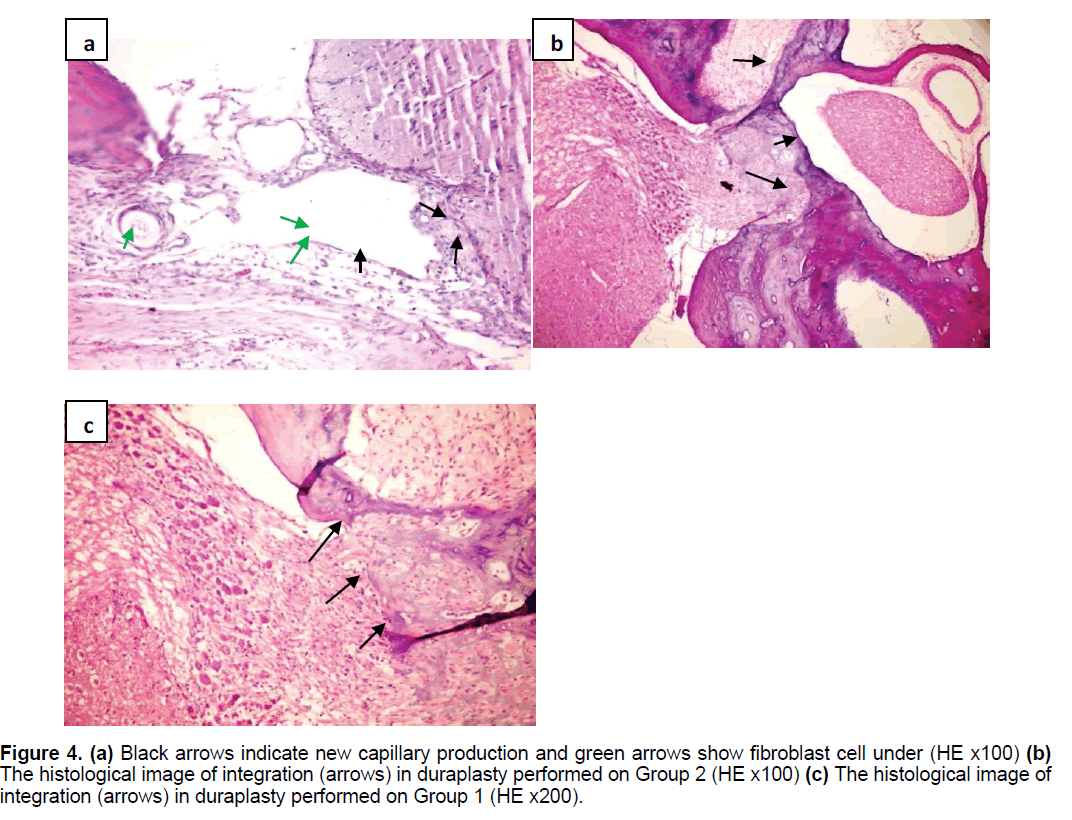
Postoperative neurological behaviors were evaluated using the Modified Garcia Score in the 1st and 4th weeks [34]. In the first month of surgery; three rats from Group 1, four rats from Group 2, three rats from Group 3,two rats from the control group and 1 from the sham group were sacrificied by intramuscular anesthesia (ketamin) following the injection of saline and then 4% paraformaldehyde into the left ventricle with a 21 G injector after thoracotomy. The transaction area of the brain was harvested and placed in a histology dish. In the 4th week postoperatively, the osteoclastic defect was observed to have ossified in varying degrees from the periphery to the center. By enlarging the defect, the whole tissue block including parenchyma, meninges and duroplasty was taken for histological examination. Specimens were kept in 4% formaldehyde for at least 7 days and were then fixed for pathology examination The primary microscopic examination was made of sections stained with Hematoxylin and Eosin (HE). The presence or absence of fibrosis was detected by Masson Trichrome (MT) staining. Microscopic evaluation with HE staining was applied to determine fibrosis/fibroplasia, capillary development, cellular reaction, capsule development, and foreign body reaction (Figure 4).
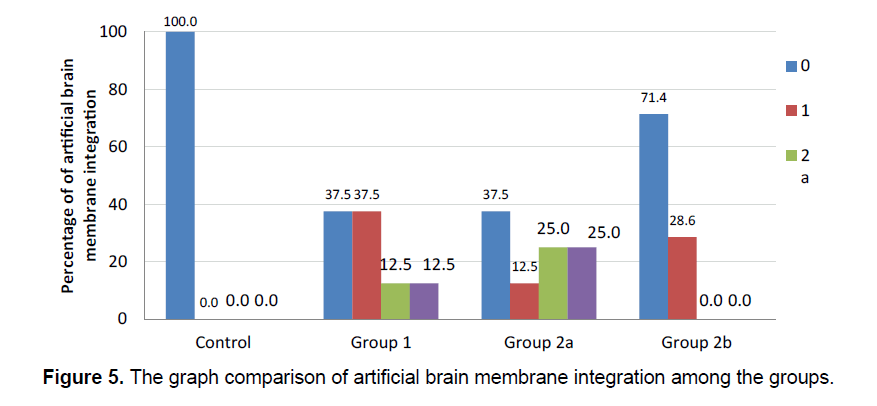
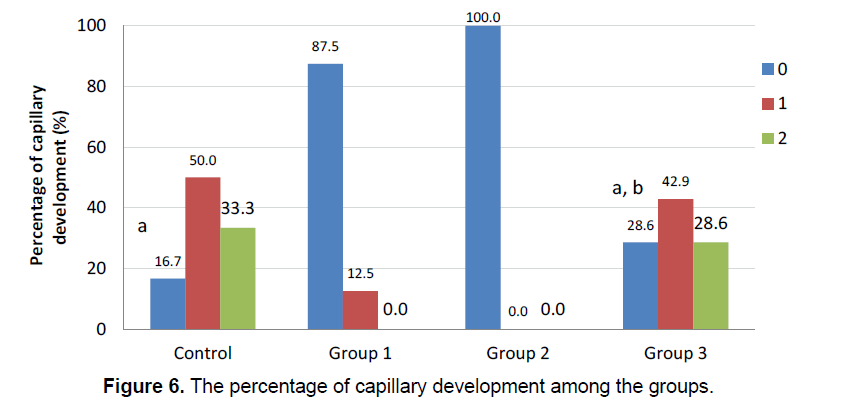
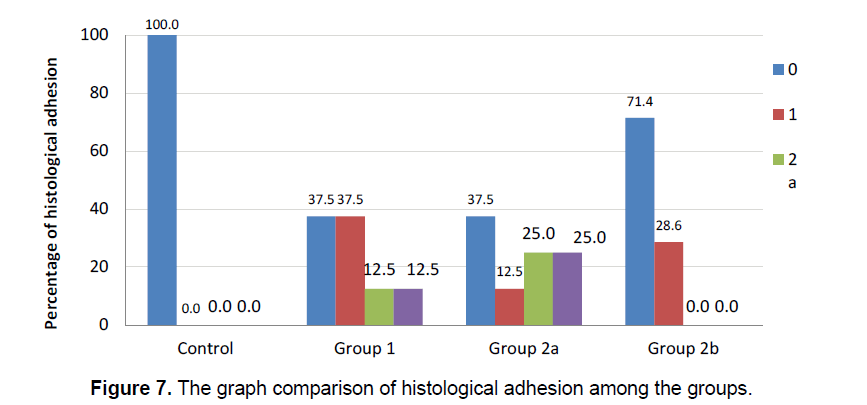
The integration of the artificial brain membrane was evaluated as follows; 0: No adhesion 1: Adhesion of the artificial brain membrane in the leptomeninges (a), in the cortex (b) 2: Adhesion of the artificial brain membrane with fibroblastic reaction and fibrosis in leptomeninges (a), in the cortex (b) (Figure 5) Capilary development and artificial brain membrane inflammation were evaluated as follows; 0:none, 1:minimal, 2: moderateextensive (Figure 6). Histological adhesion was evaluated as:0:No Adhesion, 1: Adhesion of the artificial brain membrane in the leptomeninges (a), in the cortex (b) 2: Adhesion of the artificial brain membrane with fibroblastic reaction and fibrosis in leptomeninges (a), in the cortex (b).
3. Results
Calculations and analyses of the study data were made using IBM SPSS Statistics 21.0 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) and MS-Excel 2007 software.The distributions of variables were stated as numbers (n) in the groups. The difference of sequential result variables between the groups (fibrosis, inflammation, capillary formation, ABM Intg (artificial brain membrane integration) was analyzed using the Kruskal Wallis test. In cases of statistical significance, Bonferroni correction was applied. The Fisher-Freeman Halton test was used in the comparison of categorical variables and the variables (presence-absence) were re-coded into categorical versions in the groups. A value of p<0.05 was accepted as statistically significant (Table 1).
| Groups | N | Mean (+) Rank | |
|---|---|---|---|
| F_Fibrosis | Control | 6 | 19.67 |
| Group 1 | 8 | 12.63 | |
| Group 2 | 8 | 11.06 | |
| Group 3 | 7 | 18.21 | |
| Total | 29 | ||
| I_Inflammation | Control | 6 | 15.42 |
| Group 1 | 8 | 13 | |
| Group 2 | 8 | 13 | |
| Group 3 | 7 | 19.21 | |
| Total | 29 | ||
| C_Capillary Formation | Control | 6 | 21.75 |
| Group 1 | 8 | 11.06 | |
| Group 2 | 8 | 9.5 | |
| Group 3 | 7 | 20 | |
| Total | 29 | ||
| ABM Intg_Artificial Brain Membrane Integration | Control | 6 | 9 |
| Group 1 | 8 | 17.69 | |
| Group 2 | 8 | 19.19 | |
| Group 3 | 7 | 12.29 | |
| Total | 29 | ||
- Table 1. The statistics of the findings observed among the groups (n :Number of animals inÃÆââ¬Å¡Ãâàeach groups).
Fibroblastic activity and İnflammatory reaction were observed to be similar in the study groups. (χ2=6.889; p=0.076 ve χ2=7.313; p=0.063, respectively). The presence of fibroblastic activity in the groups was statistically significant, but not significantly different between the groups. The capillary formation in the groups was different in at least one group (Figure 6) (χ2=15.183; p=0.001). The capillary formation in the control group was significantly higher than in Groups 1 and 2. In Group 3, capillary formation was significantly higher than in Group 2 and Group 1. The variables of capillary formation and ABM Intg showed statistically significant differences in Group 2 compared to the other groups (χ2=15.077; p=0.002 ve χ2=8.146; p=0.043, respectively) (Figure 5) Dural capillary formation, ABM inflammation(artificial brain membrane inflammation) and histological adhesion were statistically similar in all groups(χ2=6.493; p=0.090). Histological adhesion was not statistically significant (Figure 7).
Figure 4. (a) Black arrows indicate new capillary production and green arrows show fibroblast cell under (HE x100) (b) The histological image of integration (arrows) in duraplasty performed on Group 2 (HE x100) (c) The histological image of integration (arrows) in duraplasty performed on Group 1 (HE x200).
There was no statistically significant difference between groups in Modifiye Garcia scores for functional evaluation, applied on the 7th day and at the end of the 1st month postoperatively (χ2=8.300 p=0.149).
4. Discussion
In this study, a histological comparison was made of duroplasty applied with semi-synthetic collagenbased bovine dural material, bovine type 1 collagen dural material and autologous graft from the suboccipital fascia.
An impermeable dural repair is crucial in dural closure. Nonetheless, CSF leakage has been reported in 5%-10% of cases. Primary closure is not always possible in all cases and there may be a need for graft for full closure. Therefore, collagen-based grafts have started to be more widely used recently. These are absorbable materials and complication rates are lower [17,21-24]. As it is a direct hemostatic and coagulant initiator, the collagen matrix creates a chemical signal and attracts fibroblasts, which are effective for 10-14 days, starting on the 3rd and 4th day. Fibroblasts provide the basis of collagen structure by using the porous collagen matrix as a scaffold. Through this porosity, adequate tissue adhesion is provided and it reduces possible leaks. There is no need for extra fixation if the overlay between graft and dura is sufficient in closures made with collagen matrix. This facilitates the closure. The resorption of collagen matrix in 6-8 weeks does not cause complications as in static and non-absorbable materials. These complications include foreign body reaction, inflammatory reaction, unusual neovascularization and related bleeding and hematoma which can create nerve pressure in the long-term. In closures made with collagen matrix, there is adhesion around the graft, which depends on the membrane formed by the fibroblasts. Excess meningocerebral adhesions due to neomembrane and inflammatory cell reaction in previously used materials, caused a lack of confidence. The absence of capsule and neomembrane formation in the collagen matrix group of materials makes it more reliable in this regard. This is due to the porous structure of the collagen matrix used as a scaffold by fibroblasts. The dural graft to be used must be easy to place, impermeable, fracture-resistant and it should not require an extended surgical incision, which can cause meningoserebral adhesion and trigger local and systemic reactions. (immunological, toxic, prion infection) [2-12,21-24,27-29]. In the current study, no symptoms of infection were observed in any of the rats. No CSF leakage or fistula were detected clinically. When the synthetic grafts and periosteum were compared, an adequate barrier was seen to have been formed in all three. While the best capillary formation was seen in the control group, it was followed by the autologue (periosteum) group. This raises the question of whether vascularization and the long-term results in foreign body-based repairs are as good as in autologous repairs. Atci et al. reported similar findings and concluded that it was not very important in terms of graft function [1]. Graft integration was histologically satisfactory in all three groups at the end of the third month, but was better in Group 2 (the bovine-derived collagen matrix group). This demonstrated that the integration of synthetic grafts could be better than that of autografts. Neulen et al showed that the integration of artificial grafts was quite good [3]. No significant difference was seen between the control group and the other groups in terms of fibrosis, inflammation and adhesion.
It can be considered that stronger, more statistically significant results could have been obtained from this study with a larger number of animals. As the study was conducted on rats, there were also difficulties in terms of surgical technique and limitations to the procedures that could be applied. Further studies of a porcine model where CSF pressure can be measured would contribute an extra richness of data and different ideas. Graft survival couldn't be followed due to the difficulty of monitoring the animals for a long time and limited laboratory facilities [30,31].
The modified Garcia score is a complete functional score that assesses the general condition. That there was no statistically significant difference between the groups with the same healing process suggests that applied treatment methods are not clinically different in the short-term, but there may be differences in terms of complications [32-34].
The results of this study demonstrated the biocompatibility and integration of collagen-based artificial cerebral membranes (collagen matrix) with local tissues and suggested reasons for any complications in the process (infection, fibrosis, CSF leakage).
5. Conclusion
Better histopathologic integration can be obtained in the duraplasty process by using semi-synthetic collagen-based artificial brain membrane, than with autologous grafting (periosteal) and thus can be considered a good alternative without the negative aspects of autologous graft.
6. Acknowledgement
We would like to thank Desu Medical for providing the grafts.
References
- Atci IB, ÃÆÃâÃâââ¬âzer FD, Mesut ME, et al. (2013). Comparison of the histopathologic outcome of three different allograft used for the repair of spinal dural defect in rats. J Neur Sci. 30: 347-355.
- Mater DP, Matriksin DT. (2006) Suitability of collagen matrix as a dural graft in the repair of experimental posterior fossa dura mater defects. Tur Neurosur. 16: 9-13.
- Neulen A, Gutenberg A, TakÃÆÃâÃâács I, et al. (2011). Evaluation of efficacy and biocompatibility of a novel semisynthetic collagen matrix as a dural onlay graft in a large animal model. Acta Neurochirurgica. 153: 2241.
- Hamzaoglu V, Ozalp H, Karkucak A, et al. (2015). Comparison of the efficiency, side effects and complications of the synthetic dural grafts: Beriplast and Tissudura. J Exp Clin Med. 32: 77-82.
- Narotam PK, Jos̮̩̉̉ S, Nathoo N, et al. (2004). Collagen matrix (DuraGen) in dural repair: Analysis of a new modified technique. Spine. 29: 2861-2867.
- TTomita T, Hayashi N, Okabe M, et al. (2012). New dried human amniotic membrane is useful as a substitute for dural repair after skull base surgery. J Neurol Surg B Skull Base. 73: 302-307.
- Filippi R, Schwarz M, Voth D, et al.(2001). Bovine pericardium for duraplasty: clinical results in 32 patients. Neurosurgl Rev. 24: 103-107.
- Chaplin JM, Costantino PD, Wolpoe ME, et al. (1999). Use of an acellular dermal allograft for dural replacement: An experimental study. Neurosurgery. 45: 320.
- Narotam PK, van Dellen JR, Bhoola KD. (1995). A clinicopathological study of collagen sponge as a dural graft in neurosurgery. J Neurosurgery. 82: 406-412.
- Narotam PK, Reddy K, Fewer D, et al. (2007). Collagen matrix duraplasty for cranial and spinal surgery: a clinical and imaging study. J Neurosurgery. 106: 45-51.
- Narotam PK, Qiao F, Nathoo N. (2009). Collagen matrix duraplasty for posterior fossa surgery: Evaluation of surgical technique in 52 adult patients: Clinical article. J Neurosurgery. 111: 380-386.
- Stendel R, Danne M, Fiss I, et al. (2008). Efficacy and safety of a collagen matrix for cranial and spinal dural reconstruction using different fixation techniques. J Neurosurg. 109: 215-221.
- Chappell ET, Pare L, Salehpour M, et al. (2009). GORE PRECLUDEÃÆââ¬Å¡Ãâî MVPÃÆââ¬Å¡Ãâî dura substitute applied as a nonwatertight ÃÆâÃâââ¬Ãâà âunderlayÃÆâÃâââ¬Ãâàgraft for craniotomies: Product and technique evaluation. Surg Neurol. 71: 126-128.
- Ayhan S, Tugay C, Ortak T, et al. (2002) Effect of bioabsorbable osseous fixation materials on dura mater and brain tissue. Plastic Reconst surg. 109: 1333-1337.
- Kadioglu HH, Takci E, Arik M, et al. (2002). Immune response to dehydrated human dura mater: Evaluation in a rabbit model. Neurology India. 50: 256.
- Kelly DF, Oskouian RJ, Fineman I. (2001) Collagen sponge repair of small cerebrospinal fluid leaks obviates tissue grafts and cerebrospinal fluid diversion after pituitary surgery. Neurosurgery. 49: 885-890.
- 17.Greenberg MS, Arredondo N. (2006). Handbook of Neurosurgery. 3rd ed. Thieme.1664.
- Potter W, Kalil RE, Kao WJ. (2008). Biomimetic material systems for neural progenitor cell-based therapy. Front Biosci. 1: 21.
- Mukai T, Shirahama N, Tominaga B, et al. (2008) Development of watertight and bioabsorbable synthetic dural substitutes. Artificial Organs. 32: 473-483.
- MartÃÆÃâÃâÃÂnez-Lage JF, RÃÆÃâÃâábano A, Bermejo J, et al. (2005). Creutzfeldt-Jakob disease acquired via a dural graft: failure of therapy with quinacrine and chlorpromazine. Surg Neurol. 64: 542-545.
- Danish SF, Samdani A, Hanna A, et al. (2006) Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J Neurosurg. 104: 16-20.
- Sade B, Oya S, Lee JH. (2011). Non-watertight dural reconstruction in meningioma surgery: results in 439 consecutive patients and a review of the literature: Clinical article. J Neurosurg. 114: 714-748.
- Malliti M, Page P, Gury C, et al. (2004) Comparison of deep wound infection rates using a synthetic dural substitute (neuro-patch) or pericranium graft for dural closure: A clinical review of 1 year. Neurosurgery. 54: 599-604.
- Zerris VA, James KS, Roberts JB, et al. (2007) Repair of the dura mater with processed collagen devices. J Biomed Mater Res Part B Appl Biomater. 1: 580-588.
- Marshall LF, Marshall SB, Klauber MR, et al. (1992). The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrau. 9: S287.
- Laquerriere A, Yun J, Tiollier J, et al. (1993). Experimental evaluation of bilayered human collagen as a dural substitute. J Neurosurg. 78: 487-491.
- Esposito F, Cappabianca P, Fusco M, et al. (2008). Collagen-only biomatrix as a novel dural substitute: Examination of the efficacy, safety and outcome: Clinical experience on a series of 208 patients. Clin Neur Neurosurg. 110: 343-351.
- Sekhar LN, Sarma S, Morita A. (2001). Dural reconstruction with fascia, titanium mesh and bone screws: technical note. Neurosurgery. 49: 749-752.
- Knopp U, Christmann F, Reusche E, et al. (2005). A new collagen biomatrix of equine origin versus a cadaveric dura graft for the repair of dural defectsÃÆâÃâââ¬Ãâââ¬Åa comparative animal experimental study. Acta Neurochirurgica. 147: 877-887.
- Li GL, Farooque M, Holtz A, et al. (1999). Apoptosis of oligodendrocytes occurs for long distances away from the primary injury after compression trauma to rat spinal cord. Acta Neuropathologica. 98: 473-480.
- https://emedicine.medscape.com/article/326510-overview
- Khorasani L, Kapur R, Lee C, et al. (2008). Histological analysis of DuraGen in a human subject. Clin Neuropath. 27: 361-364.
- ParÃÆÃâÃâÃÂzek J, Mericka P, HuÃÆââ¬Â¦Ãâáek Z, et al. (1997). Detailed evaluation of 2959 allogeneic and xenogeneic dense connective tissue grafts (fascia lata, pericardium, and dura mater) used in the course of 20 years for duraplasty in neurosurgery. Acta Neurochirurgica. 139: 827-838.
- Garcia JH, Wagner S, Liu KF, et al. (1995). Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Stroke. 26: 627-35.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences