Telomerase - The Advent, Prospects and Obstacles
Rebanta Roy*, Arpita Samanta
Rebanta Roy*, Arpita Samanta
Department of Zoology, West Bengal State University, Barasat, Kolkata
- Corresponding Author:
- Rebanta Roy
Department of Zoology
West Bengal State University, Barasat, Kolkata
Tel: 9748750382
E-mail: rebantaroy.in@gmail.com
Received Date: July 21, 2021; Accepted Date: August 05, 2021; Published Date: August 12, 2021
Citation: Roy R (2021).Telomerase - The Advent, Prospects and Obstacles. Electronic J Biol, 17(S6): 240
Copyright: © 2021 Roy R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Black skin euphemistically called dark sin has become thTelomerase is an enzyme in eukaryotes which has an unique potential to solve the end replication problem, which results from the lagging replication of DNA, at the ending structures called telomeres, in one of its strands, as it copies the sequence from the parent strand. Telomerase is not found in each and every cell, but in some cells with special features. This review highlights the salient unique features of telomerase, how it evolved, and what are its effects in humans, the publications till date on this topic, to compare the collected data for the number of publications per year, at the end we have tried to give insights into the obstacles of research and have tried to propose few future dimensions into telomerase research.
Keywords
Telomerase, DNA, Telomeres, Replication, Strands
Introduction
We are all aware of the fact, that the human population has increased substantially over the past few centuries, with each century experiencing record growth of population, what we biologists call as an Exponential Rise, as on par with this, the spectrum of discoveries has also increased many folds, mysteries have been solved to many extent. Among these mysteries the end replication problem in Eukaryotes is not an exception, and scientists have evolved ways in which cells can solve the process by themselves.
In case of Prokaryotic organisms, the DNA is circular, and thus once after replication, it has no extended overhangs on the newly synthesized strands. The ends of the eukaryotic linear chromosomes are called telomeres, which are protective DNA - Protein complexes, coined by Muller [1] carrying a TG Rich sequence [2], with slight variations among different taxas of classification, they are mostly consensus [3]. This is the region solely responsible for the end replication problem [4]. From here begins the need for machinery, which would give a sustainable solution this problem. Now from the baseline of its discovery by Blackburn and Greider, telomerase has been like a pandora box which still awaits its opening to the full extent, Telomerase is a RNA Dependent DNA Polymerase, which during each round of replication is potent to stitch off the overhang produced on the 3’-5’ Lagging Strand. DNA Synthesis occurs semi discontinuously, the 5’-3’ synthesized DNA from the 3’-5’ synthesized DNA template is the leading strand and the 3’-5’ synthesized DNA from the 5’-3’ DNA template is called the lagging strand [5]. The enzyme sub family which leads the synthesis of DNA is called DNA Dependent DNA Polymerases, they cannot initiate the formation of the DNA strands from scratch and thus require assistance from another RNA Polymerase called the Primase which is potent to synthesize a 5’-3’ RNA primer, and thus this primer provides the free 3’ end for the active synthesis of the DNA strands. At the end of synthesis, another class of the DNA polymerase replaces these primers and synthesizes a short DNA fragment in place of the primer. The leading strand synthesis is thus complete but due to semi discontinuous synthesis of the DNA, in the lagging strand after the removal of primer, there is no free 3’ end for the DNA polymerase to add the nucleotides, thus there is an overhang in the 3’-5’ end [5]. In majority of the cells FEN-1 removes nucleotides from the 5’-3’ strand to keep parity with the 3’-5’ DNA strand and thus with each round of replication there is a loss of few nucleotides from the 5’-3’ end, and after completion of many rounds of replication the genes get affected, and this potential DNA damage signals the apoptotic and other related programmed cell death processes to get activated eventually leading to cell death [6]. But some cells, to get special mention, the stem cells do have this potential to retain the nucleotides and evade the removal; telomerase acts as a master enzyme here, [7]. telomerase has a sequence which acts as a template for the 5’-3’ DNA strand and few extra nucleotides are added, now again DNA Polymerase set of enzymes removes the primer and adds dNTPs, to be precise, the 5’-3’ strand acts as template for the 3’-5’ DNA, thus synthesis begins on this strand and after synthesis, the extra synthesized part is removed and no portion of the genetic material is lost [8]. Thus telomerase appeared as a “remarkable enzyme”.
Evolution of discovery of telomerase
In 1961, Hay flick noted that most cells in culture medium survive for limited time and show limited number of cell division [9] and that limitation is known to us as “Hayflick limit”. In 1970s, when a chromosome is multiplied a problem is found mainly in lagging strand (3’-5’) on DNA. Standard polymerases cannot copy the lagging strand completely. Then Olovnikov, 1971 and Watson, 1972 first pointed out the implication of this “end replication problem”. They said that chromosomes would shorten with each replication. Then finding of answer of this problem began and in 1978, Elizabeth Blackburn and Joseph Gall worked on Tetrahymena thermophila and noted that chromosome ends contain six base sequences TTGGGG repeated 20-70 times, named as telomere. In 1981, in collaboration with Jack Szostak, Blackburn revealed that telomeric function could be transferred from one organism (Tetrahymena thermophile) to another (Saccharomyces cerevisiae). Then in 1985, Blackburn and Carol Greider identified an enzymatic activity that able to extend telomeric sequence. They identified the activity of “terminal transferase” and termed it “telomerase” [10].
The enzyme with substantial uniqueness
Telomerase acts as an enzyme without the need of an exogenous template strand - Telomerase is a ribonucleoprotein, i.e. it is composed of an RNA part and a protein part, with various subunits and interlinking domains [11]. Some considered it as a DNA dependent DNA polymerase [12] but later studies said that it acts as an RNA dependent RNA polymerase in mitochondria [13] later studies say that it is an RNA dependent DNA polymerase. Other classical or canonical DNA Polymerases need a template to initiate DNA replication and to copy the base sequences onto the daughter strand, but telomerases don’t need such templates from outside, instead they focus largely on their own machinery which has an RNA component serving as a template for telomerase to add nucleotides at the extreme 3’ end of the chromosomal DNA. Thus telomerase acts as a self-sustaining system, in an unusual case of generating a single stranded DNA. Thus these characters, viz, the use of entirely single stranded DNA template, the use of the RNA part and no need of the exogenous template, gives telomerase and unique fingerprint.
Components and the domains of telomerases
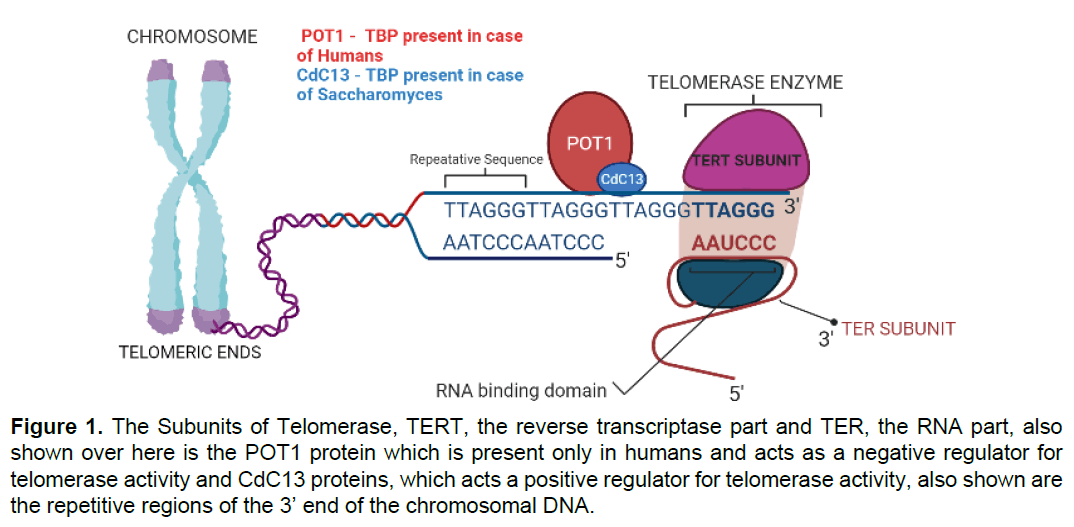
There are in general two components of the telomerase enzyme, viz. TERT or the Telomerase Reverse Transcriptase and TER or the Telomerase RNA. The telomerase acts as an RNP or ribonuclear protein and was first purified in Tetrahymena [14]. The TERT is the catalytic functional subunit of the telomerase and the TER is the RNA portion which acts as a template for synthesis of the ssDNA. The TERT it as per its name reverse transcribes the RNA template to DNA, unlike the normal transcription processes where the reverse of this phenomenon occurs. The TERT is made up of various domains that are interlinked to each other to form the TERT. The domains are as follows N-terminal (TEN) domain, the Telomerase RNA binding domain (TRBD), the reverse transcriptase (RT) domain, and the C-terminal extension (CTE) domain. The TER in humans is made up of TR is composed of functional domains viz. Pseudoknot domain, CR4/5 domain, and H/ACA domain the telomerase thus acts as a holoenzyme. The N terminal domain is responsible for its interaction with the telomeric 3’ end of the DNA. The TRBD binds the TER and the RT and CTE are responsible domains which bind to the RNA-DNA junctional area and add to the 3’ end [15] (Figure 1).
Figure 1: The Subunits of Telomerase, TERT, the reverse transcriptase part and TER, the RNA part, also shown over here is the POT1 protein which is present only in humans and acts as a negative regulator for telomerase activity and CdC13 proteins, which acts a positive regulator for telomerase activity, also shown are the repetitive regions of the 3’ end of the chromosomal DNA.
Role of Telomere Binding Proteins in Telomerase Efficacy
Telomerases are potent enzymes for polymerization and can undergo telomeric replication up to an undefined extent, so for putting on brakes to this green signal there needs to be a feedback regulation, and this negative feedback is provided by a class of several proteins which are termed as Telomere Binding Proteins or TBPs. The TBP which was discovered first was called the TTAGGG repeat factor (TRFs) in 1992 and the next was named TRF2 and TRF was renamed as TRF1 soon after this [16]. If there would have been no presence of the TBPs the telomeres would have been extended indefinably posing certain problems in front of the cells. As in case of Saccharomyces cerevisiae, a protein named CdC13 plays a major role in recruitment and binding of telomerase on the telomeric sequence repeats to extend the telomere to solve the end replication problem [17]. Again in case of humans a protein like POT1 binds to the 3’ end area of the telomeres and calls for a negative feedback in which it downregulates the activity of the telomerase enzyme and thus prevents the excessive extension. Besides this the telomeric proteins also have another property, it generally distinguishes the ends of the chromosomes i.e. the telomeres from general double stranded DNA breaks, in absence of which devastating results of chromosomal fusion would have taken place, which could have destroyed the cell as a whole. Again proteins also facilitate the t loop formation in case of the human telomeric DNA, which is considered to be an adaptive function to protect ends of the chromosomes from the repair and recombination mechanism [18] (Figure 2).
Telomerase Activity in Humans
In case of humans, telomerase activity is actually detected at blastocyst stage of development. Normally telomerase activity remains down regulated in many somatic tissues during their early development but in adult stem cells, endothelial cells and also in lymphocytes this telomerase activity becomes high, regulated by some physiological conditions. Information about these physiological conditions is still under progress now. Also some complex regulations like splicing, posttranscriptional modification are found to control this telomerase activity. However in cancer cell this activity is expressed vigorously and provides the unlimited proliferation potency.
Role of Telomerase in Apoptosis and Cancer Biology
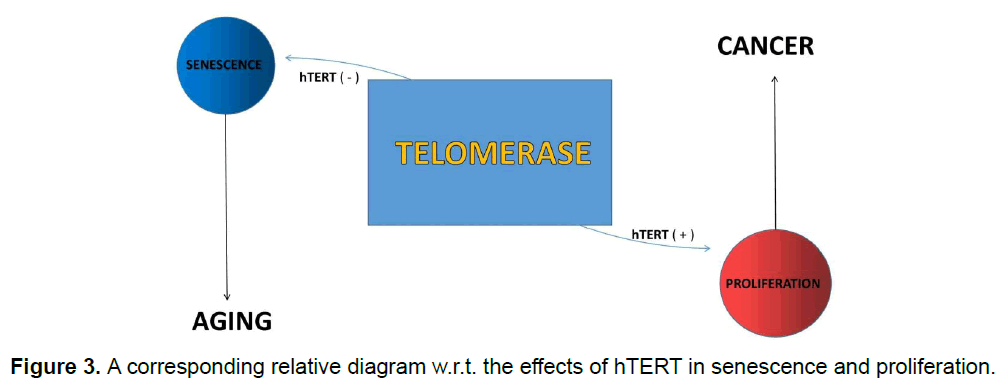
Cancer is a genetic event in which normal cells achieve the ability to replicate indefinitely due to many causes. In most cases telomerase activity is responsible for this proliferation. Due to chromosomal aneuploidy and rapid cell division in cancer cells the activity of telomerase enzyme becomes high in comparison to normal cells. If any mutation occurs in such genes that maintain the arresting process of cell cycle, like TP53, it allows the cell division even with short telomere for many generations. These cells then become immortal and new telomere will allow fixation and stabilization of chromosomal end. As these cells also carry those same mutations it will affect cell cycle in each turn, resulting that those cells undergo cancerous state. When the entropy production rate is calculated in normal individuals it decays gradually with age whereas, in metastatic carcinoma patients the rate of entropy production also diminish with age but in carcinogenic patients the entropy production rate is much higher than healthy organisms. This higher robustness can be interpreted in terms of cancer.
Relativeness between Telomerase and Aging
Aging or senescence is a programmed deterioration of physiological function that maintains the homeostasis. There so many factors that is responsible for aging procedure in which telomerase activity is more concerned point. In replicative senescence telomeres become spoiled after each cell division but telomerase plays important role in regeneration of telomere, resulting cellular longevity. Telomere remains bound by a multiprotein complex, known as shelterin, the main function of which is to prevent the access of DNA repair proteins to the telomeres. Telomere uncapping can also results from deficiencies in shelterin components. Various losses of function models for shelterin are characterized by rapid decline of regenerative capacity of tissue and accelerated aging. In humans, recent analyses have supported that there is a strong relationship between short telomere and mortality risk, particularly at younger age [19]. But cells can choose only single fate between apoptosis and senescence. So, antiapoptotic signaling induces the senescent, proinflammatory phenotype during aging process (Figure 3).
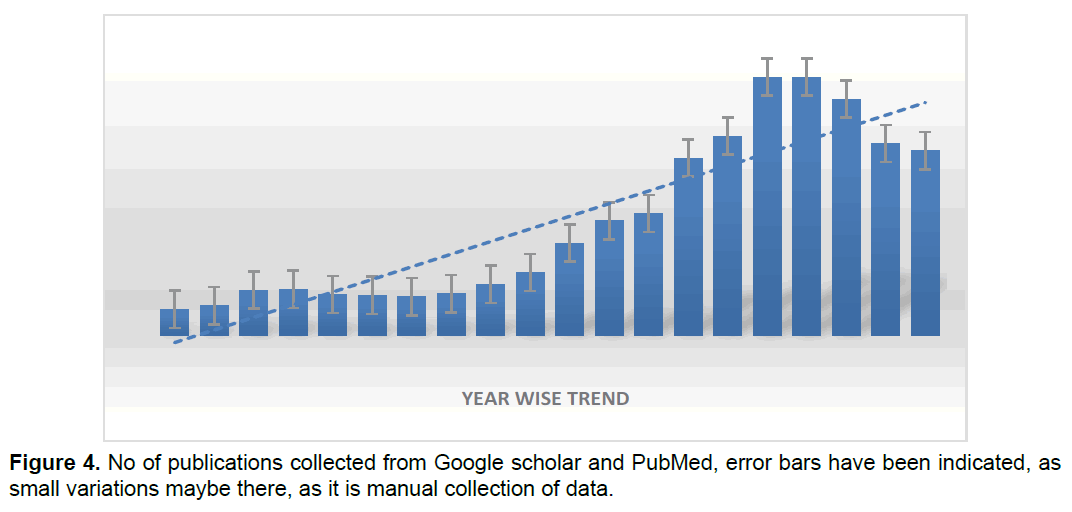
Publication Status Year by Year
Publications related to telomeres and telomerase followed a decent trend from 2000 to 2018, but there was stunted growth since 2019 and even in 2020, we have collected our data from PubMed and Google Scholar, the range of data is is indicated in the graph of (figure 4).
Future and Proposed Aspects of Telomerases
The main focus of the future would be and in fact is to up regulate the activity of telomerase by down regulating cancer or excessive cellular proliferation. Though such researches have been in our spectrum already, where cancer resistant mice models have been checked for the expression of the telomerase enzyme when it is injected. But it is already proven that telomere shortening is not only the factor for ageing, it is a multifactorial trait. Liposomal mode of genetic material, protein domain and drug transfer could be a preferable zones of research, where hydrophobic molecules are transported to target sites inside the hydrophobic core of the lipid bilayer again the hydrophilic molecules are transported to the target sites inside the hydrophilic lumen of the liposome, the telomerase catalytic domains can be cleaved and measured for its hydrophobicity or hydrophilicity and thus can be sent to potential sites of assembly, from where they can form a complete TERT subunit, and by designing an analogue to the TER, short oligomeric RNA templates can be used to synthesize the ends of the chromosomes in case of accidental or physiological telomeric end shortening and this might be a novel discovery in case of telomerase functioning.
Conclusion
Telomerases are important enzymes in controlling the replication process at the end of the DNA replication mechanism; we have seen how they have been involved in cancer and how the cancer cells have a quite surprising overexpression of this enzyme which causes the cells to proliferate to the maximum undefined extent and, it has already been established that telomere shortening is a multifactorial feature, not just a cause of ageing.
Acknowledgment
This is an independent research work, which would not have been possible without the constant guidance of the faculties of the Department of Zoology, West Bengal State University and the Department of Zoology, Barasat Government College. The authors are indebted to their parents for their constant support.
Glossary
CTE: C-terminal Extension
dNTPs: Deoxynucleotide triphosphates
POT1: Human Protection of Telomerase 1
RT: Reverse Transcriptase
TBP: Telomerase Binding Protein.
TEN: N-terminal “anchor” Domain.
TER: Telomerase RNA
TERT: Telomerase Reverse Transcriptase
TRBD: Telomerase RNA Binding Domain
References
- Chuaire L (2006). Telomere and Telomerase: Brief review of a history initiated by Hermann Müller and Barbara McClintock. Colombia Médica. 37:332-5.
- Shore D, Bianchi A (2009). Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J. 28:2309-22.
- Saintomé C, Alberti P, Guinot N, et al. (2018). Binding properties of mono-and dimeric pyridine dicarboxamide ligands to human telomeric higher-order G-quadruplex structures. Chem Comm. 54:1897-900.
- Wynford-Thomas D, Kipling D (1997). The end-replication problem. Nat. 389:551.
- Wellinger RJ (2014). In the end, what’s the problem? Mol cell. 53:855-6.
- Shammas MA (. 2011). Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 14:28.
- Hiyama E, Hiyama K (2007). Telomere and telomerase in stem cells. Br J Cancer. 96:1020-4.
- Jafri MA, Ansari SA, Alqahtani MH, et al. (2016). Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 8:1-8.
- Hayflick L, Moorhead PS (1961). The serial cultivation of human diploid cell strains. Exp Cell Res. 25:585-621.
- Greider CW, Blackburn EH (1989). A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 337:331-7.
- Egan ED, Collins K (2012). Biogenesis of telomerase ribonucleoprotein. Rna 18:1747-59.
- Legassie JD, Jarstfer MB (2005). Telomerase as a DNA-dependent DNA polymerase. Biochem. 44:14191-201.
- Jaiswal BS, Kljavin NM, Stawiski EW, et al (2005). Oncogenic ERBB3 mutations in human cancers. Cancer Cell. 23:603-17.
- Collins K, Kobayashi R, Greider CW (1995). Purification of Tetrahymena telomerase and cloning of genes encoding the two protein components of the enzyme. Cell. 81:677-86.
- Zhang Q, Kim NK, Feigon J (2011). Architecture of human telomerase RNA. Proc Natl Acad Sci. 108:20325-32.
- Ilicheva NV, Podgornaya OI, Voronin AP (2015). Telomere repeat-binding factor 2 is responsible for the telomere attachment to the nuclear membrane. Advances in protein chemistry and structural biology. 101:67-96.
- Churikov D, Charifi F, Eckert-Boulet N, et al. (2016). SUMO-dependent relocalization of eroded telomeres to nuclear pore complexes controls telomere recombination. Cell reports. 15(6):1242-53.
- Wei X, Decker JM, Wang S, et al. (2003). Antibody neutralization and escape by HIV-1. Nat. 422:307-12.
- Boonekamp JJ, Simons MJ, Hemerik L, et al. (2013). Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging cell. 12:330-2.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences