Potential Pathophysiological Crosstalk between Parkin and FBXO7 Signalling Pathways
Zhi Dong Zhou, Eng King Tan
Zhi Dong Zhou1,3,*, Eng King Tan1-3
1National Neuroscience Institute of Singapore, 11 Jalan Tan Tock Seng, Singapore
2Department of Neurology, Singapore General Hospital, Outram Road, Singapore
3Signature Research Program in Neuroscience and Behavioral Disorders, Duke-NUS Graduate Medical School Singapore, 8 College Road, Singapore
Received date: August 16, 2016; Accepted date: August 31, 2016; Published date: September 07, 2016
Citation: Zhou ZD, Tan EK. Potential Pathophysiological Crosstalk between Parkin and FBXO7 signalling pathways. Electronic J Biol, 12:4.
Abstract
Mutations of F-box protein 7 [FBXO7] and Parkin, two proteins related to Ubiquitin-Proteasome System [UPS], are implicated in the pathogenesis of Dopamine [DA] neuron degeneration in Parkinson's Disease [PD], possibly involving impairment of UPS and mitophagy. Parkin is a HECT/RING hybrid ubiquitin E3 ligase that physically receives ubiquitin on its catalytic centre and passes ubiquitin onto its substrates, whereas FBXO7 is an adaptor protein in Skp-Cullin-F-box [SCF] SCFFBXO7 ubiquitin E3 ligase complex to recognize substrates and mediate substrates ubiquitination by SCFFBXO7 E3 ligase. There are overlapping clinical features in Parkin and FBXO7 linked PD. One recent study demonstrates that FBXO7 can mediate mitochondrial translocation of Parkin under mitochondria impairment to initiate neuroprotective mitophagy. The signalling pathways of FBXO7 and Parkin may have complicated pathophysiological crosstalk, which should be implicated in PD pathogenesis and therapy. The FBXO7 may attract Parkin to impaired mitochondria to mediate ubiquitination of key substrates for mitophagy initiation. FBXO7 and Parkin may recognize each other as substrates reciprocally to promote their UPS degradation. Furthermore, FBXO7 may modulate Parkin E3 ligase activity. The aggregation-prone mutant FBXO7 has been shown to induce Parkin protein aggregation, which may lead to down regulation of available protective Parkin protein. Further studies are needed to decipher the complicated interactions between FBXO7 and Parkin.
Keywords
FBXO7; Mitochondria; Mitophagy; Parkin; Parkinson's disease; Proteotoxicity; Ubiquitin proteasome system.
Parkinson's disease [PD] is a common neurodegenerative disorder characterized by chronic and progressive loss of dopaminergic neurons in substantia nigra pars compacta [SN]. PD can affect about 2% of the population above 65 years of age [1-3]. PD symptoms include rigidity, postural instability, tremor at rest and slowness or absence of voluntary movement, and even neuropsychiatric symptoms [4,5]. The pathological hallmarks of PD include progressive degeneration of Dopamine [DA] neurons in SN as well as accumulation of α-synuclein [α-syn] positive Lewy bodies in afflicted brain regions [4,6]. Most PD cases are late onset and may be classified as Sporadic PD [SPD]. However, gene mutations or variations can lead to early onset inherited familial PD [FPD] [3,7]. Parkin gene mutations are associated with classic Levodopa responsive FPD [PARK2] [8]. However, recessive gene mutations of F-box protein 7 [FBXO7] are associated with juvenile onset Parkinsonism [PARK15], frequently accompanied by atypical features including dementia, dystonia, hyperreflexia and pyramidal signs [9,10]. Both Parkin and FBXO7 are involved in Ubiquitin-Proteasome System [UPS] and neuroprotective mitophagy process [11]. There are overlapping pathways in Parkin and FBXO7 linked disease (Figure 1).
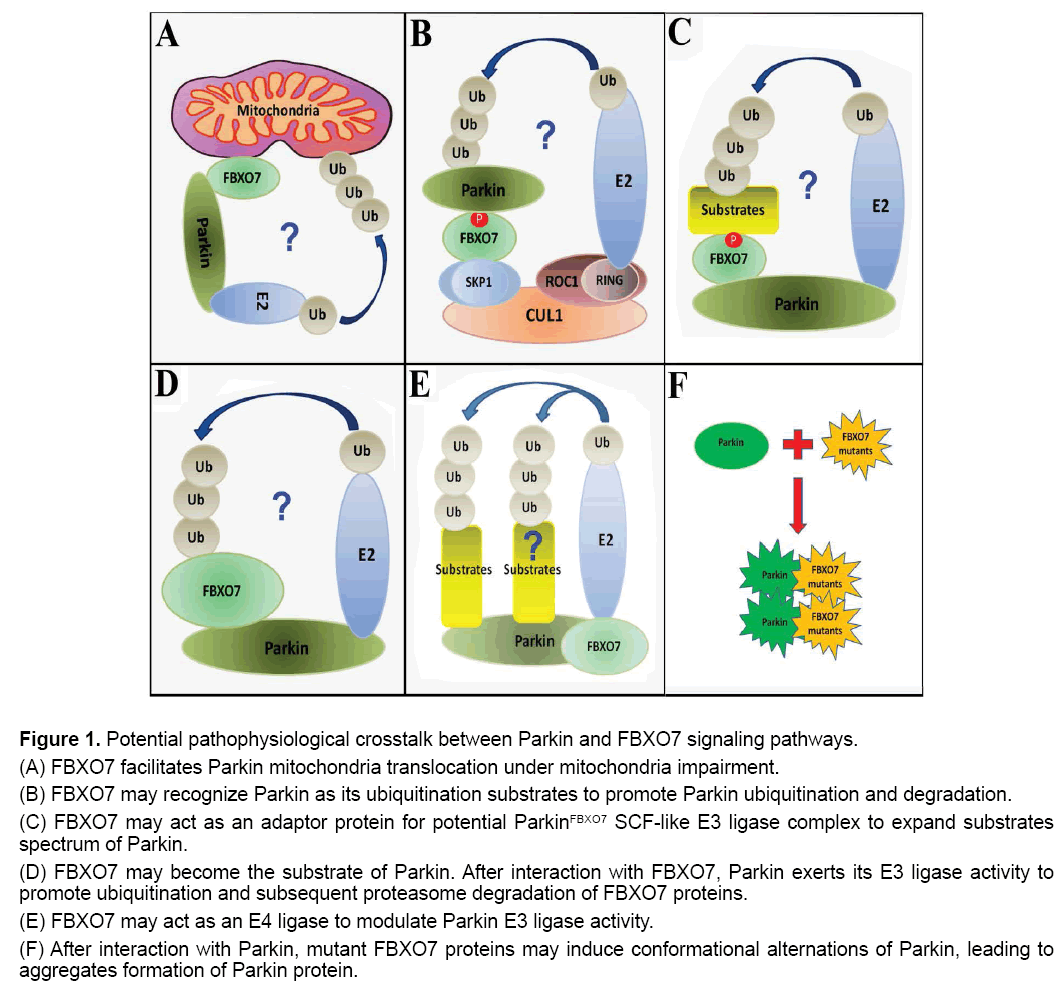
Figure 1: Potential pathophysiological crosstalk between Parkin and FBXO7 signaling pathways.
(A) FBXO7 facilitates Parkin mitochondria translocation under mitochondria impairment.
(B) FBXO7 may recognize Parkin as its ubiquitination substrates to promote Parkin ubiquitination and degradation.
(C) FBXO7 may act as an adaptor protein for potential ParkinFBXO7 SCF-like E3 ligase complex to expand substrates spectrum of Parkin.
(D) FBXO7 may become the substrate of Parkin. After interaction with FBXO7, Parkin exerts its E3 ligase activity to promote ubiquitination and subsequent proteasome degradation of FBXO7 proteins.
(E) FBXO7 may act as an E4 ligase to modulate Parkin E3 ligase activity.
(F) After interaction with Parkin, mutant FBXO7 proteins may induce conformational alternations of Parkin, leading to aggregates formation of Parkin protein.
First, FBXO7 was found to facilitate Parkin mitochondria translocation under mitochondria impairment to initiate neuroprotective mitophagy (Figure 1A) [12]. Therefore, it can be hypothesized that stress-induced mitochondrial impairment leads to exposure of certain mitochondrial targets of FBXO7. FBXO7 can be first recruited to impaired mitochondria and subsequently FBXO7 in mitochondria can further attract Parkin to damaged mitochondria. Finally, the accumulated FBXO7 and Parkin in mitochondria may act together to mediate ubiquitination of targets in impaired mitochondria and initiate mitophagy that clear away the impaired mitochondria and protect cells against stress [12]. In this case, FBXO7 and Parkin cooperate to protect DA neurons against stress, while mutations in FBXO7 and Parkin lose their functions, leading to mitophagy inhibition and DA neuron degeneration.
Second, FBXO7 may recognize Parkin as its ubiquitination substrate (Figure 1B). The FBXO7 is an adaptor protein in Skp-Cullin-F-box [SCF] SCFFBXO7 ubiquitin E3 ligase complex to recognize substrates and mediate substrates ubiquitination by SCFFBXO7 E3 ligase [11]. After binding with Parkin, the protein complex of FBXO7 and Parkin can be further recruited into SCFFBXO7 E3 ligase [formed by Cullin1, Skp1, ROC1 and FBXO7] (Figure 1B). Then Parkin can be ubiquitinated by SCFFBXO7 E3 ligase and undergo subsequent proteasome degradation. In this case, FBXO7 may promote degradation of Parkin and maintain the physiological metabolism of Parkin in DA neurons.
Third, FBXO7 may act as an adaptor protein for potential ParkinFBXO7 E3 ligase complex (Figure 1C). After binding with FBXO7, Parkin may use FBXO7 as its adaptor protein to interact with more substrates. The substrates caught by FBXO7 can be subsequently ubiquitinated by ParkinFBXO7 SCF-like E3 ligase and undergo proteasomal degradation. In this case, the adaptor protein FBXO7 can expand the substrate spectrum of Parkin. This hypothesis can be supported by finding that Parkin can interact with FBW7 protein, another F-box protein, to form SCF-like E3 ligase complex [ParkinFBW7 E3 ligase] to protect neuron against kainate toxicity [13]. In this case, FBXO7 and Parkin work together to eliminate unwanted or aged proteins, promoting DA neuron survival against stress.
Fourth, after binding of Parkin with FBXO7, Parkin may exert its E3 ligase activity to promote ubiquitination of FBXO7 (Figure 1D). This possibility can be supported by previous finding that Parkin can exert its E3 ligase activity to induce ubiquitination of FBW7 and promote subsequent proteasome degradation of FBW7 [14]. In this case, Parkin promotes ubiquitination and clearance of FBXO7, therefore controls the physiological metabolism of FBXO7 protein in DA neurons.
Fifth, FBXO7 may act as an E4 ligase to modulate Parkin E3 ligase activity (Figure 1E). The ubiquitin E4 ligase is a protein that can kinetically enhance the E3 ubiquitin ligase–mediated transfer of ubiquitin to its protein substrate [15,16]. It has been found that the E3 ligase CHIP can bind with Parkin and act as an E4 ligase for Parkin [17]. In the absence of CHIP, the E3 ligase activity of Parkin will be significantly impaired [17]. However in the presence of CHIP, the Parkin E3 ligase activity can be greatly enhanced [17]. Similarly, it is possible that FBXO7 may modulate Parkin E3 ligase activity for ubiquitination of Parkin substrates as well. In this case, FBXO7 plays a role in modulating Parkin E3 ligase activity to promote DA neuron survival under stress.
Sixth, mutant FBXO7 can interact with Parkin and promote protein aggregates formation of Parkin (Figure 1F) [18]. We recently found that overexpression of R498X FBXO7 mutant, but not WT FBXO7, promotes WT Parkin aggregates formation [18]. Our findings also demonstrated that mutant FBXO7 can form deleterious protein aggregates in mitochondria, suggesting the mutant FBXO7 can be an aggregation-prone protein [18]. There are accumulated suggestions linking deleterious protein aggregation [proteotoxicity] to disease onset of PD and other human neuron degenerative diseases [19-23]. In this case, mutated FBXO7 proteins may bind with and induce protein aggregates formation of Parkin, leading to diffusion of protein aggregation and down regulation of available functional Parkin under FBXO7 mutations, which can contribute to DA neuron vulnerability under stress.
There are increasing evidences to support interactions between Parkin and FBXO7 linked pathways. Parkin and FBXO7 are both implicated in tumorigenesis [24,25]. Both WT Parkin and FBXO7 can be cytoprotective, whereas their PD-linked mutants are all deleterious to DA neurons. Further evidence is needed to verify these hypotheses and unravel the complicated crosstalk between the signaling pathways of Parkin and FBXO7, which may open to development of newer therapeutics in PD.
References
- Obeso JA, Rodriguez-Oroz MC, Goetz CG, et al. (2010). Missing pieces in the Parkinson's disease puzzle. Nat Med.16:653-661.
- Tan EK, Skipper LM. (2007). Pathogenic mutations in Parkinson disease. Hum Mutat.28:641-653.
- Dawson TM, Ko HS, Dawson VL. (2010). Genetic animal models of Parkinson's disease. Neuron.66: 646-661.
- Petit GH, Olsson TT,Brundin P. (2014). The future of cell therapies and brain repair: Parkinson's disease leads the way. NeuropatholApplNeurobiol.40: 60-70.
- Davie CA. (2008) A review of Parkinson's disease. Br Med Bull.86:109-127.
- Kasten M, Klein C. (2013). The many faces of alpha-synuclein mutations. MovDisord.28:697-701.
- Bonifati V. (2012). Autosomal recessive Parkinsonism. Parkinsonism RelatDisord.18: S4-6.
- Tomac AC, Hoffer BJ. (2001). Assignment of the mouse Park2 (PARKIN), the homologue to a new human Parkinson candidate gene, to the telomeric region of mouse 17A3.2-3.3, by in situ hybridization. Cytogenet Cell Genet.95:120-121.
- Deng H, Liang H, Jankovic J. (2013). F-box only protein 7 gene in parkinsonian-pyramidal disease. JAMA Neurol.70:20-24.
- Lohmann E, Coquel AS, Honore A, et al. (2015). A new F-box protein 7 gene mutation causing typical Parkinson's disease. MovDisord.30:1130-1133.
- Zhou ZD, Sathiyamoorthy S, Angeles DC, et al. (2016). Linking F-box protein 7 and parkin to neuronal degeneration in Parkinson's disease (PD). Mol Brain.9:41.
- Burchell VS, Nelson DE, Sanchez-Martinez A, et al. (2013). The Parkinson's disease-linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat Neurosci.16:1257-1265.
- Staropoli JF, McDermott C, Martinat C, et al. (2003) Parkin is a component of an SCF-like ubiquitin ligase complex and protects post-mitotic neurons from kainateexcitotoxicity. Neuron.37:735-749.
- Ekholm-Reed S, Goldberg MS, Schlossmacher MG, et al. (2013) Parkin-dependent degradation of the F-box protein Fbw7beta promotes neuronal survival in response to oxidative stress by stabilizing Mcl-1. Mol Cell Biol.33:3627-3643.
- Chatterjee A, Upadhyay S, Chang X, et al. (2008) U-box-type ubiquitin E4 ligase, UFD2a attenuates cisplatin mediated degradation of DeltaNp63alpha. Cell Cycle. 7: 1231-1237.
- Huang Y, Minaker S, Roth C, et al. (2014) An E4 ligase facilitates polyubiquitination of plant immune receptor resistance proteins in Arabidopsis. Plant Cell.26:485-496.
- Paul I, Ghosh MK. (2014). The E3 ligase CHIP: insights into its structure and regulation. Biomed Res Int.918183.
- Zhou ZD, Xie SP, Sathiyamoorthy S, et al. (2015). F-box protein 7 mutations promote protein aggregation in mitochondria and inhibit mitophagy. Hum Mol Genet.24: 6314-6330.
- Hilker R, Brotchie JM, Chapman J. (2011). Pros and cons of a prion-like pathogenesis in Parkinson's disease. BMC Neurol.11:74.
- Olanow CW,Prusiner SB. (2009). Is Parkinson's disease a prion disorder?ProcNatlAcadSci U S A. 106:12571-12572.
- King OD, Gitler AD, Shorter J. (2012). The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res.1462: 61-80.
- Costanzo M,Zurzolo C. (2013). The cell biology of prion-like spread of protein aggregates: Mechanisms and implication in neurodegeneration. Biochem J. 452:1-17.
- Polymenidou M, Cleveland DW. (2012). Prion-like spread of protein aggregates in neurodegeneration. J Exp Med. 209:889-893.
- Laman H, Funes JM, Ye H, et al. (2005). Transforming activity of Fbxo7 is mediated specifically through regulation of cyclin D/cdk6. Embo J.24: 3104-3116.
- Picchio MC, Martin ES, Cesari R,et al. (2004). Alterations of the tumor suppressor gene Parkin in non-small cell lung cancer. Clin Cancer Res.10: 2720-2724.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences