Phomopsis azadirachtae ÃÆâÃâââ¬Ãâââ¬Å The Die-Back of Neem Pathogen
Girish K., Shankara Bhat S
1Department of Studies in Microbiology, Manasagangotri, University of Mysore, Mysore - 570 006, Karnataka, India
2Department of Microbiology, Maharanis Science College for Women, JLB Road, Mysore - 570 005, Karnataka, India
3Labland Biodiesel Private Limited, # 98, 7th main, Jayalakshmipuram, Mysore - 570 012, Karnataka, India
- Corresponding Author:
- Tel: +91-9341816617
E-mail: girishk77@yahoo.com
Abstract
Neem (Azadirachta indica) commonly known as ‘Indian lilac’ or ‘Margosa’, is a native tree to India. Neem finds very wide application and both wood as well as non-wood products are utilized in many ways. Neem products have antibacterial, antifungal, insecticidal and other versatile biological activities. However, neem is not free from microbial diseases though having biological activity against various microorganisms. Many bacteria and fungi are known to infect neem. A new fungus Phomopsis azadirachtae was reported on neem causing die-back. The fungus infects the neem trees of all age and size. The symptoms of the disease are twig blight, inflorescence blight and fruit rot. The disease results in almost 100% loss of fruit production.
Keywords
Azadirachta indica; Diseases of neem; Die-back of neem; Phomopsis azadirachtae.
1. Introduction
Neem is an evergreen deciduous tree. It is commonly called “Indian lilac” or “Margosa” and belongs to the Mahogany family Meliaceae. It is native to Indian sub-continent. The Persian name of the neem is Azad-Darakht-E-Hind, which means ‘free tree of India'. Over 20 million trees are found all over India. Karnataka stands third with a percentage of 5.5% trees [1]. Neem tree has adaptability to a wide range of climatic, topographic and edaphic factors and compared to other species is well adapted to stress conditions [2]. It is referred as “Tree for solving global problems”.
In India, parts of neem tree have been in use for medicinal purposes. Ayurveda regards the tree as a ‘Sarva roga nivarini’. In addition to durable wood, non-wood products like flowers, fruits, seed, oil, cake, leaf, bark and gum also find various uses [3]. Neem seeds yield 40% of deep yellow fatty oil, the well-known ‘Margosa oil’, which has medicinal properties and finds its use in treating chronic skin diseases, ulcers, leprosy rheumatism and sprain [4].
T.he seed cake is cheap and useful fertilizer. Neem tops the list of 2,400 plant species that are reported to have pesticidal properties. Over 195 species of insects are affected by neem extracts and insects that have become resistant to synthetic pesticides are also controlled with these extracts. Owing to its versatile characteristics neem is rightly called the “village pharmacy” or “Doctor tree” or “Wonder Tree of India” or “The bitter Gem”.
In spite of its well-known antifungal and antibacterial and other versatile biological activities, neem is not free from microbial diseases. Many fungal and bacterial pathogens were reported on it [4,5]. Die-back of neem is caused by Phomopsis azadirachtae Sateesh, Bhat & Devaki. The fungus affects leaves, twigs and inflorescence, irrespective of age, size and height of the tree. In severely affected trees it has resulted in almost always 100% loss of fruit production [6], which in turn has affected the availability of a highly valuable source of botanical pesticide, the seed. The disease is spreading at an alarming rate and needs to be controlled quickly. According to field survey conducted, almost all the trees in and around Mysore were affected with the fungus [3].
2. Microflora and diseases of neem
The bacterial diseases reported on neem include leaf spot disease caused by Pseudomonas azadirachtae [7], Xanthomonas azadirachtii [8,9]. The fungal diseases that have been reported on neem include pink disease caused by Corticium salmonicolor [10], twig canker and shot-hole incited by Phoma jolyana [11], leaf spot caused by Pseudocercospora subsessilis [12], blight and stem rot caused by Sclerotium rolfsii [13], leaf web-blight caused by Rhizoctonia solani [13-15], leaf spotting and blight incited by Alternaria alternata and Colletotrichum gleosporioides [16]. Root rot disease incited by Ganoderma lucidum [5] and Ganoderma applanatum [17] damping-off caused by Fusarium oxysporum [5], powdery-mildew caused by Oidium azadirachtae [5], die-back disease incited by Phomopsis azadirachtae [18], collar-rot incited by Fusarium semitectum [19] and stem-rot caused by Sclerotinia sclerotiorum [20].
Neem seed mycoflora includes Aspergillus spp., Penicillium sp. [3,21,22], Xylaria azadirachtae [5], Aspergillus ochraceus, A. niger, A. flavus, P. azadirachtae, Fusarium oxysporum, Mycelia sterilia [3,23]. Fusarium avenaceum was reported as an endophytic fungus on neem [24]. Mahesh et al. [25] isolated a total of 77 endophytic fungal isolates belonging to 15 genera from the inner bark of A. indica (Table 1).
3. Die-back of neem
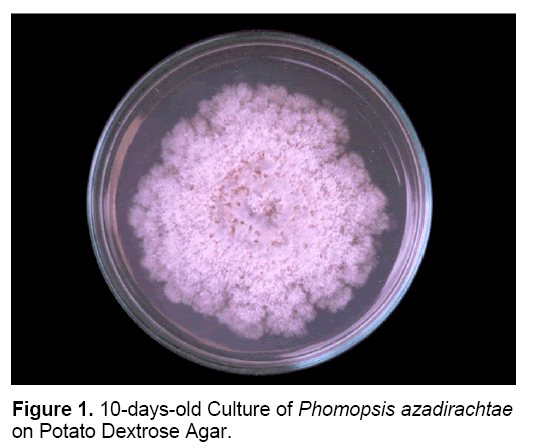
Die-back of neem is caused by Phomopsis azadirachtae Sateesh, Bhat and Devaki. The occurrence of die-back of neem was first reported from new forests of Dehra Dun, North India [26]. Sateesh et al. [18] first identified and reported the pathogen causing die-back of neem – Phomopsis azadirachtae (Figure 1). The disease symptoms include twig blight, inflorescence blight and fruit rot. At present it is the major, devastating disease of neem [6].
3.1 Disease symptoms
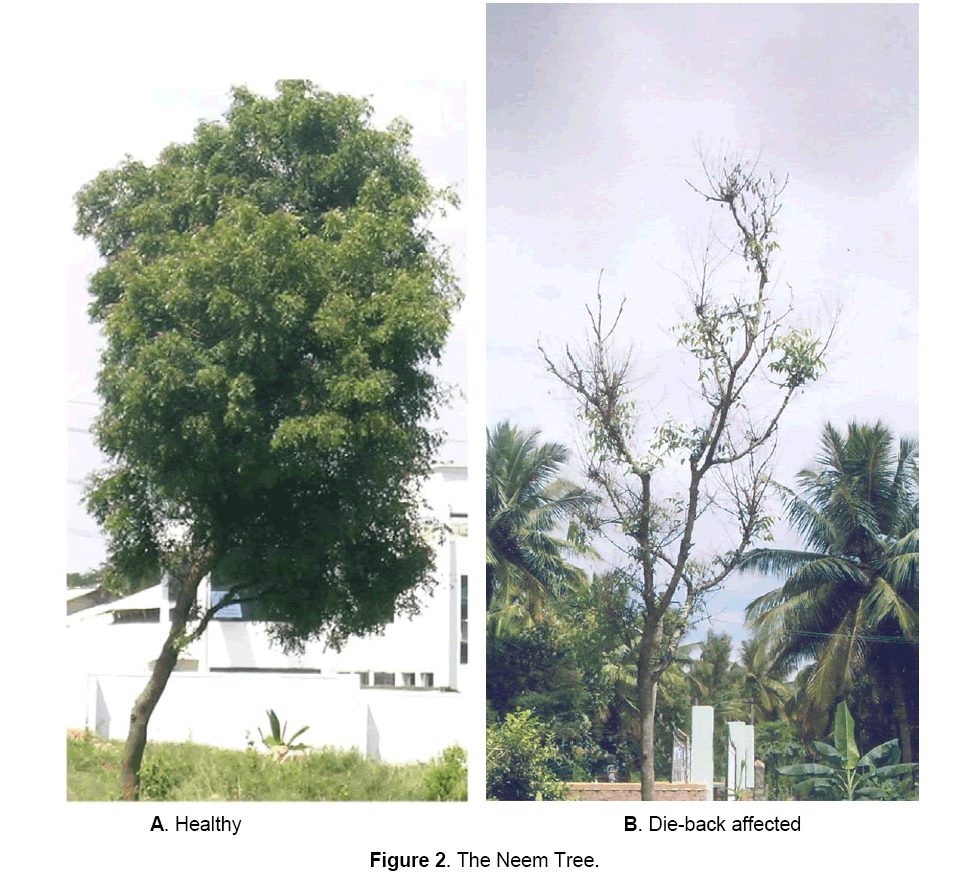
Disease has been noticed in neem trees irrespective of age, size and height. The disease is more pronounced during August to December, though can be observed throughout year. Appearance of symptoms starts with the on-set of rainy season and becomes progressively severe in later part of the rainy season and early winter season. The terminal branches are mainly affected. The disease results in the progressive death of the tree, year after year [3]. Twig blight is the major symptom (Figure 2). Disease also results in inflorescence blight and fruit rot resulting in almost 100% fruit yield loss [6]. Disease spreads through conidia that are disseminated by rain droplets and insects [3]. The pathogen is also seed-borne [23].
3.2 The Pathogen
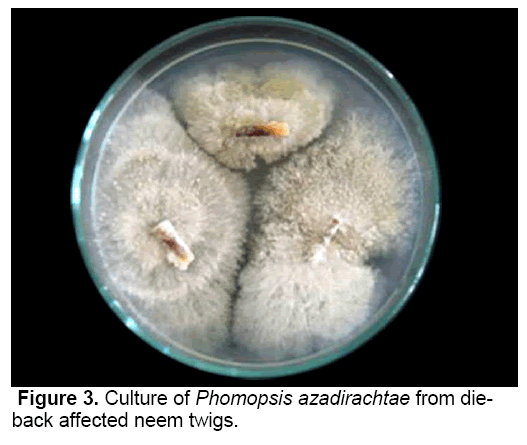
P. azadirachtae, the incitant of die-back on neem is a deuteromycetes fungus [18]. The pathogen was successfully isolated from all the twig explants collected from diseased neem trees on PDA medium (Figure 3). Though many species of Phomopsis have been reported to have teliomorph (Diaporthe), it was not identified with P. azadirachtae. It was not possible to induce teliomorphic or sexual phase in P. azadirachtae, in spite of cultivating the pathogen on specific media, under specific conditions required to induce sexual phase. No collateral host has been identified.
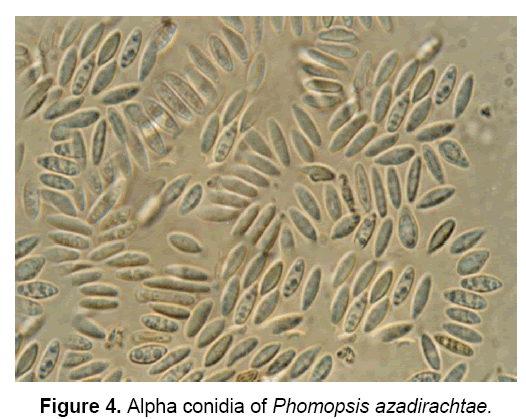
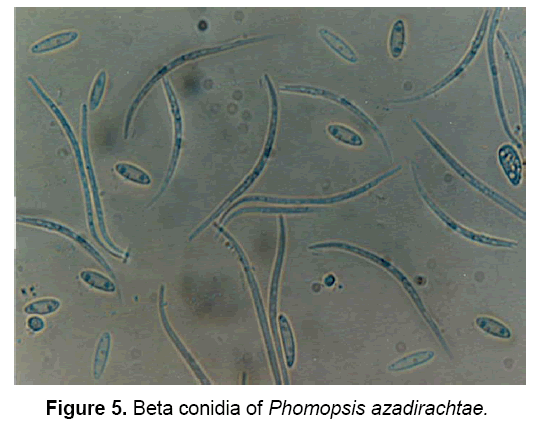
The pathogen produces two types of spores: Alpha(α) – conidia (Figure 4) and Beta(β)- conidia (Figure 5). α – conidia are fertile and germinate readily but germination of β- conidia has not been observed. The ergosterol estimation study confirmed the presence of pathogen in neem tissues [3].
The description of pathogen is as follows: “Mycelium immersed, branched, septate, profuse, colourless, becomes pale brown later. Conidiomata pycnidial, solitary or aggregate, half-immersed, pale brown to dark brown or black, ampuliform or subglobose, unilocular, thick-walled, textura annularis, uniform throughout with the endogenous basal swelling cone with lumina of bigger cells, outer layers melanised, 300-500 μm high, up to 900 μm, wide in sections, very short basal clypei, ostiole single, unilocular, circular, papillate. Conidiophores simple or branched, short or elongate, septate, filiform, hyaline, line the inner layer of locule, 12-20 X 1.6-2.0 μm conidiogenous cells phialidic, subulate or filiform, integrated or discrete, channel and collarette minute, hyaline, periclinal thickenings of variable thickness, 5-8 X 1.6-3 μm, produce both alpha-conidia and beta-conidia, conidia acropleurogenous. Conidia of two types, in a cream to dark yellow coloured slimy cirrhi: alpha-conidia hyaline, fusiform, straight, 2-4 guttulate, smooth, aseptate, 4.8-11 X 1.6-3.2 μm, germinate readily, beta-conidia hyaline, filiform, hamate, eguttulate, aseptate, 16-25.6 X 1.6-2.0 μm germination unknown” [18].
3.3 Cultural conditions
Light was found to have effect on sporulation, but not on mycelial growth. Sporulation requires proper light of about 8-12h per day along with high relative humidity. The optimum temperature for vegetative growth of P. azadirachtae is in the range of 26-28°C and the pathogen can grow in a wide temperature range of 10-35°C. Optimum pH was found to be 6 (range 4-9). Out of eleven carbon sources viz., cellobiose, cellulose, fructose, galactose, glucose, maltose, mannitol, lactose, sorbitol, starch, and sucrose - sucrose and starch were found to be the best carbon sources. Among the different nitrogen sources such as ammonium sulphate, asparagine, glycine, potassium nitrate, sodium nitrite and urea - ammonium sulphate and potassium nitrite were opted by the pathogen. Among the different vitamins supplied viz., thiamine, pyridoxine, riboflavin, nicotinic acid, biotin and inositol, P. azadirachtae grew well on thiamine, riboflavin, nicotinic acid and pyridoxine amended media. The pathogen requires about 8-10 days for complete growth of mycelial mat in a 90mm diam. Petri dish and sporulation starts after 15 days. The nature of pathogen to grow under wide range of physical conditions and to assimilate various chemical factors indicates its ability to survive in varied environmental conditions [3].
3.4 Pathogenicity studies
Conidial inoculation, mycelial inoculation and tooth-pick inoculation methods were tried. Conidial inoculation method proved to be suitable to establish the pathogen in neem plant. Establishment of pathogen resulted in the development of twig blight symptom characteristic of die-back disease. The same fungus was isolated from the twigs of all the neem plants inoculated with conidia [3]. The pathogen was unable to infect allied taxon of the same family, Melia azedarach, revealing its restricted host range to A. indica [3]
3.5 Viability of mycelia and conidia
At room temperature, aerial mycelia remained viable for about 18 months on all types of media tested. Viability of mycelium varied at refrigerated conditions with respect to different media ranging from 21-36 months [3]. The viability and germination of conidia also varied depending on the type of media and storage conditions. Conidia could be maintained viable up to 24 months at room temperature and up to 36 months at refrigerated conditions especially on Potato Dextrose Agar (PDA) and Malt Extract Agar (MEA) [3]. This ability of pathogen to retain viability of conidia and mycelia for comparatively long time in varied environmental conditions may be the reason for the reoccurrence of the disease in subsequent monsoon reasons [3].
3.6 Seed-borne nature of P. azadirachtae in neem
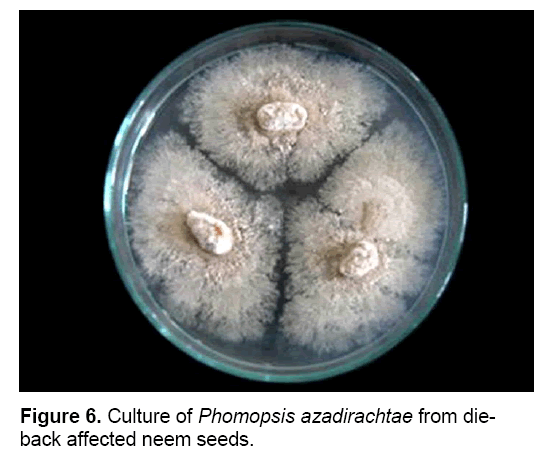
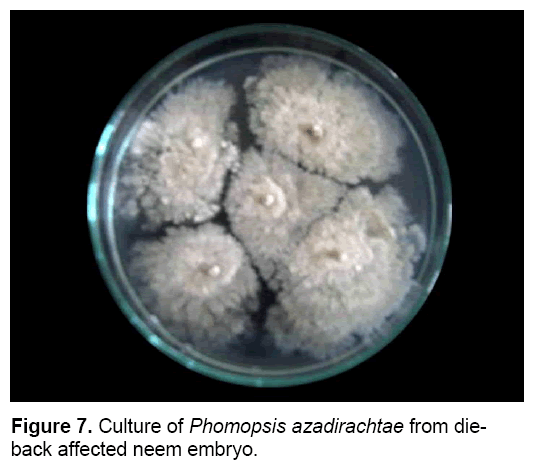
P. azadirachtae is seed-borne and seed transmitted (Figure 6). Thus it gets transmitted from seed to seedling and might result in wide spread of disease. Studies on the seed-borne nature of the pathogen revealed that P. azadirachtae was deep seated. The pathogen was present in seed coat, cotyledons and embryo. Many other fungi were isolated from seeds, except embryo, such as Aspergillus ochraceous, A. niger, A. flavus, Fusarium oxysporum, Penicillium sp. and Mycelia sterilia. Only P. azadirachtae was isolated from embryo (Figure 7). Seed to seedling transfer studies revealed that the pathogen can cause seed rot, seedling rot, formation of weak seedlings, seedlings with fibrous root system and without root system [3,23].
3.7 Biological studies
Variations among the P. azadirachtae isolates collected from different districts of Karnataka and Tamilnadu, South India were studied. P. azadirachtae isolates showed cultural, morphological, pathogenic and biochemical variation. Significant differences in the mycelial type, colour of the colony, texture, radial growth and number of pycnidia were observed among the isolates [27,28]. Isolates of P. azadirachtae collected from different geographical regions of Karnataka [29] and Tamilnadu [30] exhibited marked variations in their electrophoretic protein profile. All these parameters investigated suggest the presence of intraspecific variability among the P. azadirachtae isolates and its heterogeneous nature.
Histopathological studies of naturally infected neem tree explants and seeds showed the presence of the pathogen in diseased neem tissues. The pathogen was found to be both intra as well as intercellular. The pathogens' presence was also observed in vascular tissues revealing its systemic nature [27,31].
Fluorescence microscopy approach to evaluate the viability of conidia showed that α- conidia are more fertile than β- conidia. It was difficult to study viability of β- conidia using natural autofluorescence method as β- conidia didn’t germinate readily. The percentage germination data of α- conidia didn’t match the percentage non-fluorescing conidia data, and this might be because of presence of dormant α- conidia [27,32].
P. azadirachtae produces phytotoxins. Culture filtrate of the pathogen inhibited the germination of neem seeds indicating the production of a phytotoxic secondary metabolite that can reduce the seed vigour and seed quality [3]. The isolates of P. azadirachtae collected from different regions of Karnataka [27] and Tamilnadu [28] varied in the phytotoxic effect of their culture filtrates on the neem seed germination. Bioassay of crude toxin extract of P. azadirachtae against neem callus growth resulted in the progressive inhibition of callus growth and necrosis [28]. Callus was obtained from cotyledonary explants on Murashige-Skoog (MS) medium amended with 1 ppm BAP and 1 ppm Kinetin as per Sateesh [3].
3.8 PCR-based detection / identification of Phomopsis azadirachtae
Methods to detect P. azadirachtae using polymerase chain reaction (PCR) have been developed. rDNA sequences of many Phomopsis spp. were retrieved from the database and were subjected for multiple alignment using CLUSTAL program to select conserved sequences, which were used to design Phomopsis specific primer pairs (forward and reverse) using Primer3 Software. Specificity of the selected sequence with Phomopsis sp. was examined by comparing the DNA data to those of the GenBank database. The two primers, Phf-5'CGGATCTCTTGGTTCTGGCA-3' and Phr-5' GACGCTCGAACAGGCATGCC-3' have the potential to produce 141-bp DNA as amplified product [33] and the primer PF1 (5'-ATCTCTTGGTTCTGGCATCG) and primer PF2 (5'-GCTTGAGGGTTGAAATGACG) have the potential to produce a 154 bp DNA as amplified product [34] in PCR.
P. azadirachtae was isolated on PDA from die-back infected neem twigs. The DNA was extracted from all the isolates. Phomopsis genus-specific primers were then used for the amplification of extracted DNA by PCR. Primers, Phf and Phr produced 141-bp DNA as amplified product [33] and the primer PF1 and primer PF2 produced a 154 bp DNA as amplified product [34] confirming the isolates as Phomopsis .
P. azadirachtae was isolated on PDA from die-back infected neem twigs, seeds and embryo. The DNA was extracted from all the isolates. Amplification of DNA producing expected 141 bp product indicated the organism isolated from die-back affected neem tissues was P. azadirachtae [35]. The primers PF1 and PF2 were utilized to detect the presence of P. azadirachtae in diseased neem tissues such as neem twigs, neem seeds and embryo. DNA was isolated from die-back affected neem tissues and amplified using the mentioned primers, which resulted in the yield of a PCR product of expected size i.e., 154 bp, confirming the presence of P. azadirachtae and its systemic nature [34].
DNA samples isolated from other fungi such as Aspergillus sp., Fusarium sp., Penicillium sp., Mycelia sterilia and an unidentified bacterium isolated on neem were also amplified using the above mentioned two primer pairs. No amplification occurred confirming the Phomopsis specific nature of these primers [33,34]. DNA samples extracted from a few isolates of P. azadirachtae collected from different regions of Tamilnadu were also amplified using the primers PF1 and PF2, which amplified all the DNA samples to produce the expected 154 bp size PCR product. Thus, any isolate of P. azadirachtae can be identified using this primer pair [34].
3.9 Study of disease incidence
A disease survey of die-back of neem was done, using Global Positioning System (GARMIN 12), in different agroclimatic regions of Karnataka and Tamilnadu, India. Hand held GPS used in this study helps in continuous monitoring of the diseased trees. The details of individual tree such as height, girth, age, latitude, longitude, altitude and disease severity at each surveyed place were obtained and plotted on topographic maps, using MAPINFO software. Survey revealed that the severity of die-back disease was independent of tree age and size. A very high incidence of die-back was observed in most places of Karnataka and Tamilnadu, which was almost 100% in most of the places irrespective of climatic conditions [36,37].
3.9 Management studies
Six different systemic fungicides viz., Bavistin 50% W.P., Bayleton 25% W.P., Baynate 75% W.P., Calixin 80% E.C. and Kitazin 48% E.C., were tried. Bavistin was most effective, which completely suppressed mycelial growth, sporulation and conidial germination at 0.3 ppm. Treatment of neem seeds with bavistin resulted in the death of the seed-borne pathogen. Germination of neem seeds was not affected by bavistin even at higher concentrations (up to 2000 ppm) [3]. Carbendazim (bavistin) at 0.25 ppm and thiophanate methyl at 0.75 ppm controlled the growth of the pathogen completely [38].
Effect of bavistin on neem callus cultures was studied. Cotyledonary explants produced good callus on Murashige-Skoog (MS) medium amended with 1 ppm BAP and 1 ppm Kinetin. Exposure of neem calluses to bavistin at 200 ppm and above resulted in reduced growth and necrosis was observed on exposure to bavistin at 500 ppm and above concentrations [3].
Bacterial antagonists, Bacillus cereus, B. subtilis, Enterobacter aerogenes, Pseudomonas fluorescence and fungal antagonists, Trichoderma harzianum, T. viridae, Gliocladium virens, Aspergillus niger, A. oryzae, Penicillium chrysogenum, Chaetomium globosum were tested against P. azadirachtae by dual culture method. Bacillus subtilis showed significant inhibitory effect on P. azadirachtae. Ten times concentrated culture filtrate of B. subtilis completely inhibited the growth of P. azadirachtae. Volatile compounds of B. subtilis had no effect on the growth of pathogen. B. subtilis produced both heat labile and heat stable antibiotics. Heat labile antibiotics were found to be highly potent against P. azadirachtae [3].
The ethyl acetate fractions of culture filtrates of six antagonistic microorganisms such as Bacillus cereus, Bacillus subtilis, Pseudomonas aeruginosa, Pseudomonas oleovorans, Trichoderma harzianum and Trichoderma viride were tested for their antifungal activity against P. azadirachtae. B. subtilis and Ps. aeruginosa were highly effective in suppressing the growth of the pathogen at very low concentration i.e., at 25 ppm [28].
Effect of 24 botanical pesticides was studied. Only five plant species produced good results i.e., Lawsonia inermis, Asparagus officinalis, Bambusa arundinacea, Lantana camera and Macrosolen parasiticus. L. inermis produced greater inhibitory effect at lower concentration in comparison with other four plant species. Ethanol, methanol and aqueous extracts of L. inermis were tried and aqueous extracts were highly effective against P. azadirachtae [3].
Five essential oils – eucalyptus oil, pepper oil, nutmeg oil, coriander oil, fennel oil and two oleoresins – turmeric oleoresin and capsicum oleoresin were screened against P. azadirachtae. High activity was observed with nutmeg oil which completely inhibited the growth of pathogen at 2000 ppm [27].
4. Conclusion
The nutritional requirements, and physiological conditions required for the growth of P. azadirachtae have been understood. Isolation of the pathogen from die-back affected neem explants collected from different regions of Karnataka and Tamilnadu shows that the disease is spreading at an alarming rate and is prevalent in almost all neem growing areas. The seed-borne nature of pathogen may be one of the reason for wide spread nature of the disease.
The pathogen is highly variable. This may be because of the difference in environmental and ecological conditions of various geographical regions. The basic understanding of the biology of the pathogen would help in preventing the spread of the disease and thereby protecting the healthy neem trees from damage. Geographic information and global positioning system can be effectively used in tree disease management.
PCR methodology for identification of the pathogen in diseased neem explants has been developed. Isolation and identification of P. azadirachtae by conventional method requires about 15 to 21 days, whereas the PCR-based technique is capable of detecting very low propogules within 4 - 5 days or directly from diseased tissues in even less time. The primers developed amplify the Phomopsis specific DNA and could be utilized for rapid and reliable PCR-based detection of the pathogen in neem tissues especially in seeds. This will help to quarantine the neem seeds and prevent the spread of the disease.
The pathogen produces toxin and the toxin seems to have a role in the pathogenesis. Although pathogenesis has been understood to some extent, proper knowledge of toxin chemistry and its role in pathogenesis requires further investigations and the current investigations provide a proper base for this. The management strategy was successful in vitro against P. azadirachtae and needs field tests to go for large scale applications.
References
- Bahuguna, V.K. (1997) Silviculture and management practices for cultivation of Azadirachta indica (Neem). Indian For, 123: 379-386.
- Chari, M.S. (1996) Neem and transfer of technology. In: Neem and Environment (Vol. I), (Singh, R.P., Chari, M.S., Raheja, K., et al., eds.). Oxford and IBH publishing Co. Pvt. Ltd., New Delhi, India.
- Sateesh, M.K. (1998) Microbiological investigations on die-back disease of neem (Azadirachta indica A. Juss.). Ph.D. thesis. University of Mysore. Mysore, India.
- Anonymous. (1992) Neem – A tree for solving global problems. National Academy Press, Washington D.C., U.S.A.
- Tewari, D.N. (1992) Monograph of neem (Azadirachta indica A. Juss.). International Book Distributors, Dehra Dun, India.
- Shankara Bhat, S., Sateesh, M.K., Devaki, N.S. (1998) A new destructive disease of neem (Azadirachta indica) incited by Phomopsis azadirachtae. Curr Sci, 74: 17-19.
- Srivastava, S.K., Patel, P.N. (1969) Epidemiology of bacterial leaf spot, blight and shot-hole disease of neem in Rajasthan. Indian Phytopathol, 22: 237-244.
- Chakravarthi, B.P., Gupta, D.K. (1975) A non- pigmented strain of Xanthomonas azadirachtii Moniz and Raj causing leaf spot of neem (Azadirachta indica A. Juss.). Curr Sci, 44: 240-241.
- Moniz, L., Raj, H.H. (1967) Xanthomonas azadirachtii sp. nov. causing leaf spot disease in Azadirachta indica A. Juss. Indian J Microbiol, 7: 159-160.
- Bakshi, B.K. (1976) Forest pathology: Principles and practice in forestry. Forest Research Institute, Dehra Dun, India.
- Singh, I., Chauhan. (1984) Phoma jolyana, a new pathogen on neem (Azadirachta indica). Indian For, 110: 1058-1060.
- Castellani, E., Mohamed, M.I. (1984) Diseases of Azadirachta indica in Somalia. 1 Cercospora disease. Riv Agric Subtrop e Trop 78: 733-739.
- Sankaran, K.V., Balasundaran, M., Sharma, J.K. (1986) Seedling diseases of Azadirachta indica in Kerala, India. Eur J For Pathol, 16: 324-328.
- Mehrotra, M.D. (1990) Rhizoctonia solani, a potentially dangerous pathogen of Khasi pine and hardwoods in forest nurseries in India. Eur J For Pathol, 20: 329-338.
- Mehrotra, M.D. (1989) Leaf blight of some hardwood species in Assam and Meghalaya and its control in the nursery. Indian For, 115: 378-384.
- Mehrotra, M.D., Pandey, P.C. (1992) Some important nursery diseases of Azadirachta indica and their control. In: Monograph of Neem (Azadirachta indica A. Juss) (Tewari, D.N., Author). International Book Distributors, Dehra Dun, India.
- Chakraborthy, R., Konger, G. (1995) Root-rot of neem (Azadirachta indica) caused by Ganoderma applanatum. Indian for, 121: 1081-1082.
- Sateesh, M.K., Shankara Bhat, S., Devaki, N.S. (1997) Phomopsis azadirachtae sp. nov. from India. Mycotaxon 65: 517-520.
- Uniyal, K. (1999) Collar-rot in Azadirachta indica and its control. Indian for, 125: 513-516.
- Ghasolia, R.P., Shivpuri, A. (2004) Neem- A new host of Sclerotinia sclerotiorum. J Mycol Plant Pathol, 34: 200.
- Sinniah, D., Varghese, G., Bhaskaran, G., et al. (1983) Fungal flora of neem seeds and neem oil toxicity. Malay Appl Biol, 38: 20-25.
- Verma, N., Srivastava, K.K. (1993) Fungal species associated with seeds of some common desert tree species. In: Proceedings of 45th annual meeting. Indian Phytopathological Society, Akola, Maharashtra, India.
- Sateesh, M.K., Shankara Bhat, S. (1999) Detection of seed–borne Phomopsis azadirachtae and its transmission in Azadirachta indica (Neem). Seed Sci Technol, 27: 753-759.
- Rajgopal, K., Suryanarayanan, T.S. (2000) Isolation of endophytic fungi from leaves of neem (Azadirachta indica A.Juss.). Curr Sci, 78: 1375-1378.
- Mahesh, B., Tejesvi, M.V., Nalini, M.S., et al. (2005) Endophytic mycoflora of inner bark of Azadirachta indica A. Juss. Curr Sci, 88: 218-219.
- Khan, S.N. (1992) Record of Phomopsis twig blight in Azadirachta indica from Dehra Dun (unpublished). In: Monograph of Neem (Azadirachta indica A. Juss) (Tewari, D.N., Author). International Book Distributors, Dehra Dun, India.
- Fathima, S.K. (2004) Investigations on the biology and management of Phomopsis azadirachtae on neem. Ph.D thesis, University of Mysore, Mysore, India. pp 135.
- Girish, K. (2007) Studies on the biology and management of Phomopsis azadirachtae – the incitant of die-back disease on neem (Azadirachta indica A. Juss). Ph.D. thesis, University of Mysore. Mysore, India.
- Fathima, S.K., Shankara Bhat, S., Girish, K. (2004) Variation in Phomopsis azadirachtae, the incitant of die-back of neem. Indian Phytopathol, 57: 30-
- Girish, K., Shankara Bhat, S., Raveesha. K.A. (2007) Intraspecific variability in Phomopsis azadirachtae infecting neem. Arch Phytopathol Plant Protect, DOI: 10.1080 / 03235400701191580.
- Fathima, S.K., Shankara Bhat, S., Sateesh, M.K. (2004) Histopathology of neem seeds naturally infected with Phomopsis azadirachtae. Seed Res, 32: 93-95.
- Fathima, S.K., Shankara Bhat, S. (2004) Natural autofluorescence in the conidia of Phomopsis azadirachtae. Asian J Microbiol Biotechnol Environ sci, 6: 21-24.
- Nagendra Prasad, M.N., Shankara Bhat, S. Charith Raj, A.P., et al. (2006) Molecular detection of Phomopsis azadirachtae the causative agent of die-back disease of neem by Polymerase chain reaction. Curr Sci, 91: 158-159.
- Girish, K., Shankara Bhat, S., Raveesha, K.A., (2007) PCR-based detection of Phomopsis azadirachtae in die-back affected neem seeds. Arch Phytopathol Plant Protect, DOI: 10.1080 / 03235400701281628.
- Nagendra Prasad, M.N., Shankara Bhat, S., Charith Raj, A.P. (2007) Detection of Phomopsis azadirachtae from die-back affected neem twigs, seeds and embryo by Polymerase chain reaction. Arch Phytopathol Plant Protect, DOI: 10.1080/03235400600982584.
- Nagendra Prasad M.N., Shankara Bhat, S., Haraprasad, N. (2007) Die-back disease of neem in Karnataka and Tamilnadu, India. In: Proccedings of World neem conference. Fifth World Neem Conference, 21-24 Nov. 2007, Coimbatore, India. p 42.
- Nagendra Prasad, M.N., Shankara Bhat S., Haraprasad, N., et al. (2008) Study of die-back disease incidence of neem in Karnataka, India and PCR based identification of the isolates. Arch Phytopathol Plant Protect, DOI: 10.1080/03235400701875406.
- Girish, K., Shankara Bhat, S., Raveesha, K.A. (2007) In vitro screening of systemic fungicides against Phomopsis azadirachtae, the incitant of die-back disease of neem. Arch Phytopathol Plant Protect, (DOI 10. 1080/03235400601036646)

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences